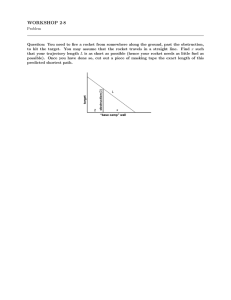
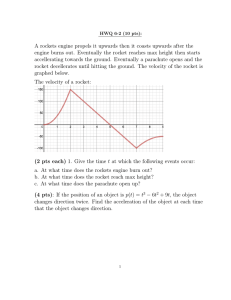
WTH-Spark Lesson Plan: Magnetism Pennsylvania Academic Standards
advertisement

WTH-Spark Lesson Plan: Magnetism Pennsylvania Academic Standards • Identify system parts that are natural and human made. • Observe and describe different types of force and motion. • Recognize and use the elements of scientific inquiry to solve problems. • Know basic energy types, sources and conversions Vocabulary List • Magnetism • Magnets • Magnetic Force Outline: 1 Hour Lesson 1. Basic Background 2. Designing and Racing Magnetic Floating Cars 3. Discussion: Recap of magnetism and magnets Lesson Plan Objective of the Lab • Students will learn the basic principles of magnetism • Students will design and then race magnetic floating cars Basic Background Some materials are attracted or repelled by other materials. These materials are called magnets. Magnets are found in nature and can also be man-made. Magnets are found everywhere around us, in modern technology like TV’s and computers to the planet we live on. Yes, Earth is one giant magnet! Some magnets last a very long time and are called permanent magnets while others only last for a short time and are called temporary magnets. Magnets have some very important properties; on each magnet there is a north and south pole. Like poles, a north and a north or a south and a south, repel while opposite poles, a north and a south, attract. Magnets are also special because they are capable of exerting repulsive or attractive (push or pull) forces without touching the object they are acting upon. These two bar magnets will exert an attractive force on each other, causing the magnets to stick together. A magnet will always have a north and a south pole; even if a magnet is cut, the resulting pieces will have north and south poles. The bar magnet has been cut into three pieces. Although the magnet was cut, each piece maintains the same north and a south pole order. Designing and Racing Magnetic Floating Cars (40 min) Material Needed: Racing Track - Made prior to the lesson by the instructor or TA • 2 ft of cardboard or other similar sturdy material to use as track (length may be adjusted to amount of material available) • Two neat rows of magnets fixed to the board with all poles facing in the same direction • Two 2 ft (or length of the board if changed) guide rails on edge of board (or the length of the board used), preferably taped, sanded or any other method to reduce friction such as greasing the rails. Floating Car • 5 in foam board (cardboard, index cards or other similar sturdy but light material may be used) cut to fit inside width of track with guide rails • Markings on underside of foam board to indicate where magnets may be placed by student to levitate board • Four magnets per floating car to levitate car • Extra magnets to use to push/pull board • Glue/Adhesive to attach magnets to car Track in Place (end view) Track with Floating Cardboard Car in Place (end view) Procedure 1) Make the racing track by affixing magnets onto the track in two neat rows. It is recommended to use about 32 square ceramic magnets per foot of the track. Attach two guide rails on the side of the track to prevent the cars from slipping. The cars will glide along the rail, so it is preferable to use more frictionless surfaces for the rails. 2) Have the students prepare racing cars in groups. Make sure they mark two lines on the bottom of their cars so that they know where to place the magnets so that the width is equal to width of the magnet rows on the track. Activity 1) Give each student one index card, four magnets to levitate the car, and a fixed amount of extra magnets to use as “engines.” 2) Have the students design the car and place magnets on the underside of their car while keeping in mind the polarity of the racing track to levitate cars and affix magnets in any way they desire to use as engines. 3) Students are not allowed to touch their cars in any way to move them; they can only use the principles of magnetism to move their cars. 4) Have the students race their cars down the track. 5) If time permits, allow students to alter the placement of their magnets and race cars again from what they have learned in the first trial. Discussion (10 min) 1) Ask the groups why their cars where able to float and ask them to explain why they used their engine magnets in the way that they did. (Answer: like poles repel, opposite poles attract) 2) Ask the students where they see magnets and magnetism used in their lives. (Answer: see basic background, ask if students have any other ideas- maybe someone will mention sticking things to refrigerator) 3) Relate magnetism with forces (repulsion/attraction to push/pull) Supplementary Background for Teachers Magnets are able to be influence other materials through space because each magnet forms what is called a magnetic field. This is how a compass works; the magnet in the compass interacts with the Earth’s magnetic field. Some animals also use the Earth’s magnetic field to navigate; for instance there is evidence that some birds are able to sense the Earth’s magnetic field and use it to help them find their destination when they migrate. Iron filings arranging themselves along the lines of the magnetic field of the magnet. The field starts from the south pole and goes to the north pole. Some materials are changed by permanent magnets to become temporary magnets, like paper clips. This is because the particles in the paper clip are influenced by the permanent magnet to align in a uniform direction. However, once the magnetic field is removed, the particles revert to a random alignment which causes it to lose its magnetic properties. Some materials are better suited for becoming a temporary magnet such as soft iron and some iron alloys. Particles before magnetization: paper clip is not a magnet Particles after magnetization: paper clip is now a magnet Magnets are used everywhere around us: compasses, TV’s (the cathode ray tube portion), credit cards, loudspeakers, VHS tapes, computers and some IPods (hard drives, but not flash drives). Magnets are used in electric generators, motors and even used in Magnetic Levitation Trains in Germany and Japan that are capable of going over 300 mph. Magnetism is linked to electricity; the two phenomena interact with each other. The interactions of magnetism and electricity form a phenomenon called electromagnetism. Electromagnetism is extremely important in our modern lives; virtually everything that has a motor or runs on electricity is in some way related to electromagnetism. Because of the interaction of magnetism and electricity, several similarities arise that may help understand the properties of both phenomena as well as electromagnetism. For instance, in electricity negative and a positive charge will attract to each other. This is similar to magnetism, where like repels and opposites attract. Electricity used in certain forms can form magnets, just as magnets used in certain ways can create electricity. The key difference between electricity and magnetism is that in electricity, a single charged particle can exist by itself. A positive charge does not require the presence of a negative charge and vice versa. This is called a monopole. However, in magnetism, every magnet must have two poles, hence magnets are dipoles. An Additional Applications For Home With Parents: Students should be able to understand and explain the properties of magnetism and attempt to discover which household items are affected by magnetism. If possible, ask students if they saw similar traits in the things that were attracted by a magnet (i.e. metallic) To demonstrate creating a temporary magnet, have the student take a magnet and attract a paper clip to the magnet. And then ask the student to try to attract a paper clip using the paper clip on the magnet. Have the student attempt to connect as many paper clips as possible to create a string of paper clips attracted to each other. Remove the magnet. Once the magnet is removed, the string of paper clips will fall apart. This is because the temporary magnets (the paper clips) require a permanent magnet to give them their properties. Also you may demonstrate to your student that magnets are able to affect each other through space by placing a piece of paper or other similar material between two magnets. Depending upon how the poles of the magnets are aligned, the magnets will still attract or repel each other through the piece of paper. **Note for advanced students: If students have thorough understanding of forces and Newton’s third law (every action has an opposite and equal reaction), then try to get the students to propel their floating cars by methods besides magnets. For instance, launching marbles from the back of the car to drive it forwards, a propeller, squirt water, etc. Also, students with an idea of aerodynamics may wish to alter their foam board to make it sleeker and more like a car/maglev train. Magnetism and Cereal Extension Materials List Each group needs: 480 ml (1 cup) of cereal with a high iron content (e.g., Total or any cereal that contains 100% of the recommended daily allowance of iron). • To share with the entire class: Magnets of varying strengths (Magnets generally used in the classroom will work. Refrigerator magnets have less strength and may not work, but will provide the students with an alternative with which to experiment.) • • Ziploc bags • Cups of water • Blender Electronic balance. The balance must be sensitive enough to be able to detect small changes in weight (at least one tenth of a gram). If this is not available to you there are alternative ways to quantify the amount of cereal removed (see procedure). • Introduction/Motivation As we have just learned, iron is one of the many minerals that are essential to our diet. Because of iron’s importance, food engineers often fortify foods with iron, meaning they add extra iron. One of the foods most commonly fortified with iron is cereal. In this activity, we will remove the iron that has been added to cereal. In this way, we will be acting as reverse food engineers. Vocabulary/Definitions Mineral: Natural compounds that are important in helping the body perform many vital functions. Iron: Mineral that is necessary to transport oxygen around the body (part of hemoglobin). Calcium: Mineral that is helpful in building strong bones. Potassium: Mineral that helps to keep muscles and nervous system working properly. Zinc: Mineral that helps your immune system. Fortify: To add one or more ingredients to a food to increase its nutritional content. Magnet: An object or device that produces a magnetic field which attracts other magnets and certain metals. Procedure Background Minerals help your body grow and stay healthy. The body uses minerals to perform several functions. For example, they help you build strong bones, transmit nerve impulses, make hormones, and maintain a normal heartbeat. One important mineral is iron. Your entire body needs oxygen to stay healthy and alive. This is because the body needs iron to transport oxygen from your lungs to the rest of your body. Iron helps this process because it’s important in the formation of hemoglobin. Hemoglobin is the part of your red blood cells that carries oxygen throughout the body. There are several foods that are iron rich including meat (especially red meat), tuna, salmon, eggs, beans, potato skins, and leafy green vegetables. People who do not get enough iron in their diet tend to become anemic. Anemia is a condition where there is not enough red blood cells in the blood. Due to the lack of red blood cells, there is also a lack of hemoglobin, which as mentioned previously carries oxygen. If the blood cannot carry enough oxygen to the rest of the body, problems will occur. The main symptom of anemia is fatigue. Also, chest pains and shortness of breath can occur. Moreover, people with anemia tend to have pale, pallid skin. Other effects of not getting enough iron include a decreased immunity to disease. Also, children who lack enough iron in their diet do not develop normally. While there are several foods that are rich in iron, many people have trouble getting enough in their diet. Therefore, food engineers often add iron to foods such as cereal. The process used to fortify the cereal is quite simple. The food engineers simply mix powdered iron with the other cereal ingredients. Several types of powdered iron can be used, but the most common types are ferrous sulfate and ferrous fumerate. When ingested, it is converted into iron that can be used by the body in your stomach. The iron mixes with the hydrochloric acid in your stomach to make iron chloride and oxygen gas. The iron chloride is absorbed by your small intestines. The amount of iron added to food is dependent on several factors. For example, the prevalence of iron deficiency in the group of the people who will eat the food and the dietary trends of their culture contribute to how much iron should be added to cereal. Food engineers also add other types of minerals and vitamins to different foods. For example, orange juice is often fortified with calcium. Because not everyone eats cereal, food engineers are thinking of ways to deliver essential nutrients to people and kids in some of the world’s poorest places. For example, some scientists and engineers have developed rice and salts that are fortified with different vitamins and minerals, including iron. This allows children all over the world to receive more nutrients in their diet because rice and salt are consumed in many different cultures. In this activity, we will show that the iron found in food is the same type of iron that is attracted to magnets. The student will discover this by engineering a process to remove the iron in cereal. Before the Activity • Obtain the materials. • Divide the materials so that each group has a set. With the Students 1. Ask the students if they have ever read the nutrition label on their foods. Also, ask them if they ever noticed things such as iron or calcium on the label. 2. Define what a mineral is and discuss some of the important minerals. Have the students write down the functions of the minerals defined above and brainstorm as a class what might happen if you did not get enough of the mineral. 3. Tell the students that iron are oftentimes added to cereal. Tell the students that they are going to design a process to remove the iron from cereal. Inform them that engineers often do the opposite. 4. Divide the class into groups of two or three students each. Groups can be larger or smaller depending on size of class and availability of materials. 5. Pass out cereal to each group and let them examine it. Have them notice that they cannot see the iron at this point. 6. Allow the groups 5-10 minutes to discuss their plan to extract the iron from the cereal. Walk around to see if their plans make sense. Don’t tell them that their plans are wrong or will not work yet. 7. Have the groups gather the materials that they chose. 8. Give the students about 15-20 minutes to try to extract the iron from their cereal. During this time, walk around the room and talk to the groups about what they are doing. Make suggestions for changes if their process is not working. a. First, give small hints such as, “think about what you could do with the water.” b. Second, explain what the different materials will do and how it may help them. For example, tell them that the water helps separate the iron from the cereal and allows it to move. c. If none of these works, begin giving more direct directions. 9. Ask the students to weigh how much iron they can remove from their iron. In order to be able to weigh the iron, first weigh a clean tissue. Then, wipe the iron off of the magnet. Keep the tissue as dry as possible. If the tissue does get wet, allow it to dry and then weigh it again. The weight of the tissue with the iron minus the weight of the clean tissue is the weight of the iron. Note: If a sensitive scale is not available you can have the students count how many specks of iron they can remove from the cereal. This process is not as accurate. However, it will give the students an idea of how much iron is removed from the cereal which is most important. 10. If time allows, give the students more time to revise their process and trade in their previous materials for new materials. Troubleshooting Tips If the students are having trouble figuring out how to remove the iron from their cereal, give them more direction. For example, suggest that the iron may be removed more easily if it is crushed. One procedure that works well is the following. Begin, by crushing the cereal into small pieces (the smaller the better). Then, put the cereal into a cup of water and stir. There should be enough water for the cereal to move freely. Next, place a strong magnet on the outside of the cup and drag it towards the top. You should see tiny black specks come up with the magnet. This is the iron that you were looking for. Alternatively, instead of crushing the cereal and then putting it in water, you could blend both together. The blender will work better. However, crushing and stirring into water should work as long as you are using a cereal with a high iron content. If you are not able to get any iron out of the cereal try the following tips. First, try a stronger magnet. The stronger the magnet, the better it will be at removing the iron from the cereal. You can also try crushing the cereal into finer pieces. Also, make sure that your cereal has enough iron in it. About 100% of your daily serving per cup (remember that serving sizes vary) should work. Investigating Questions • What procedures worked best when you tried to remove the iron from the cereal? • Why do you think the blender helped you get iron out of the cereal? • How do you think the extra iron was added to cereals? • Why do you think certain cereals are fortified with extra iron? Student Worksheet Objective: To learn the principles describing how magnets work and what magnetism is. Background: Materials which are attracted or repelled to one another are called magnets. You probably have some on your refrigerator at home and today we are going to talk about how they work. Magnets have some very important properties; on each magnet there is a north and south pole. Like poles, a north and a north or a south and a south, repel while opposite poles, a north and a south, attract. There are a couple of terms that are helpful when learning about magnets: Magnets: An object that is surrounded by a magnetic field and that has the property, either natural or induced, of attracting iron or steel. Magnetic Force: A force that exists between two electrically charged moving particles. Magnetic Cars Objective: Use what we know about magnets to make model cars that work. Materials: • Four magnets • Glue/Adhesive to attach magnets to car • Index Cards • Markers • Supplies for Decoration Methods: 1. Take the index card and discuss with your group how you can design the car so that it will move the fastest. 2. Use markers to decorate the cars. 3. Glue magnets to the bottom of the car making sure that they repel the magnets on the magnetic track. 4. Glue the magnets to the bottom of your car. 5. Test your car to make sure it floats above the track Discussion Questions 1. What happened when you raced your car on the track? ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ _____________________________________________ 2. Why does the car float? ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________ 3. What other ways can you use magnets to improve your car? ______________________________ 4. Where else do you see magnets in your life? ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________ WTH Lesson: Bottle Rocket Lesson Plan Philadelphia Standards to be Covered • Observe and describe different types of force and motion. • Compare the relative movement of objects and describe types of motion that are evident. • Know models as useful simplifications of objects or processes. Vocabulary • Natural Law • Newton’s Third Law of Motion • Weight • Lift • Thrust • Drag Outline Day 1 1. Background Day 1 2. Balloon Rocket/Soda Bottle Motor boat activity 3. Discussion about balloon rockets/ soda bottle motorboat 4. Discuss Building Rocket Design Day 2 5. 6. 7. 8. Background Day 2/ Refresher of Day 1 Building and testing of Bottle Rockets Discussion about what students learned Appendix a. Background Picture b. More advanced rocket design c. Suggested modifications Objective - Students will discover first hand Newton’s Third law of Motion. - To help students better understand the forces affecting flight. - To help students understand how propulsion can induce flight. - To encourage students to take what they observe in small scale experiments and apply it to the design of larger models. Day 1: Balloon Rocket or Soda Bottle Motorboat Activity and Planning for Day 2 Background Day 1 (10 min): Sir Isaac Newton observed and studied patterns in motion and forces. Though his studies, he developed a set of natural laws, or rules that every thing on earth must follow. One of his most well known laws, and the object of today’s experiment is Newton’s Third Law of Motion: For every action there is an equal and opposite reaction. The majority of the lesson will focus on this principle. Give real life examples of how this principle guides real-life situations: the flight of rockets, swimmer propelling themselves off the wall, car crashes, or pushing on something. In today’s experiment we will use this principle to propel our rockets. When you release the air from a balloon what happens? Why? Discuss that air also has force when pushed against a flat surface. It is this moving air and this Newton law that induces the movement in the rockets Activity (50 min): A. Rocket to Success Objective: Students should observe the propulsion created when air is expelled from a balloon. Also, they should observe the effect of differing volumes of air. Materials: balloons (bring at least 1.5 per kid due to breakage) string straws tape markers (do not let the kits write their names on the balloons, even if it is permanent it comes off) scissors clothespin (not necessary, it works much better to have the kids hold the balloon closed) Procedure: Divide students into groups of 2-3. Students will work together to construct their rockets (see Internet sites below for information on how to construct the balloon rockets). 1. Tell students to construct simple balloon rockets by attaching a balloon to a straw lengthwise with tape and stringing it on a string stretched taut. 2. The students will experiment with their rockets. Students will blow up their balloons to various sizes and observe how far their rockets travel. 3. Students will record their various measurements. 4. Students will then be able to determine that the amount of air in the balloon is relevant to the distance that the rocket travels. 5. Students will then come together as a large group and the class rocket races will take place. *Steps 3 through 5 are extremely difficult to implement, the kids do have a lot of fun letting the balloons go though. Discussion: Students should come together as a large group to discuss their observations. Students will be able to relate their observations to those of Newton's Law of Action and Reaction. Students will brainstorm ideas for other ways to demonstrate the concepts of Newton's Laws. Steer students toward ideas in designing bottle rockets. Encourage them to think about how to make a rocket fly and how to make if fly farther. Useful Internet Sites: * Balloon Rockets http://www.amnh.org/mars/balloon.html * Build a Balloon Rocket http://unmuseum.mus.pa.us/exjet.htm B. Soda Bottle Motorboats Objective: Observe Newton’s Third Law of Motion in action. Materials: • Plastic soda bottle • Toilet paper • Baking soda • Vinegar • Wading pool, bath tub, or other “body of water” Methods: 1. Wash out a 16-ounce plastic soda bottle. 2. Gather the toilet paper, baking soda and vinegar. 3. Lay out two squares of toilet paper. 4. Pour several tbsp. baking soda on the toilet paper squares and fold them to enclose the baking soda. 5. Place these toilet paper "packages" inside the bottle. 6. Have one person hold the bottle cap. 7. Pour 1/4 cup of vinegar into the bottle. 8. Place the cap on quickly and twist it only once. 9. Place the bottle in the water in the wading pool or bathtub. 10. Watch baking soda and vinegar have chemical reaction causing the bottle to move across the water. 11. Experiment with different amounts of vinegar and baking soda and note effects on boat's speed. Discussion: 1. What happened? 2. Why did the motorboats move? 3. What could make the boats move faster? If time remains after activity, begin Background of Day 2 and encourage students to think about bottle rocket plans and how they work. Day 2. Rocket Design Activity1 Background For Day 2 (15 min) Flying model rockets is a relatively safe and inexpensive way for students to learn the basics of forces ad the response of a vehicle to external forces. Just like balloon rockets and soda bottle motorboats these are governed by Newton’s third law of motion. A model rocket is subjected to four forces in flight; weight, thrust, lift and drag. There are many different types of model rockets. One of the first and simplest rockets is the bottle, or water rocket. The water rocket system consists of two main parts, the launcher and the rocket. These work much like the balloons and soda bottles in Day 1 of this experiment. There are many ways build and modify bottle rockets, let the students brainstorm ideas using the following procedure to guide them toward a working water or bottle rocket. Also, in the appendix there are examples of popular modifications such as nose cones and fins that stabilize and increase flight time, but these are add-ons. Try to get students thinking about the general propulsion before attempting to alter flight. In the appendix, is a drawing of a generic launcher and rocket. The launcher has a base to support the rocket during launch. A hollow launch tube is mounted perpendicular to the base and is inserted into the base of the rocket before launch. The launch tube is connected to an air pump by a hollow feeder line. The pump is used to pressurize the inside of the body tube to provide thrust for the rocket. We have attached a pressure gage to the feeder line to display the change in pressure in the system. This part of the system is very similar to the simple compressed air rocket. The other part of the water rocket system is the rocket itself. Usually the rocket is made from a 2liter soda pop bottle. Before launch, the bottle is filled with some amount of water, which acts as the "propellant" for the launch much like the air in a balloon or the gas that filled the motorboats. Since water is about 100 times heavier than air, the expelled water produces more thrust than compressed air alone. The base of the bottle is only slightly larger than the launch tube. When the rocket is placed on the launch tube, the body tube becomes a closed pressure vessel. The pressure inside the body tube equals the pressure produced by the air pump. When the water is allowed to escape, the pressure causes the expulsion of water and thus the propulsion of the bottle rocket. [The rocket in this link can be built for demonstration purposes as an example of advanced rocketry] http://www.lnhs.org/hayhurst/rockets/ Suggested Materials: For the class: 1 http://www.bu.edu/csp/curriculum/lessons/Lesson1%20rockets.pdf • 6 identical empty soda bottles (all from the same brand of soda so that they are • • • • • • equivalent in shape, weight, etc.) 1 bicycle pump (with pressure gauge if available) 1 “launch pad” 6 rubber stoppers for the soda bottles 1 permanent marker to mark off water levels 1 measuring tape for measuring flight distances 1 measuring cup marked off in mL Preparations to do before class (20 min): 1.) Mark each empty soda bottle with 6 water levels: 0,100,200,300,400,& 500ml. 2.) Fill each bottle to one of the 6 levels. 3.) Place a rubber stopper into each bottle – be sure to push each stopper in the same amount. Activity: (This activity should be done outside!) (35 min) 1.) Bring the students outside to an area with plenty of space (and away from any cars or windows). Be sure to bring extra water with you to refill the rockets. 2.) Before launching, have the students predict which rocket will go the farthest and why. They should write their responses in the space provided in their activity booklets. 3.) To prepare for launch, insert the bicycle pump needle into the cork of the first bottle rocket. You may need to make a small hole with a nail or tack first. 4.) Place the rocket on the “launch pad” with the stopped end facing down. Allow a student to hold the stopper with the launch pad – the bottle will fly off and leave the cork behind. 5.) Make sure everyone is clear of the launch path. Have a student pump air into the rocket until it takes off. **You may get wet** 6.) When the rocket lands, have the students take the measuring tape and measure the distance from the launch pad to the landing site and record the distance traveled by the rocket. 7.) Launch the remaining 5 rockets. Record the distances these rockets travel. Discussion Questions (10 min): 1. Which rocket traveled the greatest distance? Why? Ans. The rocket with the most liquid in the beginning should travel the farthest due to its greater expulsion and thus greater propulsion. i.e. a greater “action” creates a greater “reaction.” 2. What natural law makes it possible for bottle rockets to take off? Ans. Newton’s Third Law of Motion: every action has an equal and opposite reaction. 3. What can be done to adjust the flight of our bottle rockets? Ans. Modifications such as adding nose cones, fins, weights, or making the rocket more aerodynamic. Appendix a) Background Picture b) More Advanced Design (Appendix information / pics can be found at http://go.to/ragna-rocket#) Description This is the simplest model to begin. It has only one drawback: the landing is rather hard and after 2 or 3 flights, sometime less, the model is out of order (See Security Rules). But you must begin with something and this model is build in 30mn including the glue drying. Choose your bottle The rocket body is a soda bottle. You MUST use only soda bottles and not fruit juice or other liquid bottles because they are not specified for "high" pressure usage. Cut out the fins I use DEPRON material Currently, I use the 6mm, but the 3mm one should work but with a narrower section to glue 2 materials suit very well: - DEPRON which can be found in Do-It-Yourself shop in the isolation department. It is a kind of Polystyrene foam. It is sold by 1m plate with a 3 or 6mm thickness. I use the 6 mm, the 3mm is too fragile. - Sandwich of Polystyrene between 2 Bristol boards which is sold in handicrafts. With the same thickness, it is stronger than DEPROM but heavier. I use 3mm thick one. Both are very easy to cut in the right shapes with a cutter. Be only aware to cut perpendicularly the section to be stuck on the bottle. Draw marks on the bottle The thing to do is to draw on the bottle the marks on the bottle where will be glued the fins. At first, draw 2 circles corresponding to the high and low edges of the fins. For that wrap a paper fold around the bottle where must be the high circle and mark with a pencil along the edge of the paper. Frequently, the lowest edge of the fins is on the curved part of the bottle and the tip of the paper cannot be used. I rather measure from the first circle and I draw marks to outline the second circle, then I finish it freehand. Then, divide (with a tape measure) the high circle in three sections and with a rule lying on a table or a small plank, draw the 3 lines linking the 2 circles to materialize the place of the 3 fins. Glue the fins The DEPRON has a major disadvantage, it does not support a lot of glues like Cyanolit or Neoprene glues, but the following four work: - The Glue Gun. Be aware to heat too much before putting the glue because it may melt the DEPRON and deform the bottle. - Neoprene Glue without solvent (BOSTIK sold this glue). The pre-drying is a little bit long (>30mn) and the temperature must not be too cool. - A Special Polystyrene Glue: the transparent NOT the pasty type works very well. "BOSTIKfor Polystyrene Cornices" - The Polyurethane glue. PL PREMIUM is recommended Put Glue on the marks drawn previously, (wait a moment depending on the pre-drying of the glue) and fix the fins. A tip to ease the bonding: put the bottle in the fridge (30mn), get it out and close it rapidly with its cap. The internal temperature will rise to the ambient level and will put under pressure the bottle and then will rigidify the bottle for the gluing. If you are in a hurry you can put the bottle on the launcher and pressurize it lightly and glue fins there. Decorate your rocket c) Possible modifications After cleaning When launching, the tremendous pressure inside the bottle will cause the walls to expand. This expansion leads to a loss of energy and will make for a lack luster performance. To solve this problem take some wide strapping tape (the best), packing tape, or duct tape and pre-wrap the center of the pressure chamber. One and a half times around should do the trick. You don't want the tape to be too bulky and watch for wrinkles. This will strengthen the walls of the bottle without adding too much mass and launch altitude will increase overall. Nose Cones Nose cones and fins are not only for performance but add character and style to your rocket. Be sure to take some time thinking out the design of your rocket before committing to a plan of action. Here are several types of nose cones and the methods used to make them. These are only a few examples, so don't be afraid to be creative. The fins have been removed, in the illustrations below, for clarity. The first type illustrated here is called "The Flounder". It is the easiest to make but lacks flight stability. The cone is made from poster board or tag board. You can make the cone by simply cutting a large circle out of the poster board (about a 6 inch radius). Cut a line from the outer edge of the circle to the center on the radius. Overlap the cut edges and turn the circle while holding one edge stationary until you get the desired cone shape. Secure the cone with staples or tape. Attach to the bottle with tape or similar adhesive. As I mentioned before "The Flounder" lacks flight stability, this can be fixed to a degree by pressing a small lump( a few ounces) of clay to the inside of the nose cone. This will add mass to the cone and keep your rocket from flipping end over end while in flight. This new and improved rocket could be called "The Bullet". The "Bertha Series" nose cones are easy to make as well but require a little attention to detail. The "Bertha" nose cones are made by cutting the bottom off a spare bottle and attaching the top portion onto the pressure chamber. One strip of tape should be enough to hold the cone on. (Note: Never cut the pressure chamber) Using this method you can even make your "Bertha" rocket two or three nose cones tall! (we call that a "Big Bertha") Within the top nose cone you can add a small lump of clay to increase the mass. Pay attention to keep all the cones in a straight line. A curvy rocket will not be safe coming off the launcher. The "Space Needle" style of nose cone can be made from a spare bottle, empty paper towel roll, and a tennis ball or racket ball. This type of nose cone adds a great deal of inertial mass to the rocket and makes it really stable. Simply, attach the towel roll to the top of a single Bertha nose cone and then fix the tennis ball to the top of the roll. Take your time building this set up because you want the entire assembly to be straight as an arrow. Cut tabs in an index card and glue or tape the card to the tube. This will hold the tube upright. Then glue or tape the tabs onto the nose cone top. Fins Fins are the guidance system for your rocket. Without them a rocket would tumble end over end. Fins can give your rocket life and beauty. Fins can portray aggressive power or aerodynamic grace. However, fins tend to be the single greatest downfall of many young rocket builders. With the incredible speeds and frightful acceleration generated at launch, many fins get ripped off the rocket body within a fraction of a second. I will discuss the reasons for this later, but for now let's list some common building materials. Materials: (remember lightweight but sturdy) Index cards Clear packing tape Cardboard (cheap, plentiful, soggy when wet) Chipboard (cereal boxes) Foam core (a little tougher but more $, some water damage) Sturdy-board (like plastic cardboard, great stuff, $$, no water damage) Balsa wood (might be a little heavy, fragile on impact) Styrofoam sheets* (cheap, low mass, fragile on impact) *Requires PL Premium Construction adhesive to attach How many fins do I need? To ensure stability and safety, the minimum number of fins on a rocket is three (3). Many people choose a 3 or 4 fin design. There is no maximum number of fins you may have but keep in mind that the more fins you have the more drag you will create and drag slows a rocket down. Constructing fins 1. Be creative and cut out 3 or 4 identical fins. You can use any shape except "forward swept" fins. 2. Lay the fin on a flat surface. 3. Glue and/or tape an index card onto the side of the fin. Be sure to leave a one-inch tab on the index card. You will later bend this tab out 90 degrees to make an attachable area for the rocket. 4. Repeat the same for the other side of the fin. 5. Repeat with other fins. You should now have 3 or 4 fins each with two-index card tabs on the backside. (I like to laminate the entire fin surface with clear packing tape to reduce the amount of water damage to my cardboard fins.) Fin placement The fins of your rocket can't be placed above the halfway point of your pressure cylinder. You want to place your fins at the base of the rocket to lower or maintain the center of gravity. If you were to place the fins above the center of gravity, the rocket would tumble and spin out of control once it left the launch pad. Attaching the fins This is the tricky part. Most glues including hot glue and "Liquid Nails" type adhesives are not flexible enough when it comes to the launch. The 2-liter bottle pressure chamber might expand a millimeter or more in circumference when it is pressurized. That is enough to break the bonding seal of most glues. When the rocket is launched, the fins usually rip off. I have found that the clear packing tape or strapping tape works the best at holding the fins on the rocket. (duct tape works too) 1. Apply a piece of tape to the index card tabs and then carefully tape them to your rocket. 2. Look at your fin. Make sure it doesn't curve or it isn't crooked. It should be in a direct line with the body of your rocket. If it isn't perfect, take it off and try again. 3. Attach the other fins. If you are using 4 fins they should be at 90-degree angles. 3 fins should be at 120-degree angles. 4. Test the wiggle of the fins. Your fins shouldn't wiggle more than a few centimeters from side to side. Adding more tape to the top and bottom areas of the fin might fix this problem. Parachute Systems By now you may have spent several hours building the rocket of your dreams. It may not be completely obvious to you yet, but as the old saying goes, what goes up... must come down. It's time to think about saving all that precious work by creating a recovery system for your rocket. Here are a few ideas to get you started. Fins have been removed for clarity. (Note: you may substitute "crepe paper streamers" for "parachute" in most of the following examples) Let's make a parachute. Here is a list of materials: Tall kitchen garbage bag -orFour-grocery plastic bags Kite string -orYarn Baby Powder Scotch Tape The size and shape of your parachute can be as varied as your rocket. A good rule of thumb is to make your chute about 12 inches across. Your chute could be larger but some rocket styles have narrow nosecones and the chute could get stuck. Making the Chute Carefully cut the garbage bag along one side and the bottom seam. Open the bag along the fold and lay it flat. If you want to use the grocery bags, cut each bag in the same way but lay them out side by side 2 X 2. Use some scotch tape and tape the center seams to make a larger surface area. The Square Chute The simplest chute to make is the "Square Chute". Prepare the edges, where you will connect the strings, with scotch tape grommets. First apply a piece of tape to both sides of the plastic. Then, with a HOLE PUNCH, punch a clean hole through the center of the tape and plastic tab. Repeat this procedure for each location where you are placing a string. To complete the "Square Chute", measure two 24-inch lengths of string and tie each end to one of the grommet holes. Bring the centers of the string loops together and tie them off. The Circle Chute The "Circle Chute" is a classic, timeless engineering masterpiece. But don't let me influence you. If you have a large tire or similar round object you can trace the circle on the plastic with a marker and cut. If you don't have anything large enough to trace, fold the plastic in half, then in half again, then fold on the diagonal. This will give you a triangular wedge. With a sharp pair of scissors, cut along the section that has the open edges. You don't have to worry about cutting on the curve, just cut strait across the bottom. If you want to practice this first, try cutting a piece of paper first. Finish the edges with tape grommets as mentioned above. Lastly, tie separate 24 to 36 inch string leads to each grommet. Gather the leads together and finish the strings off with a good knot. Anti-Tangle Armature One of the unforeseeable happenings in rocket parachuting is getting the strings caught or tangled in the fins or safety lines of the rocket. An easy way to avoid this problem is to build this simple cable shield for your chute. Cut two or three straws into 4 or 6-inch sections. Slip the extra long string leads, from your parachute, into the straw sections. The armature should protect the strings as the chute deploys and then slide down out of the way when your chute opens up. (NOTE THE ILLUSTRATION IS NOT TO SCALE.) Folding the Chute Dust the chute with baby powder to ensure that it doesn't stick to the side of the rocket or itself. Grab the chute between two fingers, in the center, and pull it up toward the ceiling. Let the chute fold naturally as you lift it. Once you have the chute pulled up, fold it once in half at the center. You don't want to fold the chute too tightly but you can fold your chute into thirds if space is a factor. Wrap the excess string loosely around the chute. Fold the straw armature sections onto the chute but not part of the excess string wrap. Making a Sleeve If you find that the chute area is too small to hold your parachute, you can increase the size of container by adding a section of chipboard around the girth of the bottle. Or you can use another 2-liter bottle. Remove the top and bottom of the 2nd 2-liter and tape the new cylinder into position. This tends to make your rocket more stable by increasing the distance between the Center of Pressure and the Center of Gravity. The "Bertha Cone Chute" (3 out of 5 Stars) weighted cone and apply a pressure cylinder. In theory, (the highest point of travel), separates from the main body. enough drag to slow itself parachute deploys and the two to earth. In practice, the nose cone can main body causing a failure. too short and the cone can't With this chute system, instead of taping the nose cone on, attach a safety cord to the parachute to the top of the once the rocket reaches apogee the weighted nose cone The main body is creating down. If everything works, the pieces float gently back down sometimes get jammed on the In addition, safety cord can be clear the body. Nose Cone Tug Chute (3 out of 5 Stars) The "Tug Chute" uses the force of the lifting nose cone to pull the chute out of the sleeve or needle assembly. Attach a safety line to the nose cone or tennis ball. Attach a parachute close to the cone/ball assembly. The chute needs to be folded small enough to fit inside the needle yet not too tight to unravel. Problems with this system include; not enough line and parachute packing jams. Cone style noses with a sleeve have more success with this system. BOTTLE ROCKETS: STUDENT HANDOUT Objective: To see Newton’s Third law of Motion first hand and use it to design and build a working rocket. DAY 1: Background Day 1: Sir Isaac Newton observed and studied patterns in motion and forces. Though his studies, he developed a set of natural laws, or rules that every thing on earth must follow. One of his most well known laws, and the object of today’s experiment is Newton’s Third Law of Motion: For every action there is an equal and opposite reaction. What are some everyday examples of Newton’s Third Law of Motion in action? In today’s experiment we will use this principle to propel our rockets. When you release the air from a balloon what happens? Why? A. Experiment Day 1: Rocket to Success Objective: Use Newton’s Third Law of Motion to build a rocket with a balloon and find how the amount of air in the balloon affects its flight. Materials: balloons string straws tape markers scissors clothespin Methods: 9. Your teacher will divide you into groups of 2 or 3. 10. With your partner, put the string through the straw and then secure the ends of the string (on walls or between your partners) making it tight and straight. This will be the guide track for your rocket. 11. Blow up your balloon and hold the end tight with your fingers or the clothespin. Have a partner tape the balloon to the straw so that the balloon end is facing the same direction as the straw (see picture below). 12. Start your balloon rocket at one end of your string track and launch it by letting the air out of the balloon. 13. What happens? 14. Now repeat launching with different amounts of air in the balloon. Record your observations. What do you think is happening? B. Experiment Day 1: Soda Bottle Motorboats Objective: Use Newton’s Third Law of Motion to build a motorboat. Materials: • Plastic soda bottle • Toilet paper • Baking soda • Vinegar Methods: 1. Wash out a 16-ounce plastic soda bottle. 2. Gather the toilet paper, baking soda and vinegar. 3. Lay out two squares of toilet paper. 4. Pour several tbsp. baking soda on the toilet paper squares and fold them to enclose the baking soda. 5. Place these toilet paper "packages" inside the bottle. 6. Have one person hold the bottle cap. 7. Pour 1/4 cup of vinegar into the bottle. 8. Place the cap on quickly and twist it only once. 9. Place the bottle in the water in the wading pool or bathtub. 10. Record what you see: 11. Mixing baking soda and vinegar causes a chemical reaction that produces gas. Why would this cause the boats to move? 12. Experiment with different amounts of vinegar and baking soda. How does this affect the boat's speed? DAY 2: Background Day 2: Just like balloon rockets and soda bottle motorboats, Bottle Rockets are governed by Newton’s third law of motion. A model rocket is subjected to four forces in flight; weight, thrust, lift and drag. Weight is the downward force created by gravity. Lift is the force that acts opposite to weight and holds the airplane or rocket in the air. Thrust is the force that moves an airplane forward; it is the direct result of Newton’s Third Law and thus the object of today’s activities. Drag is the force that acts opposite thrust, pushing the aircraft backwards. Using this information and the experiment you performed in Day 1, think about how you think a rocket works. How do you think you can make a rocket out of a soda bottle? Experiment Day 2: BOTTLE ROCKETS! Objective: Use what you learned from Day 1 to build a bottle rocket and find ways to make it go farther. Materials: • • • • • empty soda bottle 1 bicycle pump 1 “launch pad” rubber stopper for the soda bottle 1 measuring tape for measuring flight distances Methods: 1. Your teacher will direct you to a Blast-Off area with six bottle rockets ready for launching. 2. Which rocket do you think will go the farthest? Why? 3. Observe each launch and after each blast-off, record the distance the rocket traveled using the measuring tape. 0 mL m 100mL 200mL 300mL 400mL 500mL m m m m m Which rocket traveled the farthest? Why do you think this happened? What else do you think can affect the flight of a rocket? WTH Lesson: The Solar System Philadelphia Standards to be Covered • Describe the composition and structure of our universe. • Identify the planets in our solar system and their general characteristics. • Know models as useful simplifications of objects or processes. Vocabulary o Solar System o Planet o Revolution o Rotation Lesson Outline – 2 hours 1. Background Information 2. Solar System Modeling Activity a. Planet creation b. Powers of Ten Video c. Modeling of solar system 3. Discussion Questions / Wrap-Up Objective - To increase knowledge about the planets and how they move around the sun To gain a perspective on the size of the universe 1. Background - 30 min Make a powerpoint or have printouts with pictures of each planet. The earth is just one of nine planets in our solar system. These nine planets plus the sun compose our solar system, or the Milky Way Galaxy. The nine planets all move on their own orbits around the sun. Listed below are the nine planets in order from closest to furthest from the sun, with additional information about each planet. Sun This king of our Solar System rules because compared to everything else, it is so large. It contains about 98% of all the mass, or rocks, dust, and gas in the Solar System. If the sun were hollow a million Earths could fit inside. Mercury is a world of extremes. Because it is so close to the Sun a visitor could easily cook to death. However because mercury spins so slowly it gets very cold in the night time, which means a visitor could also freeze to death. Venus is a hostile planet. Its thick cloud cover makes it impossible to see the surface, and traps much of the Sun's heat. This extra heat gives Venus the hottest average temperature of all the planets. In many ways Venus is similar to the Earth. It has active volcanoes, venusquakes, mountains and valleys. The major difference is that Venus's atmosphere makes the planet far to hot for life. Earth Our planet is an oasis of life in an otherwise desolate universe. The Earth's temperature, weather, atmosphere and many other factors are just right to keep us alive. Mars excites scientists because its mild temperament is more like the Earth's than any of the other planets. Evidence suggests that Mars once had rivers, streams, lakes, and even an ocean. As Mars' atmosphere slowly depleted into outer space the surface water began to permanently evaporate. Today the only water on Mars is either frozen in the polar caps, or underground. Jupiter great red spot visible in the picture to the right is where a giant storm has been raging for at least 300 years. It's super hurricane winds blow across an area larger than the Earth. This giant planet has no solid surface. Under its atmosphere is a large liquid ocean of hydrogen and water. What lies in between that ocean and the atmosphere? Actually there is no in between. The atmosphere slowly gets thicker and thicker until it becomes part of the ocean. In other words Jupiter's ocean has no surface on which you could float a boat. The sky slowly becomes the ocean. Saturn is a favorite object for many observers. Its beautiful rings are 169,800 miles wide, but less thick than a football field. In many ways Saturn is similar to Jupiter, but it is much smaller. Under the clouds of methane and helium the sky gradually turns into liquid until it becomes a giant ocean of liquid chemicals. Uranus is very odd. Unlike all the other planets and most of the moons in our Solar System Uranus spins on its side. It is believed that long ago a very large object smashed into this planet. The crash was so powerful that it completely changed the direction of Uranus' spin. Uranus may have an ocean of water beneath its clouds. It has a large rocky core, and because of the tremendous pressure could possibly contain trillions of large diamonds. Uranus is almost identical to the planet Neptune. Neptune has a giant storm much like the storm on Jupiter. This storm is often called The Great Dark Spot. We do not know how long this storm has been active, because it is so far away that we could not get a good view of Neptune until modern times when we began to send robot explorers. For many centuries people did not know that this planet even existed. It was discovered by Johann Galle and Heinrich D'Arrest in 1846. Neptune has six rings which circle the planet. Pluto is smaller than 7 of the moons in the Solar System. Because it is so small many scientists don't consider it a planet at all. In 1999 a group of scientists attempted to redesignate Pluto as a comet. Pluto is the only planet that has never been visited by space craft. Because we have never sent probes to this planet we know very little about it. 2. Solar System Modeling Activity (90 min) PLANET CREATION (30 min) Materials: - Cool Trading Cards (http://amazingspace.stsci.edu/resources/explorations/trading/images/trading-cards.pdf) - 9 (w/o sun) or 10 (w/ sun) spherical foam in various sizes (Playdoh can also work) - A set of water colors / crayons - Chalk or string to mark orbits -Measuring Tape Methods: 1. Show “Powers of Ten” video. (http://www.youtube.com/watch?v=qrUQboKx_KE) 2. Discuss that the modeling activity will help the students better understand the size of the solar system. 3. Divide class into 9 or 10 groups. Discuss briefly (pick 1 or 2 items from the back of trading card?) each planet while handing out one trading card to each group (by asking groups to guess which planet is which based on picture on card?). 4. Explain to groups that they will be making models of the planets based on which trading card they obtained using the foam and coloring materials. The diameter of each planet is as follows: 3. Ask class to help you model the size of the solar system. Locate an (open) area (preferably with a surface that is amenable to chalk-drawing, else use string) and work with the students to mark the orbits as follows (note first 4 planets are a little cramped): 4. Introduce concept of “orbit”. Stress that planets NEVER leave their orbits. Begin the experiment by having children walk in their path or "orbit" around the sun. Stress that the planets never leave their own orbits. This travel around the sun is called "revolution". This term should now be introduced. 5. After the children have orbited the sun once, bring in the added concept of "rotation". While moving around the sun, the children should also start to spin around like tops. (Caution them against becoming dizzy.) This demonstrates "rotation". Tell children that it takes one year for the earth to revolve around the sun, and it takes one day for the earth to rotate on its own axis. 6. Point out that "rotation" or spinning on one's own axis takes much less time than going all the way around the sun, "revolution." 7. Take a picture / videotape the real life solar system model. 1. Discussion Questions (20 min) TYING IT ALL TOGETHER: After returning to the classroom, demonstrate the principle just learned with the globe. Let someone spin the globe and walk around a "sun" to show "rotation" and "revolution". For children who have trouble keeping "rotation" and "revolution" straight, here is a tip: the middle sound of "rotation" has the same vowel sound as "day" and it takes the earth one day to rotate. This same type of activity can be used to show the relationship between the moon and the earth. Role play is an excellent way to teach primary children and makes these abstract concepts come to life. The Solar System STUDENT HANDOUT Objective: To understand the concepts of relative size and distance in our solar system. Background Our solar system had 9 planets which circulate around the sun. Each planet has its own unique path which it follows to travel around the sun. These paths are used by scientists to determine the speed at which a planet moves and the location it will be at during any given time. Vocabulary Planetary Rotation: Spinning on ones own axis Planetary Revolution: Doing a loop around the sun. Experiment: Making Ice Cream Materials: • Planet Cards • Foam ball • A set of water colors / crayons • Chalk Methods: Which planet were you given to model? Describe this planet. 1. Take your small foam ball and work with your group to design it to look like the planet. 2. Go to the model of the solar system and place your planet the correct distance from the sun, using your measuring tape and the chart below: 3. Look at where the other planets are and how big they are compared to yours. 4. Walk around the sun in your planets orbit . What shape is the orbit for your planet? ________________________________________________________ Questions for Discussion 1. How long does it take the planet you have to make a rotation? _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ ________________________ 2. How long does it take the planet you have to make a revolution around the sun? _____________________________________________________________________ _____________________________________________________________________ ____________ 3. Write the names of the planets from smallest to largest using the model we made. _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ ________________________ 4. How are models helpful? What does it mean when I say that our model is made to scale? _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ ______________________________