Organic Molecules Version 2
advertisement

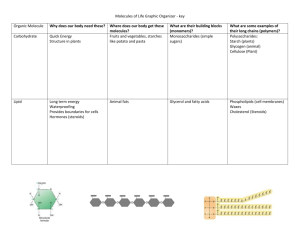
An Introduction to Organic Molecules Organic Molecules Organic molecules are made primarily of four elements : C, N, O, H Backbone is C Polymers are built of small units called monomers. Monomers and Polymers Monomers Polymers Monosaccharide Polysaccharide (Fatty acids)+glycerol Lipids Amino acid Protein Nucleotide Nucleic acid Monomers Build Polymers Polymers are built through a process called dehydration synthesis. Dehydration Synthesis builds POLYMERS Breaking Down Polymers • Polymers are broken down through a process called hydrolysis. Hydrolysis breaks down POLYMERS The FOUR major organic compounds (polymers): Carbohydrates Lipids Proteins Nucleic Acids Carbohydrates - all types of sugars Monosaccharides : Simple Sugars • All monosaccharides have the basic chemical formula CH2O • Function: Immediate Energy!!! Common sugars are glucose, fructose and galactose. Disaccharides : Double Sugars All disaccharides are made of 2 monosaccharides linked together Function: Energy Polysaccharides : Complex sugars (aka. Complex carbs) • All polysaccharides are built of monosaccharides • Two functions of polysaccharides are storage and structure. Energy Storage Polysaccharides Starch: plant Glycogen : animals Structural Polysaccharides Cellulose : plants Chitin: animals (invertebrates) Review of Carbohydrates • • • • Monomers : Polymers: Structure: Functions: Proteins Proteins - chains of amino acids • Proteins make up 50% of the dry weight of most cells (that’s a LOT!!) • Proteins build structure and carry out cell metabolism • Proteins are built of amino acids. • There are 20 types of amino acids. Types of Proteins • • • • • • • Structural Storage Transport Hormonal Contractile Antibodies Enzymes Structural : elastin, collagen, keratin Storage : ovalbumin & casein Transport : hemoglobin & membrane proteins Hormonal : insulin & growth hormones Contractile : actin & myosin (both in muscle fibers) Enzymes (speed up chemical reactions): amylase & protease Review of Proteins • • • • Monomers : Polymers: Structure : Functions : LIPIDS Polymers that don’t mix with water! This means they are hydrophobic. Groups are FATS, PHOSPHOLIPIDS & STEROIDS Fats = 1 Glycerol + 3 Fatty Acids Fats are large molecules made of 2 monomers : glycerol + fatty acids. There are two types of fatty acids - saturated and unsaturated. Functions of fats include : insulation, energy storage, shock absorber for internal organs (like bubble wrap!) Saturated Fatty Acids Characteristics: Solid at room temperature (can pack together tightly) Mostly found in animals No double bonds between Carbons (filled with Hydrogens) Examples : Unsaturated Fatty Acids Characteristics Liquid at room temperature (can’t pack together tightly) Found mostly in plants Double bonds found between Carbons (not filled with Hydrogens) Examples : Phospholipids = 2 Fatty Acids + Phosphate • Phospholipids are found in the cell membrane. • Phospholipids have polar (charged) & nonpolar (not charged) ends. The phosphate end is HYDROPHILIC. The fatty acid end is HYDROPHOBIC. • The unique structure (polar & nonpolar) contributes to the function of these molecules in the cell. Steroids = Carbon skeleton with 4 fused rings not just anabolic steroids!! • Steroids are a natural and important components of the cell membrane in many organisms. • An example is cholesterol - found ONLY in animal tissues. It is used to help construct other important hormones in organisms. Steroids (continued) • Examples of hormones created using steroids are estrogen, progesterone and testosterone. • Anabolic steroids are a synthetic form of testosterone to increase muscle mass. Both men and women who take testosterone increase the masculine physical features normally triggered by varied levels of testosterone in the body. Review of Lipids • Monomers of FATS: • Functions of fats: • Monomers of PHOSPHOLIPIDS: • Functions of phospholipids: • Structure and function of steroids: Nucleic Acids: the polymers built of nucleotides DNA RNA Nucleotides - the building blocks of nucleic acids DNA - deoxyribonucleic acid • Characteristics – – – – Double helix Four nitrogenous bases : A, T, C, G Deoxyribose sugar (5-carbon sugar) Function : storage of genetic codes RNA - ribonucleic acid • Characteristics – – – – Single stranded Four nitrogenous bases - A , U, C, G Ribose sugar (5-carbon sugar) Function : transcribe and translate DNA into proteins Review of Nucleic Acids • • • • Monomers : Polymers : Structures: Functions :