Atoms: The Building Blocks of Matter The Atom: From Philosophical
advertisement

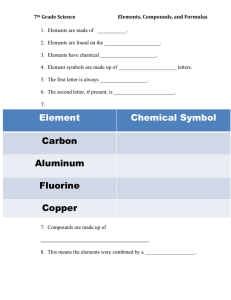
Atoms: The Building Blocks of Matter The Atom: From Philosophical Idea to Scientific Theory Opinion or Theory? Young people should not smoke. Smoking at an early age may make it more difficult to quit smoking later. Early Views of Matter • Democritus (450 B.C.) – proposed that matter was not infinitely divisible; that matter could be broken down into tiny particles, which were not divisible. • These particles were referred to as atomos. • Aristotle (~400 B.C.) – believed that matter was continuous. Foundations of Atomic Theory • Antoine Lavoisier – (late 1700’s) used a balance to show that matter was not created or destroyed during chemical reactions – Law of Conservation of Matter (Mass). • Joseph Proust (1799)- showed that compounds always contain exactly the same proportion of elements by mass– Law of constant composition a.k.a. Law of definite proportion For example: The mass of water (H2O) always contains exactly 88.9 % oxygen and 11.1 % hydrogen. Dalton’s Atomic Theory • John Dalton – (early 1800’s) proposed the atomic theory of matter Compound 1 Compound 2 Mass of oxygen that combines with 1 gram of Carbon 1.33 g 2.66 g • Compound 2 has exactly twice as much oxygen as Compound 1 Dalton’s Atomic Theory This can be explained in terms of atoms. Compound 1: CO (Carbon monoxide) Compound 2: CO2 (Carbon dioxide) Law of Multiple Proportions If 2 or more elements form a series of compounds, the ratios of the masses of elements can be reduced to small whole #’s Dalton’s Atomic Theory 1. Matter is composed of tiny particles called atoms. 2. Atoms of a given element are identical in size, mass, and properties. Atoms of different elements are fundamentally different. 3. Atoms cannot be divided, created, or destroyed. 4. Atoms of different elements combine in simple whole-number ratios to form chemical compounds. 5. In chemical reactions, atoms are combined, separated, or rearranged.