PLANKTON COMMUNITIES OF ARTIFICIAL LAKES CREATED ON IRISH CUTAWAY PEATLANDS
advertisement

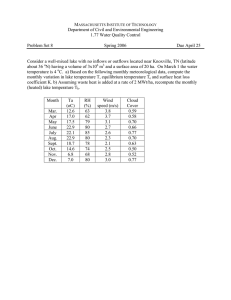
PLANKTON COMMUNITIES OF ARTIFICIAL LAKES CREATED ON IRISH CUTAWAY PEATLANDS Tara Higgins, Henry Kenny and Emer Colleran ABSTRACT Lake creation represents a major post-harvesting land-use option for industrial cutaway peatlands in Ireland, yet little is currently understood about the ecology of cutaway water-bodies, particularly at the microbiota level. The current paper describes for the first time the littoral zooplankton community in three contrasting artificial cutaway lakes and one natural bog lake. The study lakes, which were sampled on five occasions in 2003, contrasted strongly in their physico-chemical characteristics, nutrient states and trophic classifications. These trends reflected variations between sites in sediment types, water supply sources and catchment land uses. In the absence of fish, invertebrate predation by cyclopoid copepods (Tropocyclops prasinus ) and cladocerans (Polyphemus pediculus) appeared to play an elevated role in structuring the zooplankton communities in the study lakes, in terms of abundance, species composition and size structure. Tara Higgins (corresponding author, e-mail: tara. higgins@nuigalway. ie), Department of Zoology, National University of Ireland, Galway; Ireland; Henry Kenny and Emer Colleran Environmental Microbiology Research Unit, Department of Microbiology, National University of Ireland, Galway. Received 26 May 2006. Accepted 15 March 2007. Published 20 July 2007. BIOLOGY AND INTRODUCTION Of the 80,000 hectares of peatlands coming out of commercial production in Ireland by 2030, Bord na Móna, the Irish Peat Board, envisages that more than 50% will be designated as semi-natural wilderness areas for conservation and recreation uses (Egan 1999). This will include an estimated 20,000 hectares of shallow lakes and wetlands. The scale of the proposals is vast, representing one of the largest wildlife habitat creation opportunities to emerge in Europe in modern times. An initial series of experimental lakes has been created within a 2000ha cutaway raised bog site in the Irish midlands, in a pilot rehabilitation project called the Lough Boora Parklands. Smaller-scale lake creation trials are also being conducted at Bord na Móna’s Oweniny Atlantic blanket bog complex near Bellacorick in County Mayo. Approaches used when developing lakes on industrial cutaway peatlands have varied enormously in terms of the degree of peat removal, basin construction, hydrological manipulation and post-flooding management. Cutaway lakes, as a consequence, differ markedly in their water chemistry characteristics and trophic statuses, ranging from alkaline, oligotrophic angling lakes with inorganic sediments to highly acidic, hypertrophic waterbodies underlain exclusively by peat (Higgins and Colleran 2004; 2006). Because cutaway lakes are new and highly complex systems, very little is understood about their ecology as yet, particularly at the level of the microbiota, and there are at present no methods for predicting development ENVIRONMENT: PROCEEDINGS OF THE that are based on experience. As part of a wider investigation into the potential conservation value of cutaway lakes (Higgins and Colleran 2006), the current research provides the first ever assessment of the littoral zooplankton communities of three contrasting cutaway lake types and a natural, intact bog lake. The primary factors governing zooplankton distribution in cutaway lakes are elucidated in this paper, and the wider significance of these findings for cutaway lake ecology is assessed. METHODS STUDY SITES AND FIELD SAMPLING Three artificial cutaway peatland lakes and one natural blanket bog lake were selected for this study. The study lakes differed in terms of their location, age, type of underlying sediments and littoral vegetation cover (Table 1). Two of the cutaway lakes, Turraun (Fig. 1c) and Clongawny (Fig. 1a), were located on cutaway raised bog in mid-west County Offaly (for detailed descriptions, see Higgins and Colleran (2006)). The third cutaway lake, Bellacorick cutaway lake, was located on cutaway blanket bog at Bellacorick in north-west County Mayo. The fourth study site, regarded as a reference bog lake, was situated on intact Atlantic (lowland) blanket bog at Bellacorick, Co. Mayo. Samples for zooplankton analysis were collected on a monthly basis between June and October 2003. Samples were collected from the two Bellacorick lakes on 25 June, 24 July, 20 ROYAL IRISH ACADEMY, VOL. 107B, NO. 2, 77 85 (2007). # ROYAL IRISH ACADEMY 77 BIOLOGY AND ENVIRONMENT August, 25 September and 30 October, while samples from the midland lakes were collected on 23 June, 22 August, 19 August, 23 September and 29 October. Six-litre surface water samples (0.2m) were collected from between eight and ten different sampling stations along the littoral zone of each lake. Samples were screened though a 90mm mesh in a filtering cone, to give a single composite sample for each lake. Composite samples were gently washed with 100% ethanol into 500ml wide-mouth plastic storage bottles. PH and conductivity were determined on site using a field kit (WTW P4 Multiline). Composite 2l water samples for physico-chemical, nutrient and phytoplankton analysis were also collected from each lake. WATER CHEMISTRY ANALYSIS Dissolved colour was read spectrophotometrically at 465nm on samples filtered through GF/C filters, while alkalinity was calculated using a standard H2SO4 titration method (APHA 1998). Soluble inorganic carbon was measured on filtered samples using a Shimadzu TOC-5000A analyser (APHA 1998), and silicate was determined on filtered samples using a low-range heteropoly blue method (Hach 2001). Soluble reactive phosphorus (SRP) and total phosphorus (TP) were analysed using the ascorbic acid reduction method (Murphy and Riley 1962), involving persulphate digestion of unfiltered samples for TP. Ammonium, nitrate and nitrate were determined spectrophotometrically on filtered samples using low-range indophenol blue (Chaney and Morbach 1962), cadmium reduction and diazotisation methods, respectively (Hach 2001). The sum of nitrate, nitrite and ammonium is reported as dissolved inorganic nitrogen (DIN). Table 1 * ZOOPLANKTON AND PHYTOPLANKTON ANALYSIS Zooplankton subsamples were enumerated in a custom-made, mechanically rotating, circular counting tray and binocular dissecting microscope. At least 100 individuals of the more common species were counted. Identifications of zooplankton to the genus or species level, where possible, were based on standard identification keys, with expert verification where necessary (M. Holmes and E. De Eyto, pers. comm.). Size determinations were made using a micrometer fitted in one of the microscope eyepieces; biovolumes were calculated by comparing individual species to geometric shapes and applying the relevant geometric formula. Size measurements were taken of at least five individuals of each species from every sample counted. Species richness (S ) was estimated as the total number of species recorded (MacIntosh 1967). Species diversity was assessed using Simpson’s index (D) (Simpson 1949), which ranks samples on a scale of 0 (a community comprised of a single species) to 1 (all species in a community are present in equal proportion). Phytoplankton cells were preserved in Lugol’s iodine and were identified, measured and counted Characteristics of the four study lakes, Turraun cutaway lake, Clongawny cutaway lake, Bellacorick natural lake and Bellacorick cutaway lake. Location Year constructed Size (ha) Mean depth (m) Lake sediments Littoral vegetation biomass (kg m 2) Dominant littoral vegetation 78 Samples for chlorophyll a analysis were filtered immediately on return to the laboratory through GF/C filters, extracted using a mixture of 90% acetone and dimethly sulfoxide (1:1 v/v) (Burnison 1980) and measured spectrophotometrically with correction for phaeopigments. All water chemistry analyses were performed in triplicate; data presented are overall means for each lake9the standard error of the mean (SEM). Turraun cutaway lake Clongawny cutaway lake Bellacorick natural lake Bellacorick cutaway lake 07844?W 53815?N 1991 55 0.5 Phragmites peat, shell marl 38 07853?W 53810?N 2001 12 1.0 Sphagnum peat, woody fen peat 0.6 54806?N 9834?W n/a 10 1.5 Cyperaceous peat 35 54807?N 9835?W 1995 6 0.8 Highly humified cyperaceous peat 18 Typha Phragmites Juncus Phragmites Juncus Schoenus Molinia Calluna Juncus Eriophorum Carex PLANKTON COMMUNITIES OF CUTAWAY PEATLAND LAKES * Fig. 1 (a) Clongawny cutaway lake, showing the paucity of recolonising vegetation and the bare, unconsolidated nature of the peaty sediments at the site; (b) the industrial peatland landscape, pre-flooding; (c) flooded cutaway peatland at Turraun, showing the success and extent of revegetation at this site. with an inverted microscope. Biovolumes were calculated by comparing individual cells to simple geometric shapes and applying relevant standard formulae (Rott 1981). Phytoplankton species diversity was assessed using Simpson’s index of diversity (Simpson 1949). RESULTS A number of distinct trends are evident from the physico-chemical and biological data for the four study lakes presented in Table 2. Turraun was an alkaline, largely clear water, mesotrophic eutrophic lake, with a moderately diverse phytoplankton assemblage comprising a mixture of chlorophytes, cyanophytes and diatoms. Clongawny was an acidic, highly stained, eutrophic hypereutrophic lake in which minute, unicellular chlorophytes were overwhelmingly dominant. Bellacorick natural lake was an acidic, highly stained, mesotrophic lake in which flagellated unicellular chlorophytes characterised the phytoplankton community. Bellacorick cutaway lake was a neutral, very highly stained, mesotrophic lake with a diverse phytoplankton community consisting of diatoms and chlorophytes. The study sites also contrasted strongly in zooplankton abundances. Zooplankton densities were highest in Clongawny, particularly in September, when a peak of 1237 organisms l 1 was recorded (Fig. 2a). Bellacorick natural lake records showed the second greatest abundance of zooplankton (13 141 organisms l 1) (Fig. 2d), while zooplankton numbers were consistently low in Bellacorick cutaway lake (1 12 organisms l 1) (Fig. 2c) and Turraun (0.04 7 organisms l 1) (Fig. 2b). Species richness and diversity levels were highest in Bellacorick natural lake (18 species, D 0.613), lowest in Turraun (nine species, D 0.395) and intermediate in Clongawny (twelve species, D 0.431) and Bellacorick cutaway lake (twelve species, D 0.520) (Table 3). The zooplankton community in Clongawny was dominated by chydorids, chiefly Chydorus sphaericus , in June, July and October (Fig. 2a and Table 3). Cyclopoid copepods, represented mainly by Tropocyclops prasinus , were recorded in Clongawny in high numbers during August (347 organisms l 1) and September (108 organisms l 1). These high densities of Tropocyclops prasinus corresponded with reduced rotifer densities in the 79 BIOLOGY AND ENVIRONMENT lake, while the reverse was true in October when rotifer densities increased to 1087 organisms l1 (72% of total zooplankton biovolume) and cyclopoid copepod densities declined (Fig. 3a). In Turraun, the Bosminidae was the only family consistently present. Rotifers, and to a lesser extent bosminids, accounted for the higher zooplankton abundance observed in Turraun in June (7 organisms l1) (Fig. 2b). The calanoid copepod Diaptomus gracilis was notably present in the lake in August and September, while the macrothricid species Ilyocryptus sordides was significant in October (Fig. 2b and Table 3). The zooplankton fauna in Bellacorick natural lake was dominated by cladocerans. Dominant cladoceran species in Bellacorick natural lake included the large predatory polyphemid species Table 2 * Polyphemus pediculus in June, July and August, bosminids in June and July, chydorids in June, July and October, daphnids such as Ceriodaphnia setosa and Scapholeberis mucronata in July, August and September, and the large-bodied macrothricid Acantholeberis curvirostris in August and September. The cyclopoid copepod Eucyclops serrulatus was significant in Bellacorick natural lake in October (Fig. 2c and Table 3). An inverse relationship was discernable between densities of Polyphemus pediculus and small cladocerans in Bellacorick natural lake over the course of sampling, with peaks in the density of P. pediculus in June (28 organisms l1) and August (22 organisms l1) corresponding to low numbers of cladocerans, with the reverse being true in July, September and October (Fig. 3b). Nutrient status and phytoplankton characteristics of the Turraun cutaway lake, Clongawny cutaway lake, Bellacorick natural lake and Bellacorick cutaway lake. Values shown are the mean of five monthly samples collected between June and October 2003, 9SEM. Turraun cutaway lake PH Conductivity (mS cm 1) Colour (mg Pt. Co. l 1) Alkalinity (mg CaCO3 l1) Silica (mg l1) Dissolved inorganic N (mg l1) Soluble reactive P (mg l1) Total P (mg l 1) Chlorophyll a (mg l1) Trophic status1 Phytoplankton biovolume (mm3 l1) Phytoplankton composition (%)2 Bellacorick natural lake Bellacorick cutaway lake 4.590.09 7792 14495 0.990.4 0.0790.02 1.291.0 5.492.0 66.198.0 126.795.8 Eutrophic hypertrophic 77,60096,642 4.490.06 9697 16697 2.090.6 0.0690.02 6.491.6 6.190.5 13.191.2 3.991.2 Mesotrophic 903988 7.190.04 119917 18499 30.594.1 0.2590.15 11.396.8 6.091.4 14.591.2 2.990.8 Mesotrophic 1,0019329 30% Cyanophyta 30% Chlorophyta 27% Bacillariophyta 5% Dinophyta, 5% Chrysophyta Dominant phytoplankton groups Chlorococcaleans Naviculoid diatoms Coelosphaerium 90% Chlorophyta 5% Dinophyta 2% Cryptophyta 48% Bacillariophyta 34% Chlorophyta 9% Chrysophyta 4% Dinophyta Phytoplankton diversity (D )3 0.256 60% Chlorophyta 18% Dinophyta 6% Chrysophyta 4% Bacillariophyta Chlamydomonas Dunaliella Pteromonas Gymnodinium Mallomonas Dinobryon 0.473 1 8.390.05 261912 4593 114.797.7 0.4290.25 9.297.0 3.090.6 27.394.1 10.192.6 Mesotrophic eutrophic 4,40091,049 Clongawny cutaway lake 0.501 Cosmarium pygmaeum Chlorella ; Peridinium Based on total phosphorus and chlorophyll a concentrations (OECD 1982). Based on mean (n 5) percentage contribution of phytoplankton groups to total phytoplankton biovolume. 3 Values are Simpson Diversity Indices. 2 80 Cyclotella Tabellaria Achnanthes Chlorella Kirchnerialla Dinobryon 0.705 PLANKTON COMMUNITIES OF CUTAWAY PEATLAND LAKES * Fig. 2 Composition of zooplankton in the four study lakes between June and August 2003, in terms of density (organisms l 1) and relative biovolume (%). In Bellacorick cutaway lake, the polyphemid Polyphemus pediculus dominated the zooplankton between June and September (0.12 9 organisms l1), while bosminids were significant in July and September and the cyclopoid copepod Eucyclops serrulatus was abundant in July, September and October (Fig. 2d and Table 3). Despite the lower densities of Polyphemus pediculus at this site compared with Bellacorick natural lake, a similar inverse relationship between small cladocerans and P. pediculus abundance was apparent in Bellacorick cutaway lake (Fig. 3c). DISCUSSION Differences in physico-chemical, nutrient and trophic statuses among the four study lakes corresponded with large variations in littoral zooplankton density, composition and size structure. Clongawny cutaway lake was an acidic, highly stained lake, reflecting the presence of unconsolidated Sphagnum and woody fen peat sediments at this site and an absence of hard water inflows. High phosphorus levels in Clongawny supported extremely high phytoplankton growth 81 BIOLOGY AND ENVIRONMENT rates, and the lake contained bloom quantities of minute, unicellular Chlorophytes such as Chlorella and Cosamarium pygmaeum . The eutrophication of Clongawny has resulted from phosphorus leaching Table 3 * from adjacent commercial forestry plantations (Higgins et al . 2006), highlighting the vulnerability of lakes created on unvegetated, bare cutaway peatlands to nutrient runoff. Presence and relative abundance of zooplankton species in the four study lakes. Turraun cutaway lake CLADOCERA Chydoridae Chydorus sphaericus (Müller) Chydorus piger (Sars) Alona affinis (Leydig) Alona rustica (Scott) Alona costata (Sars) Alonella exigua (Lilljeborg) Alonella nana (Baird) Alonopsis elongata (Sars) Graptoleberis testudinaria (Fischer) Bosminidae Bosminia spp Daphnidae Daphnia spp Ceriodaphnia setosa (Matile) Scapholeberis mucronata (Müller) Polyphemidae Polyphemus pediculus (Linnaeus) Macrothricidae Acantholeberis curvirostris (Müller) Drepanothrix dentate (Eurén) Ilyocryptus sordides (Liénen) Clongawny cutaway lake Bellacorick natural lake Bellacorick cutaway lake ROTIFERA Keratella spp Total species number Simpson’s D 9 0. 395 12 0. 517 18 0.613 12 0.520 COPEPODIA Cycloida Eucyclops serrulatus (Fischer) Tropocyclops prasinus* (Fischer) Calanoida Diaptomus gracilis (Sars) Calanoid spp Harpacticoida Bryocamptus pygmaeus (Sars) * rare species in Ireland (M. Holmes, pers. comm.) 55% of biovolume. 6 15% of biovolume. 16 50% of biovolume. 51% of biovolume. 82 PLANKTON Org a ni sms l 400 Rotifera Sml. cladocera T. prasinus* 300 200 −1 1,000 800 600 400 200 0 Orga nisms l −1 a 1,200 COMMUNITIES OF CUTAWAY PEATLAND LAKES 100 0 June July Aug Sept Oct Clongawny cutaway lake 10 1.0 Polyphemus pediculus 0.8 Small Cladocera* 6 0.6 4 0.4 2 0.2 −1 O r g a ni sm s l 8 O rga ni sms l −1 b 0.0 0 June July Aug Sept Oct Bellacorick natural lake c O r ga ni s ms l −1 100 80 Polyphemus pediculus Small Cladocera 60 40 20 0 June July Aug Sept Oct Bellacorick cutaway lake * Fig. 3 Distribution of dominant zooplankton groups in Clongawny cutaway lake, Bellacorick natural lake and Bellacorick cutaway lake between June and August 2003 (*series plotted on secondary y -axis). Although Chydorus sphaericus was abundant in Clongawny (1056 8842 organisms ml1), similar to other productive Irish lakes (De Eyto et al. 2000; De Eyto 2001; Irvine et al . 2001), it proved ineffective in controlling phytoplankton production in Clongawny. Large numbers of the predatory cyclopoid copepod Tropocyclops prasinus also occurred in Clongawny. This species is rarely recorded in Ireland (M. Holmes, pers comm.), although it is widely reported in bog pools in the US (Sanderson and Frost 1996) and Canada (Masson and Pinel-Alloul 1998). The success of predatory cyclopoid copepods in bog pool is caused by an absence of fish predation, and it results in zooplankton communities characterised by largebodied invertebrates, reduced overall foodweb complexity and stronger interactions between zooplankton and invertebrate predators (Arnott and Vanni 1993; Gibbons 1998). The omnivorous T. prasinus has the potential to selectively capture a wide variety of suitably sized invertebrate prey, particularly given enriched food availability; studies have documented the effectiveness of T. prasinus in reducing populations of small cladocerans, (Melao and Rocha 2004) and rotifers (Dieguez and Gilbert 2002; Lapesa et al. 2002). Its abundance in Clongawny appeared to impact on the populations of small cladocerans and rotifers recorded in the lake, whose susceptibility to predation by T. prasinus was likely to have been enhanced by the lack of shielding macrophytes at this bare, unvegetated site (Fig. 1a). The structure and composition of the zooplankton communities in Bellacorick Natural and Bellacorick cutaway lakes, as in Clongawny, reflected an elevated level of invertebrate predation in these fishless systems. Despite strongly contrasting physico-chemical environments indicative of contrasting peat sediment types, the zooplankton community in Bellacorick cutaway lake was broadly similar to that of the nearby indigenous bog lake, Bellacorick natural lake. Both sites contained large populations of the large-bodied predatory cladoceran Polyphemus pediculus , a species that feeds selectively on protozoans, rotifers and small cladocerans across a wide range of pH environments (Berzins and Bertilsson 1990; Packard 2001). The three coloured lakes, Bellacorick natural lake, Bellacorick cutaway lake and Clongawny lake, all contained high densities of detritivorous chydorids, as is characteristic of bog pools (Crisp and Heal 1998; Irvine et al. 2001). Chydorids are also successful pioneering species, and were historically the characteristic fauna of post-glacial lakes following the retreat of the ice sheets (Harmsworth 1968; Duigan and Birks 2000). In Turraun cutaway lake, zooplankton densities were consistently low, despite of the high food base in the form of an abundant and edible phytoplankton population. The low zooplankton densities in Turraun, relative to the rich food base, were probably symptomatic of a rich macroinvertebrate population. Although not quantitatively assessed in the current study, the well-developed macroinvertebrate population in Turraun has been previously described by O’Connor (2000), O’Connor et al. (2000) and Trodd (2003). Macroinvertebrate predation by specialised grazer guilds is known to have substantially greater effects on the dynamics and structure of zooplankton communities in habitats where vertebrate predators are scarce or absent (Herwig and Schindler 1996; Wissel and Benndorf 1998; McNaught et al. 1999). Age and subsequently colonisation time were likely to have been major factors influencing the proportionately greater role of macroinvertebrate predation in Turraun, relative to the other sites. Turraun, which was created in 1991, is the longest established cutaway lake in Ireland, and it contains extensive cover of submerged, emergent, littoral and terrestrial macrophytes (Fig. 1c and 83 BIOLOGY AND Table 1). While studies have shown that populations of phytoplankton (Feehan and Donovan 1996), protozoans (Buttler et al. 1996), and microinvertebrates (Van Duinen et al . 2003) can establish very quickly after cutaway peatlands are flooded, macroinvertebrate populations take a considerable length of time to develop (Van Duinen et al. 2004). This is because macroinvertebrates have more complex life cycles and make higher demands on their environment (O’Connor 2000; Trodd 2003; Van Duinen et al . 2003; 2004). Age increases both the length of time over which colonisation can proceed and also influences sediments, vegetation cover and plant species richness. The paucity of recolonising vegetation at Clongawny (Fig. 1a and Table 1), in contrast to Turraun and Bellacorick cutaway lake sites, reflects its more recent establishment and related physical and biological features, such as the propensity of bare, unconsolidated peat sediments to desiccate and the absence of viable seed banks (Curraun and MacNaeidhe 1986), which collectively hamper plant invasion. Given the considerable timescales involved in ecosystem establishment and stabilisation, further monitoring and in-depth research is needed in order to assess long-term successional trends in artificial cutaway peatland lakes. ACKNOWLEDGEMENTS The authors wish to thank Bord na Móna for funding this research. The assistance of Mark Holmes (Natural History Division, Natural History Museum, Dublin) and Elvira De Etyo (Marine Institute, Furnace, Newport, Co. Mayo) in confirming zooplankton identifications is also appreciated. REFERENCES APHA 1998 Standard methods for the examination of water and wastewater . Washington, DC. American Public Health Association, American Water Work Association, Water Environment Federation. Arnott, S.E. and Vanni, M.J. 1993 Zooplankton assemblages in fishless bog lakes: influence of biotic and abiotic factors. Ecology 74, 2361 80. Berzins, B. and Bertilsson, J. 1990 Occurrence of limnic micro-crustaceans in relation to pH and humic content in Swedish water bodies. Hydrobiologia 199, 65 71. Burnison, B.K. 1980 Modified dimethyl sulfoxide (DMSO) extraction for chlorophyll analysis of phytoplankton. Canadian Journal of Fisheries and Aquatic Science 37, 729 33. Buttler, A., Warner, B.G., Grosvernier, P. and Matthey, Y. 1996 Vertical patterns of testate amoebae 84 ENVIRONMENT (Protozoa: Rhizopoda) and peat-forming vegetation on cutover bogs in the Jura, Switzerland. New Phytologist 134, 371 82. Chaney, A.L. and Morbach, E.P. 1962 Modified reagents for the determination of urea and ammonia. Clinical Chemistry 8, 130 32. Crisp, T. and Heal, O.W. 1998 Diversity and distribution of aquatic meso- and micro- fauna in mires. In V. Standen, J.H. Tallis and R. Meade (eds), Patterned mires and mire pools . London. The British Ecological Society. Curraun, P.L. and MacNaeidhe, F.S. 1986 Weed invasion of milled-over bog. Weed Research 26, 45 50. De Eyto, E. 2001 Chydorus sphaericus as a biological indicator of water quality in lakes. VerhandlungenInternationale Vereinigung fuer Theoretische und Angewandte Limnologie 27, 3358 62. De Eyto, E., Irvine, K. and Free, G. 2000 The use of members of the family Chydoridae (Anomopoda, Branchiopoda) as an indicator of lake ecological quality in Ireland. Biology and Environment: Proceedings of the Royal Irish Academy 102, 81 91. Dieguez, M.C. and Gilbert, J.J. 2002 Suppression of the rotifer Polyarthra remata by the omnivorous copepod Tropocyclops extensus : predation or competition. Journal of Plankton Research 24, 359 69. Duigan, C.A. and Birks, H.H. 2000 The late-glacial and early-Holocene palaeoecology of cladoceran microfossil assemblages at Kråkenes, western Norway, with a quantitative reconstruction of temperature changes. Journal of Paleolimnology 23, 67 76. Egan, T. 1999 A landscape uncloaked: Lough Boora Parklands, The National Centre of Cutaway Boglands Rehabilitation in Ireland. Policies and Priorities for Ireland’s Landscape , 119 32. Dublin. The Heritage Council. Feehan, J. and Donovan, G. 1996 The bogs of Ireland: an introduction to the natural, cultural and industrial heritage of Irish peatlands . University College Dublin. The Environmental Institute. Gibbons, D. 1998 Acid bog pools: from observation to experiment. In V. Standen, J.H. Tallis and R. Meade (eds), Patterned mires and mire pools . London. The British Ecological Society. Hach 2001 DR/4000 Spectrophotometer procedure manua . 10th edn. Loveland, Colorado. Hach Company. Harmsworth, R.V. 1968 The developmental history of Blelham Tarn (England) as shown by animal microfossils, with special reference to the cladocera. Ecological Monographs 38, 223 41. Herwig, B.R. and Schindler, D.E. 1996 Effects of aquatic insect predators on zooplankton in fishless ponds. Hydrobiologia 324, 141 7. Higgins, T. and Colleran, E. 2004 A comparative assessment of water quality in four artificial lakes created on Irish cutaway peatland. In J. Päivänen (ed.), Wise use of peatlands , Proceedings of the12th International Peat Congress, vol. I, Tampere, Finland, 6th 12th June 2004, 372 8. Jyväskylä. International Peat Society. Higgins, T. and Colleran, E. 2006 Trophic status of experimental cutaway peatland lakes in Ireland and implications for future lake creation. Journal PLANKTON COMMUNITIES OF CUTAWAY PEATLAND LAKES of Environmental Science and Health Part A 41, 849 63. Higgins, T., Colleran, E. and Raine, R. 2006 Transition from P-limited to secondary N-limited phytoplankton growth in an artifical wetland created on flooded cutaway peatland in Ireland. Applied Vegetation Science 9, 223 30. Irvine, K., Allot, N., De Etyo, E., Free, G., Whyle, J., Caroni, R., Kennelly, C., Keaney, J., Lennon, C., Kemp, A., Barry, E., Day, S., Mills, P., O’Riain, G., Quirke, B., Twomey, H. and Sweeney, P. 2001 Ecological assessment of Irish lakes: final report. Wexford. Environmental Protection Agency. Lapesa, S., Snell, T.W., D.M. Fields, D.M. and Serra, M. 2002 Predatory interactions between a cyclopoid copepod and three sibling rotifer species. Freshwater Biology 47, 1685 95. MacIntosh, R.P. 1967 An index of diversity and the relation of certain concepts to diversity. Ecology 48, 392 404. Masson, S. and Pinel-Alloul, B. 1998 Spatial distribution of zooplankton biomass size fractions in a bog lake: abiotic and (or) biotic regulation? Canadian Journal of Zoology 76, 805 23. McNaught, A.S., Schindler, D.W., Parker, B.R., Paul, A.J., Anderson, R.S., Donald, D.B. and Agbeti, M. 1999 Restoration of the food web of an alpine lake following fish stocking. Limnology and Oceanography 44, 127 36. Melao, M.G.G. and Rocha, O. 2004 Life history, biomass and production of two planktonic cyclopoid copepods in a shallow subtropical reservoir. Journal of Plankton Research 26, 909 23. Murphy, J. and Riley, J.P. 1962 A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27, 31 6. O’Connor, Á. 2000 The development of aquatic macroinvertebrate communities in two artificial ponds on Irish cutaway bog, with particular reference to Corixidae (Hemiptera Heteroptera). Unpublished PhD thesis, Trinity College Dublin. O’Connor, A., Kavanagh, B. and Reynolds, J.D. 2000 Aquatic macroinvertebrate colonisation of artificial water bodies on cutaway oceanic raised bog in Ireland. In L. Rochefort and J.Y. Daigle (eds), Proceedings of the 11th International Peat Congress , Sustaining our peatlands, Quebec, August 6 12, 2000, 742 50. Edmonton. International Peat Society. OECD 1982 Eutrophication of waters: monitoring, assessment and control . Paris. Organization for Economic Co-Operation and Development (OECD). Packard, A. 2001 Clearance rates and prey selectivity of the predaceous cladoceran Polyphemus pediculus . Hydrobiologia 442, 177 84. Rott, E. 1981 Some results from phytoplankton counting intercalibrations. Schweizerische Zeitschrift für Hydrologie 43, 43 62. Sanderson, B.L. and Frost, T.M. 1996 Regulation of dinoflagellate populations: relative importance of grazing, resource limitation, and recruitment from sediments. Canadian Journal of Fisheries and Aquatic Science 53, 1409 17. Simpson, E.H. 1949 Measurement of diversity. Nature 163, 688. Trodd, W. 2003 Assessment of aquatic habitats in Turraun Nature Reserve for restoration management and use of Chironomidae (Diptera: Insecta) as indicators of water quality. Unpublished PhD thesis. Dublin. University College Dublin. Van Duinen, G., Brock, A., Kuper, J., Peeters, T., Verberk, W., Zhuge, Y. and Esselink, H. 2003 Restoration of degraded raised bogs: do aquatic invertebrates tell a different story? In A. Järvet and E. Lode (eds), Ecological processes in northern wetlands , Selected Papers of the International Conference and Educational Workshop, Tallinn, Estonia, 2003, 255 61. Tallinn. Tartu. Van Duinen, G.A., Brock, A., Kuper, J., Peeters, T. and Esselink, H. 2004 Do raised bog restoration measures rehabilitate aquatic fauna diversity? A comparative study between pristine, degraded and rewetted raised bogs. In J. Päivänen (ed.), Wise use of peatlands , Proceedings of the12th International Peat Congress, vol. 1, Tampere, Finland, 6 12th June 2004, 399 405. Jyväskylä. International Peat Society. Wissel, A. and Benndorf, J. 1998 Contrasting effects of the invertebrate predator Chaoborus obscuripes and planktivorous fish on plankton communities of a long term biomanipulation. Archiv fur Hydrobiologie 143, 129 46. 85