Thermal DSC DTA TG all slides Oct 28 2002
advertisement

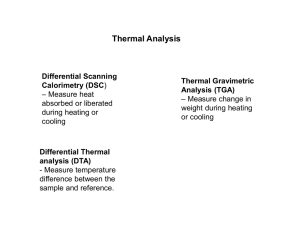
Analysis of Materials (Polymers) by Thermal Methods: DSC, TG/DTA Instructor: Ioan I. Negulescu CHEM 4010 Tuesday, October 29, 2002 Thermal Methods: Thermal methods are based upon the measurement of the dynamic relationship between temperature and some property of the system such as mass and heat absorbed or evolved by/from it. • Differential Scanning Calorimetry, DSC • Differential Thermal Analysis, DTA • Thermogravimetry, TG are the most important thermal methods used in characterization of polymers. The temperature increase, T, of a body which is heated is directly proportional to the amount of heat absorbed, Q, and inversely proportional to its mass, m, and its capacity C to store heat: T = Q/m C Eq. 1 Consider the temperature increase of two different samples of the same mass m1 = m2 for which the same amount of heat was given Q1 = Q2 If their heat capacities are different C1 C2 they do not experience the same temperature increase, i.e., T1 T2 A greater heat flow (dQ/dt, where t is time) will always flow into the sample whose heat capacity is higher, in order that the steady-state heating rate be maintained. The heat capacity at constant pressure (Cp) of a material is defined as the temperature increase of a unit of substance (mass) as a result of the supply of a unit of heat at constant pressure. For the same substance, Cp is dependent upon its aggregation state, i.e., it is different for the liquid state as compared to loose gaseous or to more compact solid state. A polymeric material has different heat capacities for amorphous or crystalline morphologies. For amorphous polymers, the heat capacity for the glassy state (i.e., below glass transition, Tg, where only vibrations of atomic groups occur) is different from that characterizing the leathery (short range diffusional motion, i.e., of chain segments), rubbery (retarded longrange motions), rubbery flow (slippage of long-range entanglements) or liquid state. Figure 1. Temperature - molecular mass diagram for amorphous polymers: (1) Glass transition (Tg); (2) Diffuse transition zone; and (3) Thermal decomposition. Figure 2. Temperature - Molecular Mass diagram for (semi) -crystalline polymers: (1) Glass transition (Tg); (2) Melting point (Tm); (3) Diffuse transition zone; and (4) Thermal decomposition. For amorphous polymers, the glass-rubber transition temperature is of considerable importance technologically. It (Tg) determines the lower use limit of a rubber (e.g., polydienes, Tg -50°C) and the upper limit use of an amorphous thermoplastic material(e.g., polystyrene, Tg 100 ° C). In the case of (semicrystalline) linear polymers it is possible to identify a melting temperature (Tm). Above this temperature the polymer may be liquid, viscoelastic or rubbery according to its molecular mass, but below it, at least in the high molecular mass range, it will tend to be leathery and tough down to the glass transition temperature. POLYHYDROXYALKANOATES R can be hydrogen or hydrocarbon chains of up to around C13 in length, and x can range from 1 to 3 or more. Varying x and R effect hydrophobicity, Tg, Tm, and level of crystallinity POLYHYDROXYALKANOATES Fig 4: The Family of PHAs has Physical Characteristics that Allow it to be Used Across a Wide Spectra of Applications Detailed information on glass transition, crystallization, and melting, is therefore critically important in formation, processing and utilization of polymers. Differential thermal methods (DTA and DSC) have been widely applied to the study and characterization of polymeric materials. In DTA the heat absorbed or emitted by a system is observed by measuring the temperature difference (T) between that system (the sample) and an inert reference material (often alumina), as the temperature of both is increased at a constant rate (usually 5-10 C/min). Figure 3. Schematic diagram of a typical DTA apparatus. • In DSC, the sample and the reference are also subjected to a continuously increasing or decreasing temperature. • In the scanning operation the sample and the reference show different temperature independent heat capacities. • Heat (dQ/dt) is added to the sample or to the reference as necessary to maintain the two identical temperatures. Bottom: (b) measured curve. m is the measured heat flux. bl is the heat flux corresponding to the base line and t is time. • The ordinate is usually represented by the heat flux (denominated as or dQ/dt) or by the variation of the heat capacity. • The glass-to-rubber transition, or shortly the glass transition (Tg) is a phase change reminiscent of a thermodynamic second order transition (melting and crystallization being first order transitions) for which a plot of specific heat versus temperature shows a sudden jump. • The first order transitions appear as peaks. DSC curve of a polymeric sample: (1), (3) and (5) are base lines; (2) is glass-to-rubber transition, Tg; (4) is the interpolated base line; and (6) is the first order transition peak. The glass transition region in cooling (a) and subsequent heating (b) mode showing some commonly used definitions of glass transition, Tg. Poly(Lactic Acid) -[-O-CH(CH3)-CO-]n Two of the most attractive features of poly(lactic acid), PLA, polymers are: • they are easily synthesized from renewable resources (corn!) • they are both hydro- and biodegradable Poly (Lactic Acid) Glass transition temperature, Tg. -200 Glass Transition, Tg DSC, W DSC -400 DDSC, W/min 240 200 160 120 80 -600 40 DDSC 0 -800 -40 30 40 50 60 70 o Temperature, C 80 90 DSC traces for melting and crystallization of a polymer sample. Melting of polyoxymethylene with superheating. DSC analysis of a poly(ethylene terephthalate) sample quenched from the melt. DSC traces of Low Crystallinity PLA treated in Water at 70C and 100C. The higher the crystallinity achieved at 100 C, the higher and the less defined the Tg 2000 Same Melting Pattern Crystallization Before Melting W eak Cold Crystallization Strong Tg DSC 70C W 0 Melting DSC 100C W W eak Tg 1000 0 -2000 o 1hr@ 100 C o 1hr@ 70 C 0 50 -1000 100 150 o Temperature, C 200 Melting of two semicrystalline HDPE samples. DSC, W -5.0k ENDO 0.0 H: 132 mj/mg -10.0k o 132 C H: 165 mj/mg -15.0k HDPE Detergent Bottles HDPE Milk Bottles o 134 C -20.0k 110 120 130 140 o Temperature, C 150 Considering H = 200 mJ/mg as the enthalpic change for the melting of a 100% crystalline HDPE sample, from DSC data of these two recyclable HDPE it can be found that: • the polymer derived from detergent bottles was (132/200)x100 = 66% crystalline • the polymer used for milk bottles was (165/200)x100 = 82.5% crystalline. Determination of the HDPE content in a blend with inorganic filler from DSC data. HDPE Detergent Bottles ENDO Heat Flow (m W ) 0 H = 106 mJ/mg -5 % HDPE=( H/ H 100 )x100 H 100 =132 mj/mg -10 % HDPE=(106/132)x100 HDPE = 80.3% 40 60 80 100 o 132.8 C 120 140 o Temperature, C 160 180 Polyhydroxylated Nylons Similarity of Nylon 6,6 and the poly hydroxylated counterpart: DSC Thermal Transitions in Polyhydroxylated Nylon 6,6 3.5k 6k Decomposition 5k H 4k DSC, W 2.5k 3k 2.0k Tm 1.5k 2k Tg 1k DDSC 1.0k 0 500.0 DDSC, W /m in 3.0k -1k DSC 0.0 -2k 0 20 40 60 80 100 120 140 o Temperature, C 160 180 200 Thermogravimetric (TG) analysis is concerned with the change in weight of a material as its temperature changes. This indicates: • the temperature at which the material loses weight through evaporation or decomposition • the temperature at which no weight loss takes place is revealed, which indicates stability of the material. TG Measurement Principle of Seiko TG/DTA Thermobalance Thermal Degradation of Polyhydroxylated Nylon 6,6 15.0k W eight Loss (TG), % o 100 C o 150 C o 200 C o 235 C -20 -6.3% -6.9% -19.0% -50.0% -40 o 10.0k DTG TG 0 205 C o 425 C -60 5.0k -80 DTG 0.0 -100 0 100 200 300 400 o Temperature, C 500 600 Poly (4-dodecyl-1-4-aza heptamethylene-D-glucaramide). Thermal decomposition. 2 o 166 C 0 o 372 C o 1.3%/ C -20 o DTG (%/ C) TG (% Weight Loss) o -1.3%@150 C o 188 C o 0.6%/ C -40 -60 0 -80 o -97.5%@400 C -100 TGpercent 0 100 200 300 400 o Temperature, C 500 600 Initial decomposition of linear polymers. Initial sample weight: 10 mg. Heating rate: 5C/min. Thermogravimetric analysis of a polymeric blend containing HDPE and an inorganic filler (phosphogypsum) 0 % weight loss -10 -20 -30 -40 -50 -60 TGpercentL 0 -62.8% 100 200 300 400 500 600 700 800 o Temperature, C Almost any measurement that can be done at different temperatures can be expanded into thermal analysis; and any series of thermal analysis techniques can be combined with other non-thermal technique for valuable multiple-parameter information. Coupled techniques, such as Thermogravimetry, Differential Thermal Analysis and Mass Spectrometry (TGDTA-MS) or evolved gas analysis of polymers by coupled Thermogravimetry, Gas Chromatography, Fourier Transform Infrared and Mass Spectrometry (TG-GCFTIR-MS) are just two examples often used in industrial laboratories.