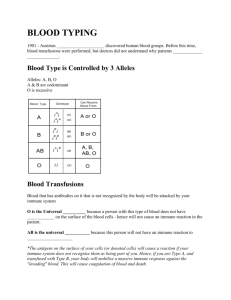
Document 14778543
advertisement

T 3 T cells, or T lymphocytes, develop in the thymus, and are a subfamily of circulating leucocytes that play an important role in the adaptive immune response and furthermore serve as crucial effector cells through antigen-specific cytotoxic activity and the production of soluble mediators (cytokines/chemokines). The characteristic feature of all T cells are clonal antigen-specific heterodimeric receptor molecules on the surface (T cell receptor; TCR). The accessory molecules CD4 and CD8 define the effector function and MHC restriction of T cells. Some T cells migrate to various locations throughout the body and interact with antibody production of B cells. Subsets of T cells (CD4+) have been classified as type 1 or type 2 T helper cells, depending on the cytokines they produce. Another subset is formed by the (CD8+) cytotoxic T cells. Cancer and the Immune System Streptococcus Infection and Immunity Mast Cells case letters (a, b). In the WHO nomenclature, human locus and gene are given a combination of capital letters and numbers (e.g. (TCR)BV8S1). Roman fonts indicate gene products and italics the genes. The antigen-binding sites of the chains are formed by protein loops called complementarity determining regions (CDR), which are connected by conserved framework regions. CDR1 and CDR2 and a fourth hypervariable loop, which is involved in superantigen binding, are V gene encoded. The CDR3 are VDJ or VJ encoded. The high variability of the CDR3 results from the combination of the V and J , or the V, D, and J genes, as well as additional events such as introduction of so-called N- or P-nucleotides, and imprecision of recombination. The number of different TCR which can be created by these mechanisms exceeds that of lymphocytes in the body. Superantigens Chronic Beryllium Disease Mucosa-Associated Lymphoid Tissue Cell-Mediated Lysis Cytotoxic T Lymphocytes Helper T Lymphocytes Lymphocyte Proliferation 3 T Cell 3 3 3 3 3 3 3 3 T Cell Antigen Receptor (TCR) T Cell Antigen-Specific Receptor The TCR is a molecule on surface of T cells composed of two polypeptides (α and β chains) of nearly equal molecular weights. Similar to antibody molecules the TCR has N-terminal variable amino acid sequences which combine to provide the individualized specificity (idiotype) shared by all TCR of a single cell of a single clone; the C-terminal portion is common to all α and β chains of the TCR. The antigen-specific receptor recognizes and binds peptides of thymus-dependent antigens (proteins) when presented by class I and II molecules which are encoded in the major histocompatibility complex (MHC). The peptides are generated by antigen-processing and antigen-presenting cells like macrophages, dendritic cells and B cells. Metals and Autoimmune Disease Idiotype Network 3 3 The clonally distributed antigen receptor of T cells is composed of a heterodimer consisting of α and β or γ and δ polypeptide chains, each containing one N-terminal variable (V) domain and one plasma membrane inserted constant (C) domain. Both heterodimers are expressed in association with the signal transducing CD3 chains. Most T cells are CD4+ or CD8+, and express the αβ TCR. They recognize foreign peptides presented by self - MHC molecules. A small number of T cells express the γδ TCR, which recognizes different types of mostly unknown ligands. Antigen recognition by γδ bearing T cells is not MHC restricted. The antigen-binding domain of α or γ chains is encoded by the V (variable) and J (joining) genes, that of β or δ chain by V, D (diversity) and J genes, which are recombined during T cell development. The gene names are usually numbered, with a Greek letter as a suffix for the chain (e.g. Vβ 8.1 for the variable gene 8.1 of the β chain). Alleles are designated by lower- T Cell-Dependent Antibody Response Assays for Antibody Production 3 T Cell-Dependent Antigen An antigen that requires the presence of T cell help to stimulate the B cell to secrete antibody. Such antigens do not elicit a productive antibody response by B cells unless the B cell receives help from a CD4 T cell. Help is generally supplied in the form of both a contactdependent signal via CD40 plus specific T cell-secreted cytokines. Generally, protein antigens are T-dependent antigens. Plaque-Forming Cell Assays Memory, Immunological Immunoassays The other six chains form the CD3 complex and consist of CD3γ which forms a heterodimer with CD3ε, CD3δ which also forms a heterodimer with CD3ε and a homodimer of CD3ζ. The CD3 chains are the targets for kinase phosphorylation. It is the CD3 complex that propagates the signal from the TCR complex to downstream signaling cascades. Signal Transduction During Lymphocyte Activation T Cell Selection 3 T Cell-Dependent Antibody Response 3 626 Thymus: A Mediator of T Cell Development and Potential Target of Toxicological Agents 3 3 An antigen that can stimulate B cells to secrete antibody in the absence of T cell help. Plaque-Forming Cell Assays 3 T Cell Oligoclonality This describes the restriction of an antigen-specific T cell response to one or several T cell receptor configurations. Following HLA–antigen–TCR interaction, this limited number of responder T cells undergo clonal expansion and increase the size of their TCR specific subpopulations. Chronic Beryllium Disease T cell receptors are composed of two different polypeptide chains, α and β chains (αβT cells) or, in a minor population of T cells, of γ and δ chaisn (γδT cells). The maturation of T cells occurs either in the thymus or, under special circumstances, (γδT cells) extrathymically. The first critical stage in T cell maturation is the successful rearrangement of the TCR β chain (pre-TCR complex). A functional TCR β chain initiates the arrangement of the TCR α locus and expression of both the CD4 and CD8 molecules (CD4+CD8+ double positive thymocytes). In contrast to these subsets of αβ T cells only little is known of the physiological effector function or antigen specificity of γδT cells. The receptor of these T cells is much more homogenous compared to αβ T cells. They are found in epithelia from where they do not recirculate. One hypothesis is that they participate in the pre-adaptive immune response. Cancer and the Immune System 3 3 T Cell-Independent Antigen γδT Cells 3 T Cell Receptor (TCR) Antigen Presentation via MHC Class II Molecules T-Dependent Antibody-Forming Cell Response 3 3 T Cell Receptor (TCR) Complex T Helper 1 Cells 3 The T cell receptor (TCR) complex consists of eight transmembrane chains that are expressed on the surface of T cells. The TCRα and TCRβ chains bind antigenic peptide when presented to them in the contact of MHC (major histocompatability) molecules. Plaque Versus ELISA Assays. Evaluation of Humoral Immune Responses to T-Dependent Antigens Helper T lymphocytes T Lymphocyte CD4 T cells that produce cytokines such as interleukins IL-4 and IL-5 but not IL-2 and interferon IFNγ. By this they direct humoral immune responses. Helper T lymphocytes Flow Cytometry 3 Cytokines such as IFN-α and IL-2, are autocrine and paracrine signaling molecules produced by CD4+ T cells in response to MHC class II antigen stimulation, and stimulate growth and activation of immunocytes and other inflammatory cells. Chronic Beryllium Disease T Helper 2 (Th2) Cells 3 T Helper 1 Cytokines (Th1) 627 3 T Helper 2 (Th2) Response CD4 T cells that produce cytokines such as interferon (IFN)γ, and interleukin IL-2 but not IL-4 and IL-5. By this they direct cellular immune responses. Helper T lymphocytes Flow Cytometry 3 3 T Helper 1 (Th1) Response A reaction mediated by CD4-positive T cells that serves to activate macrophages and promote digestion of intracellular bacteria. Th1 cells secrete cytokines such as interferon-γ (which activates macrophages) and lymphotoxin-α (which activates macrophages, inhibits B lymphocytes, and is directly cytotoxic to some cells). Lymphocytes Chronic Beryllium Disease A reaction mediated by CD4-positive T cells that kill infected cells and direct the destruction of extracellular pathogens by activating B cells. Th2 cells secrete cytokines such as the interleukins IL-4 and IL-5 (which activate B lymphocytes) and IL-10 (which inhibits macrophage activation). Lymphocytes 3 T Helper 1 (Th1) Cells T Helper Cell CD4+ helper cell subgroups that are defined by a different pattern of cytokine release. The Th1 subgroup produces a cytokine profile to induce inflammation and cell-mediated immunity. The Th2 subgroup produces a cytokine profile to induce antibody synthesis. Both subgroups act antagonistically to each other to secure an enhanced, but balanced immune response. Cytokines Maturation of the Immune Response Leukocyte Culture: Considerations for In Vitro Culture of T cells in Immunotoxicological Studies Food Allergy 3 3 3 3 3 3 T Helper 1–T Helper 2 Balance 3 Helper T lymphocytes Maturation of the Immune Response T Helper Lymphocyte 3 T Helper 2 Cells T Helper Cell Polarization 3 Balance in immune response after contact with antigen, especially important for response to allergens where T helper activity drives immune response either to cellular (delayed-type hypersensitivity, Th1) or antibody (IgE, Th2) mediated allergic reaction. Rats prone to a T helper 1 reaction (Lewis rats) showed more resistence to Salmonella infection compared to rats prone to a T helper 2 reaction (Brown Norway rats). Salmonella, Assessment of Infection Risk Trace Metals and the Immune System T Lymphocyte White blood cell with characteristic appearance, cellsurface markers, and function. They undergo differen- T 3 3T3 Neutral Red Uptake (NRU) Test tiation in the thymus. T lymphocytes control most aspects of the immune response, and are involved directly in attack on virus-infected cells and aberrant cells, such as malignant cells and cells originating from a different individual (as in a transplanted organ). CD Markers Canine Immune System Delayed Type Hypersensitivity Tachypnea Increased number of breaths per minute. Septic Shock 3 628 3 3 3 TAPA-1 (Target of an Antiproliferative Antibody-1) Trace Metals and the Immune System 3 Tachycardia Heart rate above 100 beats per minute. Septic Shock The cytotoxic activity of immune cells is targeted towards specific cell types, which vary depending on the cytotoxic cell type involved. Limiting Dilution Analysis Target Cell Killing Cell-Mediated Lysis Targeted Mutant Mouse 3 3 T Suppressor Lymphocyte Target Cell 3 T regulatory or suppressor T cells play important roles in the regulation of immune responses and mediate a dominant immunologic tolerance. The mechanisms by which naturally occurring Tregs are able to suppress CD4+ and CD8+ T cell proliferation are not yet known. The CD4+CD25+ Tregs represent a subset of suppressor T cells and have been shown to play a critical role in the prevention of organ-specific autoimmunity and allograft rejection. Transforming Growth Factor β1; Control of T cell Responses to Antigens A DNA probe (labeled with a fluorescent reporter dye and a fluorescent quencher) used to detect specific sequences in PCR products. When amplification occurs the Taqman probe is degraded by the 5' exonuclease activity of Taq DNA polymerase, thus separating the quencher from the reporter. The increase of reporter dye fluorescence is used to determine the presence of specific gene sequences. Polymerase Chain Reaction (PCR) 3 3 T Regulatory Cells (Tregs) Taqman 3 The in vitro 3T3 neutral red uptake (NRU) phototoxicity test was developed and validated in a joint EU/ COLIPA project (1992–97). The aim was to establish a valid in vitro alternative to the available in vivo tests. The parameter for the detection of cell viability and for measuring the total activity of a cell population is based on the uptake of the vital dye neutral red into cellular lysosomes of living murine BALB/c 3T3 fibroblasts. Three-Dimensional Human Skin/Epidermal Models and Organotypic Human and Murine Skin Explant Systems TAPA-1 (CD81) is a 26 kDa surface protein expressed on the surface of B cells as well as T cells. It binds several different integrins and is believed to be involved in activation, cell adhesion and migrations. Signal Transduction During Lymphocyte Activation 3 3T3 Neutral Red Uptake (NRU) Test Knockout, Genetic 3 Tests for Autoimmunity TbAT1 Genes Trypanosomes are unable to synthesize purines de novo and rely on nucleoside transporters. The Trypanosoma brucei adenosine transporter 1 (TbAT1), also described as the trypanosomal P2-transporter, enables adenosine uptake. In addition, it confers susceptibility to antitrypanosomal drugs such as arsenicals. Various point mutations have been identified in the TbAT1 gene of resistant trypanosomes. Trypanosomes, Infection and Immunity 629 tures. 2,3,7,8-TCDD has been assigned a toxic equivalency factor (TEF) of 1.0. TEF values for individual congeners of dioxins, furans, and biphenyls in combination with their concentration can be used to calculate the total TCDD toxic equivalents concentration (TEQs) contriubted by all dioxin-like congeners in the mixture using appropriate equations. Compounds are included in the scheme and assigned a TEF if they show structural relationships to PCDD or PCDF, bind to the aryl hydrocarbon receptor, elicit aryl hydrocarbon receptor mediated biochemical and toxic responses, and persist and accumulate in the food chain. Dioxins and the Immune System 3 3 TCDD Dioxins and the Immune System Teratogen 3 Any substance or exposure that causes birth defects. Birth Defects, Immune Protection Against 3 Telomeres 3 TEQ/TEF The complex nature of polychlorinated dibenzo-p-dioxins, dibenzofurans and biphenyls, which are usually generated together in occupational or environmental exposure complicates the risk evaluation for humans. This is a concept introduced to facilitate risk assessment and regulatroy control of exposure to these mix- Testosterone 3 Telomeres are the physical ends of chromosomes. They are specialized nucleoprotein complexes that have important functions, primarily in the protection, replication, and stabilization of the chromosome ends. In most organisms telomeres contain repeated simple DNA sequences composed of a G-rich strand and a Crich strand (called terminal repeats). These terminal repeats are highly conserved—in fact all vertebrates appear to have the same simple sequence repeat (up to 2000 times) in telomeres (TTAGGG)n. After each cell division, telomere shortening takes place. Telomere length is therefore indicative for the numbers of divisions a cell has been through. Critically short telomeres trigger replicative senescence and cell cycle arrest. The innate immune system provides the first line of defence against many microorganisms and is essential for the control of common bacterial infections. It comprises macrophages, neutrophils, and natural killer cells. These cells of the innate immune response play also a pivotal role in the initiation of a subsequent adaptive immune response. Aging and the Immune System Steroid Hormones and their Effect on the Immune System Tests for Autoimmunity Raymond Pieters Head Immunotoxicology Institute for Risk Assessment Sciences (IRAS) Yalelaan 2 P.O. Box 80.176 3508 TD Utrecht The Netherlands Short Description A considerable number of chemicals, including many drugs, are capable of inducing autoimmune-like diseases in man (1–3). Autoimmunogenic chemicals rarely induce similar clinical adverse effects in test animals and are hardly ever identified in general toxicity testing. Hence, autoimmune-like symptoms often become apparent only after introduction to the market. In combination with the fact that these symptoms can induce very serious or life-threatening conditions, the autoimmunogenicity of chemicals, and drugs in particular, poses a huge problem to certain sectors of society—patients, clinicians, pharmaceutical companies, and governmental T 630 Tests for Autoimmunity agencies. Conceivably, there is an urgent need for screening tests to identify such chemicals. The main reason for the inability to assess a chemical's potential to cause autoimmune-like diseases is that the underlying mechanism is very complex, and can involve the interplay of many predisposing factors. An important factor is the genetic make-up, the major histocompatability complex (MHC) haplotype, nonMHC regulatory genes, metabolic polymorphisms, and gender; but many environmental factors (such as ongoing infections and food ingredients) are also known to co-influence autoimmune phenomena (Figure 1). The complexity of the etiology may be the reason that only a few drug-using patients develop autoimmunelike derangements, but also explain why symptoms suddenly appear after a long period of symptom-free drug usage. A set of rational criteria to establish autoimmune etiology of diseases in man was postulated in 1962 and is reviewed in (4). One of the criteria requires the presence of circulating antibodies or cell-mediated autoimmunity. Others require that the corresponding autoantigen should be identified, and—more importantly —that the disease can be reproduced by passive transfer of that antibody or the self-reacting cells or by immunization with the self antigen. Today, it is realized that autoreactivity (both autoreactive B as well as T cells) is a normal and necessary property of a healthy immune system and that only few self-reactive autoantibodies or autoreactive lymphocytes may be considered pathogenic (i.e. directed against a pathologically relevant autoantigen and capable of causing tissue damage and reproducing disease in experimental animals). The frequency of these pathogenic autoreactive antibodies or lymphocytes may be significantly higher in diseased compared to control population (2,3). Tests for Autoimmunity. Figure 1 Representation of risk factors that are possibly involved in development of autoimmune derangements. Adapted from (7). Although autoreactivity is a healthy phenomenon and changes in autoimmune-linked parameters do not necessarily result in an autoimmune disease, it is important to note that changes in such parameters may be used to flag a chemical as possibly autoimmunogenic. Characteristics At present no clearly defined screening tests for autoimmunity in animals exist. The popliteal lymph node assay (PLNA) is a simple straightforward local lymph node assay that may be useful to screen for initial immunostimulating capacity of chemicals. But this assay can only be regarded as a first screening test for immunosensitizing potential and to indicate that a chemical might induce autoimmune-linked symptoms. It is preferable for screening tests for autoimmunity to use relevant exposure routes and demonstrate systemic changes in parameters indicative of autoimmunelinked responses. Diagnosis of autoimmune-linked diseases in test animals, like rats or mice, may be based on a combination of general well-being, routine clinical tests and (immuno)histology. Clinical investigations should include general hematology (e.g. to check for anemias) as well as tests for complement activity, or acute-phase proteins, and for erythrocyte sedimentation. Liver and renal impairment should be monitored biochemically (3). Morphologically, a wide range of organs should be checked for indications of inflammation, overt apoptosis (in the thymus in particular). Peripheral immunologic organs should be checked for indications of activation (e.g. hyperplasia or formation of germinal centers) (5). Morphological indications of tissue inflammation, activated immune organs or immunomodulation (e.g. thymus atrophy) should be followed up by more thorough investigations into alterations of autoimmune parameters (5). Because development of actual autoimmune disease depends on a complex interplay of (non)inherent factors (see Figure 1), relevant changes in any of the animals should be considered as an alert to pursue further investigations. This is certainly the case in outbred animals which are used for evaluation of toxicity, but also in inbred animals which are also not always 100% responsive. The initial focus in follow-up studies should be on detection of autoantibodies, which can be directed against a wide spectrum of autoantigens (2). In case the target autoantigen is not yet known, and particularly for screening purposes, the indirect immunofluorescence (IF) technique may be useful. The immunofluorescence technique, which is also used in the clinic, has been used successfully in animal studies. Briefly, cryosections or isolated cells grown on microscopic slides (for instance HepG2 tumor cells for antinuclear antibodies (ANA) or freshly isolated granulocytes for antineutrophil cytoplasmic antibodies Tests for Autoimmunity (ANCA)) are an incubated with serum suspected to contain autoantibodies followed by a incubation with fluorochrome-labeled second-step antibody. Interestingly, the immunofluorescence technique can be applied to cryosections of a range of relevant organs (such as kidney, thyroid, liver, skin, adrenals, and sex organs), although false-positive staining (perhaps as a result of antibody binding to Fc receptors) particularly in inflamed tissue has to be taken into account. When the specificity is known, autoantibodies can be detected by various other techniques (most notably enzyme-linked immunoassay, ELISA). In many cases, it may suffice to perform an ELISA for ANA. Compound-specific lymphocyte transformation tests (LTT) can be used in cases of drug allergy or chemical exposure (see for instance Schnyder et al 2000) but detection of autoreactive T cells in case of chemicals is much more difficult. This is mainly due to the fact that the relevant autoantigen (chemically altered or previously cryptic epitopes) is hardly ever known, and also because specific autoreactive T cells are relatively scarce even in clinical situations. A solution would be to immortalize selected self-reactive T cell clones, but this is not easy to incorporate in a general testing model for autoimmunity. Pros and Cons All of these methods may at best provide circumstantial evidence for autoimmune effects and/or etiology in animals. The advantage of these methods is that they can all be used in animal toxicity studies without interference with the study per se, and only ask for more extensive analyses of samples (blood, serum and organs) that are already to be isolated at dissection. But, importantly, as the immunological effects depend greatly on genetic make-up, autoimmune effects may be easily missed when small groups of outbred test animals are used. So animal tests for autoimmunity (including the parameters discussed here) should be done with larger test groups and should be performed over relatively long periods of exposure (> 90 days). Importantly, adverse effects which are indicative of autoimmunity—even if they occur in only one animal—should already be taken as an alert to execute follow-up studies with inbred animal strains, such as the frequently used high-IgE-responding Brown Norway (BN) rat. To date, only a limited number of compounds (e.g. HgCl2, gold salts, d-penicillamine, nevirapine, hexachlorobenzene) have been shown to induce autoimmune-like phenomena in this rat strain. Relevance to Humans Chemical-induced autoimmune effects detected in animals can be predictive for the human situation. However, as in humans the prevalence of autoimmune effects will be low in (outbred) animals as well. Studies 631 with particularly sensitive rat strains, for instance, such as the BN rat, may identify much better the hazard of autoimmunogenic potential of a chemical. Notably, such a sensitive rat has to be regarded as a representative of very susceptible humans. Regulatory Environment At present guidelines for detection of autoimmunogenic capacity do not exist. It should be realized that none of the present animal models—including the BN rat model—is capable of detecting autoimmunogenic potential of a wide range of different chemicals. The popliteal lymph node assay (PLNA) is an animal model that may indicate whether a chemical is immunostimulatory. Immunostimulation may result in sensitization of the immune system and is considered one of the prerequisites for inducing autoimmunity. A number of the parameters proposed here, however, could be easily or are already incorporated in existing guidelines. For instance, the OECD guideline 407 includes the hematology, clinical biochemistry and pathology of a series of organs. But without further analyses of (auto)antibody levels, larger test groups of inbred animals and long exposure periods (> 90 days) a chemical's potential to induce autoimmunity will hardly ever be detected in these toxicity studies. So, future research to design predictive protocols and screening models is greatly needed. This could be initiated by thorough research into the relevance of the above-mentioned parameters in repeated-dose studies over a relatively long period with inbred strains of rats (e.g. BN and Lewis strains) as well as mice (e.g. SJL and C3H/He strains), but also in outbred animals that are normally used in toxicity studies. Such studies should first be performed in a limited number of well-equipped laboratories, and should be followed by more extensive ring studies. References 1. D'Cruz D (2000) Autoimmune diseases associated with drugs, chemicals and environmental factors. Toxicol Letters 112–113:421–432 2. Verdier F, Patriarca C, Descotes J (1997) Autoantibodies in conventional toxicity testing. Toxicology 119:51–58 3. D'Cruz D (2002) Testing for autoimmunity in humans. Toxicol Letters, 127:93–100 4. Shoenfeld Y, Isenberg D (eds) (1990) The Mosaic of Autoimmunity, Factors Associated with Autoimmune Disease. Introduction. Research Monographs in Immunology, Volume 12. Elsevier, Amsterdam 5. Frieke Kuper C, Schuurman H-J, Bos-Kuijpers M, Bloksma N (2000), Predictive testing for pathogenic autoimmunity: the morphological approach. Toxicol Letters 112–113:433–442 6. Schnyder B, Burkhart C, Schnyder-Frutig K et al. (2000) Recognition of sulphamethoxazole and its reactive T 632 2,3,7,8-tetrachlorodibenzo-p-dioxin metabolites by drug-specific CD4+ T cells from allergic individuals. J Immunol 164:6647–6654 7. Kammüller ME, Bloksma, N, Seinen W (1989) Immune disregulation induced by drugs and chemicals. In: Kammüller ME, Bloksma N, Seinen W (eds) Autoimmunity and Toxicology. Elsevier, Amsterdam, pp 3–25 Three-Dimensional Human Skin/ Epidermal Models and Organotypic Human and Murine Skin Explant Systems Hans-Werner Vohr . Eckhart Heisler 2,3,7,8-tetrachlorodibenzo-p-dioxin Dioxins and the Immune System PH-PD, Toxikology Bayer HealthCare AG Aprather Weg 18 D-42096 Wuppertal Germany 3 Synonyms Tetravalent Vanadium Tetravalent vanadium is the ionic form of vanadium when four outer shell electrons (that is, two from 4s and two from 3d orbitals) have been shed, thereby giving the atom an overall charge of +4. Vanadium and the Immune System 3 TGF-β1 Transforming Growth Factor β1; Control of T cell Responses to Antigens human skin recombinants, reconstructed human skin/ epidermis, 3-D human skin/epidermal equivalents, in vitro engineered skin/epidermal substitutes, artificial skin/epidermis, organotypic murine or human skin explant system, MSE, HSE, hOSEC Definition Human full-thickness skin models and reconstituted epidermal equivalents are in vitro-engineered tissue cultures that provide a three-dimensional architecture which is biochemically, morphologically and functionally comparable to human epidermal tissue/skin in vivo. Organotypic skin explant systems are based on ex vivo skin removed from humans or mice and subsequently cultured in toto. All the models were shown to be useful in screening for topically applied irritating, corrosive or photocytotoxic compounds. Results from experiments with systemically applied compounds have already been published with such models, too. Furthermore, in recent studies it was demonstrated that 3-D skin models also provide the capacity to further characterize and screen for substances with a sensitizing potential. 3 3 Characteristics Reconstructed Human Epidermal Models Reconstructed human epidermal models are built up from proliferating, differentiating and cornifying keratinocytes which are airlift-cultured on a porous polymeric membrane. The design of the cell culture conditions (air-liquid interphase and medium/ingredients) drives the cells to differentiate and form a three-dimensional (3-D) epidermal multilayer with a functional and stratified surface. Most of the key structural elements of native epidermis like keratins, transglutaminase and lipid composition that characterize the status of keratinocyte differentiation are present in 3-D human epidermal equivalents. 3 An important mechanism in the immune regulation involves homeostasis between the T helper 1 (Th1) and T helper 2 (Th2) activity of CD4+ T helper cells expressing different cytokine patterns. T helper cells showing Th1 activity are more prone to induce a cellmediated immunity whilst T helper cells obtaining Th2 activity are more prone to induce a humoraltype immune response. T helper cells showing either Th1-type or Th2-type reactivity are exclusively characterized by differences in cytokine expression. Briefly, Th1 reactivity is predominantly connected to interferon (IFN)-γ, IL-2, and IL-12 secretion. In contrast Th2 cells express mainly IL-4, but also IL-5, IL6, IL-10 and IL-13. The Th1/Th2 balance is integrated in the immune regulation in a dynamic and reversible manner, depending also on kinetics and dose–response of the immune response. Cancer and the Immune System 3 Th1/Th2 Balance 3 Three-Dimensional Human Skin/Epidermal Models and Organotypic Human and Murine S 633 Three-Dimensional Human Skin/Epidermal Models and Organotypic Human and Murine Skin Explant Systems. Figure 1 Two different reconstructed tissues: H&E stained sections of untreated reconstructed human full thickness skin model (Advanced Cell Systems, AST-2000) and epidermal model (Skinethic RHE ). Both models are comercially available. (Picture of AST-2000 by kind permission of Advanced Cell Systems, St. Katharinen, Germany). Organotypic Skin Explant Systems Organotypic skin explant systems from human, rats or —to a lesser extent—mice have also been established for evaluating percutaneous absorption and penetration. However, in comparison to reconstructed skin models the explant cultures naturally provide a physiological cell composition and micro-architecture including immunocompetent cells (e.g. Langerhans cells). For toxicological and immunotoxicologic research, both topical treatment (application of compounds to the dry stratum corneum) as well as systemic-like treatment (application of substances directly into the cell culture medium) are possible using 3-D skin mod- els. Hazard identification is based on the measurement of decreased cell viability and changes in tissue morphology after treatment (histological examination; see below). In recent studies it was also shown that topical and systemic-like treatment of 3-D skin models with hazardous compounds often results in induced expression and/or release of immunomodulating proteins (cytokines, chemokines, matrix metalloproteinases, growth factors, and other parameters which are involved in a variety of biochemical pathways; see below). The determination of these parameters gives a detailed overview of the cell status which can additionally confirm the results from viability testing and histological examinations (multiple endpoint analysis; MEA). Screening for Irreversible Cutaneous Toxicity (Corrosion) Screening for Acute Irritation Both artificial skin models and organotypic skin explant systems are suitable for screening for dermal irritation induced by topically applied irritating or photo-irritating compounds and formulations. Most likely in this situation is that in vivo substances with a strong irritant potential provoke severe destruction of the reconstructed or explanted tissues and affect the integrity of residential cells. The use of these systems to test chemicals, compounds, or formulations according to their irritant properties depends on the measurement of cell viability after topical treatment with compounds and additional time-related incubation. Cytotoxic and photocytotoxic effects cause a significant 3 Full-Thickness Human Skin Substitutes Full-thickness human skin substitutes additionally provide a dermal layer that usually consists of a collagen matrix which is populated by living fibroblasts. In an early state of research the use of de-epidermized human dermis as the backbone of full-thickness skin equivalents has been discussed as well. In comparison to single-cell culture systems, the most predominant feature of these artificial skin models is the existence of a physiological and functional barrier (the stratum corneum) that regulates percutaneous absorption/penetration of compounds as well as transepidermal water loss. Although the barrier functions of artificial skin models are different from the situation in vivo, the results from studies evaluating the penetration properties of various reference test compounds have shown a good correlation to in vivo data. T Three-Dimensional Human Skin/Epidermal Models and Organotypic Human and Murine S decrease in cell viability. For this reason, determination of cell viability is essential for the assessment of compound biocompatibility using artificial skin models or organotypic skin explant systems. However, in most of the published test protocols MTT conversion is used as a single endpoint parameter for the determination of cell viability and consequently the degree of cytotoxicity caused by irritation and photoirritation. Recently the identification of more specific parameters allows a multiple endpoint analysis (cell viability, histological examination, release of IL-1α;). Expression of Immunomodulating Proteins and Screening for Dermal Sensitization Both irritation and sensitization of the skin are related to the expression and release of immunomodulating proteins such as cytokines, chemokines and cell surface proteins, especially within the epidermis. The local immune system of the skin in vivo is based on the interactions between epidermal keratinocytes, epidermal Langerhans cells, and dermal fibroblasts. Once activated by antigen uptake and processing, Langerhans cells undergo morphological changes and start to migrate to the local draining lymph nodes. There T cells become activated upon successful antigen presentation. In cases of cutaneous irritation causing epidermal cell damage, keratinocytes release a cocktail of proinflammatory proteins from their intracellular reservoirs. This finally results in a non-specific activation of the skin’s immune system (see also contact hypersensitivity section). Considerable efforts have been made to integrate Langerhans cells into reconstructed human skin models. However, there is still no complex in vitro system available that provides functional antigen-presenting cells in the epidermis or dermis. Nevertheless, keratinocytes are also thought to be involved in the initial steps of irritation and sensitization. Topical treatment of artificial skin models with irritating compounds leads especially to the release of interleukins IL-1α and IL-8 by keratinocytes. Furthermore, the subsequent analysis of cell culture supernatants by different ELISA techniques (enzyme-linked immunoassay) additionally show an induced release of different chemokines and cytokines as shown in Table 1. The profile of released proteins depends on the kind of model used for the experiments. In comparison to reconstructed epidermal models, full-thickness skin models provide a set of parameters that are related to the interaction between epidermal keratinocytes and dermal fibroblasts. In recent studies carried out with sensitizing substances the ratio between IL-1α and IL8 release after topical treatment with the compounds revealed promising results that suggest that reconstructed human skin models are capable of discriminating sensitizers from compounds with an exclu- sively irritant potential. Other studies identified promising parameters (increased release of the chemokines monocyte chemoattractant protein 1 (MCP-1) and interferon-inducible protein (IP-10) from a human full-thickness skin model AST-2000 after treatment with the standard sensitizer (oxazolone) that certainly can contribute to a successful discrimination between sensitizers and irritants in vitro. From an immunological point of view, however, it is of prime importance for sensitization testing to analyze parameters ( MIG, Langerin, TARC, etc.) that are characteristic for the cross-talk between keratinocytes, fibroblasts and antigen-presenting cells in their natural setting. For this reason, research on skin sensitization (screening, mechanistic) is particularly focussed on the use of organotypic skin explant systems as well as the development of skin recombinants that incorporate functional antigen-presenting cells. 3 634 Pros and Cons Experimental Strategies Methods used in in vitro dermal toxicology are often based on single-cell culture systems, which in turn are built up from either freshly isolated primary cells derived from cosmetic surgery, foreskins or well-established cell lines. Methods for cytotoxicity and photocytotoxicity testing, like the 3T3 neutral red uptake (NRU) test, have been successfully validated. However, test principles based on single-cell cultures are subject to some limitations due to their lack of a physiological barrier. For this reason they are usually restricted to soluble substances and therefore fail when it comes to testing hydrophobic compounds or formulations. Furthermore, the concentrations of compounds inducing irritation in single-cell cultures are significantly lower than those determined in in vivo experiments. Due to the absence of a stratified surface, false positive results may also occur, because substances may be classified as (photo)cytotoxic by 3T3 NRU although they are physicochemically unable to pass through the physiological barrier (the stratum corneum). By using 3-D skin models it is possible to overcome these problems, and they offer a promising test system for topical and systemic-like compound administration. Furthermore, artificial skin models and organotypic skin explant systems may be suitable for screening for sensitizing properties of compounds in vitro. With respect to this last point research is still in progress, but a convincing system may be available in the near future. Test Principles As already mentioned, MTT testing is often used as a single-endpoint parameter for predicting the irritant potentials of substances, although cytotoxicity is not 3 3 Three-Dimensional Human Skin/Epidermal Models and Organotypic Human and Murine S 635 Three-Dimensional Human Skin/Epidermal Models and Organotypic Human and Murine Skin Explant Systems. Table 1 Expression/release of immunomodulating proteins from 3-D skin models Parameter Expression/Release Release Inducible Interleukin-1α ++ (a,b,c) Yes Interleukin -1β + (b,c) Slightly Interleukin -6 +++ (+a,b,c) Yes Interleukin -8 +++ (+a,b,c) Yes Tumor necrosis factor-α + ((a),b,c) Slightly Monocyte chemoattractant protein MCP-1 +++ (+b,c) Yes MIG + (c) Slightly Interferon-inducible protein IP-10 + (b,c) Slightly Macrophage inflammatory factor MIP-3α + (c) Slightly Matrix metalloproteinase MMP-3 ++ (+b,c) Slightly Matrix metalloproteinase MMP-9 ++ (b,c) Yes a, epidermal model; b, full thickness skin model; c, organotypic skin explant system; +, low level; ++, medium level; +++, high level; (+), high background. a sufficient stand-alone parameter for predicting cutaneous irritation. In vitro testing associated with MTT conversion is always subject to some limitations, because the test principle is based on a chemical redox reaction which may also run without any participation of living cells. This may lead to false positive results. Another problem with MTT, especially concerning 3D skin models, was observed when test results were compared to histological examinations of reconstructed skin models after compound treatment. Due to cellular activity, formazan crystals were found to be formed especially in the cells from the basal layer. For Three-Dimensional Human Skin/Epidermal Models and Organotypic Human and Murine Skin Explant Systems. Figure 2 H&E stained section of Skinethic RHE after treatment with 0,4% SDS and 24 hours of incubation (5% CO2, 37°C, max hum.) The area marked with the red arrow shows massive destruction of cells in the upper epidermal layers. However, the basal layer (blue arrow) is not affected. Here, MTT test gave false negative results. Although cell viability was correctly determined, the integrety of the cells in the upper epidermal layer was hardly affected. This effect however, was undetectable by MTT alone (By kind permisson of SkinEthic Laboratories, Nice, France). this reason, it is not possible to detect undesired compound-related effects on cells from the stratum spinosum or stratum granulosum by MTT (see Figure 2). Other test principles for the determination of cell viability are based on the quantitative analysis of enzymes from the cytosol of cells. When cells lose their integrity through damage to the plasma membranes, the leakage of these proteins can be recorded and quantified by bioluminometric or other optical enzymatic test systems. In this context the measurement of lactate dehydrogenase (LDH) and/or adenylate kinase leakage is often discussed as a defined parameter for the analysis of substance-related cytotoxic effects on in vitro cell systems. Finally, the induced release of proinflammatory mediators like IL-1α additionally serves as a good parameter for the characterization of skin irritation, because IL-1α was found to be released from cells which are influenced by irritating chemicals. Although MTT is a reliable and valid parameter for the analysis of cell viability, the results should be supplemented and verified additionally by multiple endpoints, such as expression and release of proinflammatory mediators, decrease of the barrier function determined by transepidermal water loss (TEWEL) and/or evaluation of morphological changes (histologic examination). Comparison to In Vivo Test Principles The replacement of in vivo methods for corrosivity and irritancy according to Draize by in vitro reconstructed skin models is often discussed, especially from an ethical point of view. In addition, the use of 3-D skin models is less time-consuming than in vivo T 636 Three-Dimensional Human Skin/Epidermal Models and Organotypic Human and Murine S testing, and if the costs for animal health and care are taken together, reconstructed skin models are less costeffective than animal testing, too. However, a ranking between strong irritation and weak or mild irritation based on experimental results from testing with reconstructed skin/epidermis still seems to be questionable. The establishment of in vitro test methods for sensitization is not that easy, although protein fingerprinting of cells from organotypic skin explant systems and reconstructed epidermal/skin models revealed promising results that contribute to the in vivo situation. In recent studies it was shown that the expression and release of immunomodulating proteins (Table 1) serve as good parameters for the characterization of compounds with sensitizing properties. However, the use of these parameters as criteria for predicting sensitization has not been validated so far. For this reason, guinea pig assays like those described by Buehler or Magnusson and Kligman are still the most reliable methods for sensitization testing, even though they are based on visible subjective parameters like the formation of erythema. In this context, another valid method is LLNA/IMDS (local lymph node assay/integrated model for the differentiation of (chemical)-induced skin reactions) which characterizes sensitizing compounds with the help of cellular parameters, but is still based on animal treatment. Predictivity Irritation of the skin caused by exposure of individuals to different kinds of hazardous compounds or formulations is the most common non-specific immune reaction observed in human skin. In vivo (animal) test principles according to the methods of Draize are frequently used for the identification of substances with irritant potential. For several reasons, however, these test methods are questionable. The analysis of substances according to Draize testing is mainly based on the evaluation and scoring of macroscopic parameters such as overcasting of the rabbit eye cornea or redness of the skin after treatment with the compounds being tested. As far as this point is concerned, it has recently been shown that the choice of endpoints for the assessment of acute skin irritation according to international standards (methods according to Draize) may lead to misclassification of substances. Furthermore, the transfer of established data from animal testing to the human situation in vivo is still controversially discussed. For this reason the human patch test was established. This ideally meets the requirements, but patch testing in human is restricted to weak or moderate irritating compounds. These pragmatic disadvantages of in vivo animal and human testing for skin corrosion or acute skin irritation are furthermore accompanied by the discussion of the ethical justification of animal testing in toxicological research. With the use of reconstructed tissue models it is possible to overcome most of the problems described above. From multiple endpoint analysis (see Characteristics) reliable parameters are available that are simple to determine, while the output is more stringent than visual evaluation of results. Furthermore, artificial skin models were proven to be reproducible in intra- and interlaboratory multicenter studies. As mentioned above, the predictivity of reconstructed tissue models is limited. In comparison to human in vivo skin the different physiological barrier function of the reconstructed stratified surface may cause problems because the risk of false positive results cannot be totally excluded. In addition, distinguishing between weak and moderate irritating compounds is sometimes not easy. However, research is focussing on new parameters that could help to solve these problems. Despite this early state of affairs, it is possible to state that human reconstructed tissue models exhibit acceptable predictivity in screening for corrosive compounds (sensitivity and specificity > 80%). Although the validation and catch-up validation studies for acute irritancy of topical applied formulation and/or raw materials are still in progress, a high correlation of sensitivity has already been estimated by the relevant ECVAM Task Force. Another main topic of interest concerns alternative in vitro models for skin sensitization. At present, no reconstructed tissue model is available that meets the guideline criteria for adequate screening. However, considerable efforts have been made to search for parameters (cytokines, chemokines) which specifically characterize the complexity of the processes leading to skin sensitization (skin penetration, formation of protein-hapten complexes, antigen uptake and processing, migration of LC to the local draining lymph nodes, presentation of antigen to T cell populations in the draining lymph nodes). In the light of this complexity, the use of organotypic skin explant systems seems to be very promising, because they provide the same micro-architecture and the same cell composition as in vivo skin and are therefore potent tools for mechanistic studies. Relevance to Humans The test results from animal testing for irritancy and corrosion according to Draize are controversially discussed among toxicologists. In cases of acute irritation these test methods have never been validated and they principally depend on a collection of cross-connected empirical clinical and preclinical data. For this reason, the use of reconstructed human tissues is of particularly great value, because the cells used for these skin constructs are of human origin. Although some differences in the characteristic barrier function have been Three Rs described, the experimental design closely matches the human situation in vivo. Unfortunately, screening for sensitization in vitro is even more complex because artificial tissue structures are necessary which must in addition provide immunorelevant cross-talk activities. For hazard identification, on the other hand, fingerprinting of proteins released from 3-D in vitro skin models has already been evaluated and some of these parameters were shown to hold key positions in immunological pathways (IL-8, MCP-1, IL-1α, IL-6, etc.). These may therefore help to screen for compounds with a sensitizing potential in vitro. As mentioned earlier, human skin explant systems in particular are believed to be very suitable models for further characterization of immunorelevant parameters. In the heat of discussion about testing for sensitization, one should keep in mind that in vivo animal testing (guinea pig assays or the (modified) local lymph node assay) or human patch testing, as well as all possible in vitro models which are going to be established and validated in the future, are not capable of taking all parameters influencing the induction of skin sensitization into account (individual parameters such as genotype, age, sex, side of contact/penetration and of course the overall condition of the skin). Regulatory Environment Skin Irritation/Corrosion The international standards for skin irritation and corrosion are still based on in vivo test principles according to the methods of Draize et al. (1944). However, the considerable efforts of organizations like ECVAM, ICCVAM, COLIPA, the Steering Committee on Alternatives to Animal Testing (SCAAT) have had a favorable and lasting influence on the establishment of in vitro test methods of international guidelines. The use of several in vitro human skin models for skin corrosion was validated by ECVAM in 2000. For acute skin irritation, however, a first prevalidation study failed but the process of improving the use of reconstructed tissue models especially in this field of toxicologic research is strictly ongoing. Guidelines for Determination of Substance-Induced Skin Corrosion * OECD Guideline 402: Acute Dermal Tox. * OECD Guideline 404/405: Acute Dermal/Occular Tox Irritation and Corrosion * OECD Guideline 410: Repeated Dose Dermal Tox. * OECD Guideline 430: In Vitro Skin Corrosion— Rat TER (Trans Epidermal Resistance) Test * OECD Guideline 431: In Vitro Skin Corrosion— Human Skin Models * Annex V of Directive 67/548/EEC (1997) * US Code of Federal Regulations (1991) 637 Sensitization Up to now no in vitro screening model has been available to correctly predict exclusively sensitizing properties of compounds. From an immunological point of view this is not surprising because of the lack of antigen-presenting cells in most of the reconstructed human tissues. However, the induced release of immunomodulating proteins indicates promising parameters for successful discrimination between irritating and sensitizing substances. As long as none of the reconstructed or organotypic models match the criteria for a successful prevalidation study, immunotoxicologic research must rely on guinea pig test principles according to Bühler, Magnusson and Kligmann, or on a refined test assay like the LLNA or the integrated model for the differentiation of (chemical)-induced skin reactions (IMDS). Guidelines for Determination of Substance-Induced Sensitization * OECD Guideline 406: Skin Sensitization (1992) * OECD Guideline 429: LLNA (2002) * U.S. EPA-OPPTS Harmonized Test Guideline 870.2600 on Skin Sensitization (1998) * FDA (CDER) (Draft) Immunotoxicology Evaluation of Investigational New Drugs (2001) * CPMP/SWP/398/01 (Draft) Note for Guidance on Photosafety Testing (2001) (as modified LLNA) References 1. Botham PA, Earl LK, Fentem JH, Roguet R, van de Sandt JJM (1998) Alternative methods for skin irritation testing: the current status. ECVAM Skin Irritation Task Force Report 1. ATLA 26:195–211 2. Zuang V et al. (2002) Follow-up to the ECVAM prevalidation study on in vitro tests for acute skin irritation. ECVAM Skin Irritation Task Force Report 2. ATLA 30:109–129 3. Spielmann H et al. (2003) Report of the Second SkinEthic Workshop: In Vitro Reconstructed Human Tissue Models in Applied Pharmacology and Toxicology Testing, Nice, France 4. Coquette A, Berna N, Vandenbosch A, Rosdy M, De Wever B, Poumay Y (2003) Analysis of interleukin-1 alpha (IL-1 alpha) and interleukin-8 (IL-8) expression and release in in vitro reconstructed human epidermis for the prediction of in vivo skin irritation and/or sensitization. Toxicol In Vitro 17:311–321 5. Heisler E, Ahr HJ, Vohr HW (2001) Local immune reactions in vitro: Skin models for the discrimination between irritation and sensitization. Exp Clin Immunobiol 204:1–2 Three Rs Reduction (fewer animals), refinement (less severe T Thrombin Thrombin is a multifunctional serine protease that has procoagulant activities when diffusable in the blood stream. But it loses this ability and initiates a potent anticoagulant pathway when bound to its endothelial cell receptor thrombomodulin, thereby mediating generation of the anticoagulant enzyme-activated protein C. The cellular activities of thrombin on platelets, endothelial or smooth muscle cells are mediated through G protein-coupled protease-activated receptors (PAR) that are initially cleaved by thrombin before a newly generated peptide motif of the receptor can serve as an internal tethered ligand for initiation of cell signaling. Blood Coagulation Thymus: A Mediator of T Cell Development and Potential Target of Toxicological Agents Thymocyte Education Thymus: A Mediator of T Cell Development and Potential Target of Toxicological Agents Thymocyte Selection 3 3 Thrombin Thymocyte Development 3 procedures), and replacement (in-vitro alternatives) of animal experiments, first proposed by Russel and Burch in 1959. Canine Immune System 3 638 Thymus: A Mediator of T Cell Development and Potential Target of Toxicological Agents Thymus 3 C Frieke Kuper Thrombocytopenia Thrombocytopenia is a condition in which the normal concentration of platelets (thrombocytes) in the blood is decreased. A significant shortage of platelets can result in bruising and easy bleeding. Leukemia Antiglobulin (Coombs) Test Toxicology and Applied Pharmacology TNO Food and Nutrition Research Zeist The Netherlands Synonyms Thymus, thymus gland, sweetbread (when used as food) 3 3 Definition Thrombocytopenic Purpura A rare autoimmune disorder characterized by a shortage of platelets, leading to bruising and spontaneous bleeding. Approximately half of the cases are idiopathic (unknown cause). Other cases are caused by drugs, infections or autoimmune disorders such as lupus erythematosus. Interferon-γ 3 Thymic Hypoplasia The thymus is a primary lymphoid organ in vertebrates; in mammals it is located in the cranioventral mediastinum and lower part of the neck. The prime functions of the thymus in mammals are the development of immunocompetent T lymphocytes from bonemarrow-derived stem cells, the proliferation of mature naive T cells to supply the circulating lymphocyte pool and peripheral tissues and the development of immunological self-tolerance. The thymus elaborates a number of soluble factors (thymic hormones) which regulate several immune processes, including intrathymic and post-thymic T-cell maturation, and neuroendocrine processes such as the synthesis of neuroendocrine hormones by the central nervous system. Characteristics An immunodeficiency that selectively affects the T lymphocyte limb of the immune response. There is lymphopenia with diminished T cell numbers. Trace Metals and the Immune System Anatomy and Histology The thymus is located in the cranioventral mediastinum and lower part of the neck, whereas small islands of thymic tissue may be present near the thyroid and 3 Thymus parathyroid glands. In young animals it is roughly pyramid-shaped with its base located ventrally. The gland consists of two lobes, fused in the midline by connective tissue. The two thymic lobes are enclosed by a fibrous capsule from which septa traverse into the organ, dividing it into lobules. The lobules have basically the same architecture, with a subcapsular area, a cortex, corticomedullary junction and a medulla. The cortex is easily recognizable in hematoxylin and eosin(H&E)-stained sections by its high density of thymocytes (immature lymphocytes) and therefore darker appearance when compared with the less densely populated medulla. The framework of the thymus is formed of epithelial reticular cells in which the bone-marrow-derived lymphoid (thymocytes/lymphocytes) and non-lymphoid cells (macrophages, dendritic cells) are packed. The vast majority of lymphocytes are T cells, but accumulations of B cells do occur. Epithelial aggregates with centrally located cell debris, the so-called Hassall’s bodies, are a characteristic feature in the medulla. The different thymic compartments are associated with different T cell maturation processes, namely early (cortical) maturation and late (medullary) maturation, which in turn are associated with differences in the marker expression and cytology of epithelial cells, lymphocytes, macrophages and interdigitating cells (Figure 1). Moreover, the capacity of epithelial cells to synthesize thymic hormones differs, the major site of hormone synthesis being the medullary epithelium (1). A characteristic and unexplained microenvironment is formed by the cortical and medullary areas which are devoid of epithelial cells but full of thymocytes, the so-called epithelial-free areas or EFAs (2). The function of these EFAs is unknown, although medullary EFAs may be associated with autoimmune diabetes. Foci of myelopoiesis are found in the connective tissue septa, within the lymphoid tissue at the outer rim of the lobules, and at the corticomedullary zone. Hemoglobin-containing cells can be found among the myelocytic series in the interlobular septa, at the outer rim of the lobules. In the medulla no erythroid precursors have been observed. Blood vessels enter the lobules via the interlobular trabeculae/septa and branch at the corticomedullary area to supply the cortex and medulla. Postcapillary venules in the corticomedullary region have a specialized cuboidal epithelium similar to that of the high-endothelial venules of the lymph node, which allows passage of lymphocytes into and out of the thymus. Sheaths of connective tissue and an epithelial cell layer with its basement membrane are found around the blood vessels. The space between the epithelial basement membrane and the vessel lining is often quite broad around the corticomedullary vessels and is called the perivascular space. 639 This space may contain all kind of blood cells and most often contain fine lymphatics. Nerves course along the blood vasculature. During ontogeny, hematopoietic progenitor cells migrate into the thymic epithelial primordium between days 11–13 of fetal life in mice. Small lymphocytes can be found in the thymic primordium at about day 14 (mouse) or day 15 (rat) of fetal life. The thymus is fully developed, meaning a cortex and medulla can be distinguished, at day 17 of fetal life in the mouse and by days 19–21 in rats, and the organ grows considerably immediately after birth. This growth is caused by the immense postnatal antigen stimulation; at that time large numbers of mature T cells are demanded. The thymus starts to involute after adulthood is reached. With age, the two thymic lobes diverge caudally and in old animals are almost completely separated; the thymus is then restricted to the area cranially to the aortic arch. The number of lymphocytes decrease, especially in the outer cortex. Although areas with different lymphocyte density, suggesting the presence of cortex and medulla, are often present in advanced age, the general arrangement of the cortex enclosing the medulla is not strictly maintained. This gives the thymus an irregular appearance. The expanding perivascular connective tissue meshwork and increasing perivascular lymphocyte accumulations may further disturb the normal pattern. The septa and capsule harbor increasing numbers of adipose cells, which eventually invade the thymic parenchyma. In addition to the expansion of the connective tissue component, epithelial cords and tubules are large and numerous in the old thymus and the epithelial Hassall’s bodies become relatively more prominent though in absolute numbers they decrease. Adrenergic innervation of the gland is maintained in old animals. Thymic involution may be related to changes in the hormonal status of the individual; circulating thymic hormone is reduced to very low levels in adults. The consequences of age-related involution are obvious: the emigration of lymphocytes from the thymus shows a dramatic decrease. Apparently, the persistent generation of new antigen-recognition repertoire in the T cell population of adults is not needed. Instead, the body can defend itself using the established repertoire and extra-thymic self renewal of the T cells. Pregnancy in rodents results in radical, but reversible changes. After an initial rise in thymic weight in early pregnancy, involution starts with lymphocyte cell death in the cortex. In wild populations, cyclical enlargement and regression is documented. For instance, most birds showed an involuted thymus at the time of mating and laying, whereas on subsequent egg incubation the thymus size is increased. T 3 3 3 640 Thymus Thymus. Figure 1 Schematic presentation of a thymus lobule with cortex, corticomedullary region, medulla and an epithelial-free area (EFA). In the lobule, a simple overview of thymocyte maturation is presented: round cells representing T lymphocytes (T) with their membrane markers CD4 and/or CD8 T-Cell Maturation T cells reside in the thymus during their maturation from progenitor cells to immunocompetent T cells. The process of T-cell maturation includes a number of steps which are associated with location in different microenvironments (3). (See Figure 1). The immature cells, which enter the lobules by the blood vasculature at the corticomedullary junction, first move to the outer subcapsular cortex, where they appear as large lymphoblasts. They then pass through the cortex where the cells become small lymphocytes with scanty cytoplasm. Finally, the cells move to the medulla, where they appear as mediumsized lymphocytes. These translocational stages in development can be monitored by the immunologic phenotype: cells change from CD4−CD8− (double negative) at a very immature stage into a CD4+CD8+ (double positive) phenotype, which is characteristic for almost all lymphocytes in the cortex. In the medulla, T cells have the phenotype of relatively mature cells, with distinct CD4+CD8− (about 70%) and CD4+CD8+ (about 30%) populations. This phenotypic change is accompanied by a crucial aspect of intrathymic T-cell maturation: the genesis of the T cell receptor (TCR) consisting of the alpha-beta heterodimer (4). The DNA genomic organization encoding these chains is in germ-line configuration, with a variety of gene segments encoding the variable part of the receptor molecule. Before transcription and translation into TCR becomes possible, combinations have to be made of gene segments encoding the variable and constant parts of the TCR. This process of gene rearrangement requires the thymus microenvironment. The cell can synthesize the receptor after completion of this gene rearrangement. The receptor is then expressed on the cell membrane with the CD3 molecule, which acts as the transmembrane signal-transducing molecule after TCR stimulation. Even when the TCR has not yet been synthesized, this CD3 molecule is already present in the cytoplasm of the cell. T cells at this stage of maturation Thymus can be recognized by cytoplasmic staining with CD3 reagents. TCR gene rearrangement is similar to the rearrangement of genes encoding immunoglobulin heavy and light chains that takes place in the bone marrow microenvironment. However, after surface expression of the TCR, the cell undergoes a process unique to T cells, namely, specific selection on the basis of recognition specificity. First, the cell is examined for its affinity for its own major histocompatibility complex (MHC; self restriction). T cells with an intermediate affinity for self MHC peptides are allowed to expand (positive selection). Secondly, T cells with a high affinity for self MHC are deleted (negative selection). In this way, the random pool of antigen-recognition specificities of T cells is adapted to the host's situation. The T cell repertoire in germ-line configuration cannot be fully expressed but is influenced by the individual's own MHC haplotype. It is generally accepted that the epithelial microenvironment of the thymic cortex plays a major role in positive selection. This microenvironment expresses MHC class I and class II products and morphologically (at electron microscopic level) shows close interactions with lymphocytes. This close interaction is reflected by the complete inclusion of lymphocytes inside the epithelial cytoplasm (thymic nurse cell). Negative selection has been ascribed to either the epithelial compartment or the medullary dendritic cells. The cortex can be considered a primary or central lymphoid organ because of its antigen-free microenvironment. In contrast, antigens can move relatively freely into the medulla and encounter antigen-presenting dendritic cells as well as antigen-reactive T cells. Thus the medulla has properties of a secondary lymphoid organ. 3 Preclinical Relevance The dynamics of the thymus with ongoing reactions of cell proliferation and differentiation, and gene amplification, transcription and translation makes it highly susceptible to toxic insults. Compounds that interfere with these processes are often immunotoxic. Therefore, a decrease of thymus weight in preclinical studies is often a first indicator of toxic action of a xenobiotic agent on the immune system, although some compounds, like cyclosporine, profoundly alter thymic histophysiology, without apparent effect on thymus weight. The dynamic nature of the immune system provides it with great regenerative capacity: the original architecture of the thymus is restored rapidly following involution induced, for example, by irradiation, or treatment with glucocorticosteroids or organotin compounds. Thymus in aged or immunocompromised animals may hardly be visible. For histology adipose and connec- 641 tive tissues from the cranioventral mediastinum, which contains thymic tissue, should then be collected. The thymus is also very susceptible to acute (glucocorticoid-related) stress (5). It is conceivable that with age the thymus becomes less sensitive to toxic insults and that toxic effects on the thymus with age have less functional importance, because of age-related thymic involution. However, the components that constitute the various thymic compartments are still present in healthy old animals, as was shown by reconstitution studies. Therefore, a decreased sensitivity to toxic compounds may not be a general property of the involuted thymus in aged animals. Relevance to Humans The use of data obtained in laboratory animal species for man presents difficulties when species differ in organ anatomy and histophysiology and sensitivity. The thymus is present in all vertebrates, possibly with few exceptions, and there are only a few structural differences between the species (6). Anatomical differences relate to thymus location and number of thymic lobes, the prominence of epithelial aggregates with centrally located cell debris, the so-called Hassall’s bodies, and the presence of B cell follicles. During the third month of gestation the thymic primordium becomes colonized by marrow-derived stem cells. When these stem cells are indeed thymocyte precursor cells, their migration into the thymic primordium at that time is considerably earlier—relative to gestation time—in humans than in mice or rats. Differences in immunotoxicity between laboratory animals and man appear to depend predominantly on differences in toxicokinetics and metabolism of substances. Moreover, the interindividual differences and the age-related intraindividual variations are probably more marked than interspecies differences. It should be emphasized that the “normal” architecture of the thymus, as known from textbooks, can be expected only between the late gestational period and young adulthood, and before pregnancy. The universality of the immune system observed in mammals and the data obtained so far indicate that data from laboratory animals can be extrapolated quite well to humans. Regulatory Environment Regulatory toxicity testing, which uses immune parameters, is still under development. This applies to pharmaceuticals and industrial substances as well. Nevertheless, most guidelines recognize the importance of the thymus. For instance, the European Union guidelines on repeated-dose toxicity testing with pharmaceuticals require the macroscopic and microscopic examination of the spleen, thymus, and some lymph nodes with respect to the immune system. T Thymus: A Mediator of T Cell Development and Potential Target of Toxicological A Moreover, a multilaboratory, 28-day oral toxicity study (OECD guideline 407) with the model immunotoxicants azathioprine and cyclosporine demonstrated that the most consistent effects were observed in the thymus (7). References 1. Dabrowski MP, Dabrowski-Bernstein BK (1990) Immunoregulatory Role of the Thymus. CRC Press, Boca Raton 2. Bruijntjes JP, Kuper CF, Robinson J, Schuurman H-J (1993) Epithelium-free area in the thymic cortex of rats. Dev Immunol 3:113–122 3. Van Ewijk W (1991) T-cell differentiation is influenced by thymic microenvironments. Ann Rev Immunol 9:591– 615 4. Werlen G, Hausmann B, Naeher D, Palmer E (2003) Signaling life and death in the thymus: Timing is everything. Science 299:1859–1863 5. Godfrey DI, Purton JF, Boyd RL, Cole TJ (2000) Stressfree T-cell development: glucocorticoids are not obligatory. Immunol Today 21:606–611 6. Zapata AG, Cooper EL (1990) The immune system: comparative histophysiology. In: The Thymus. John Wiley, Chichester, pp 104–150 7. International Collaborative Immunotoxicity Study (ICICIS) Group Investigators (1998) Report of validation study of assessment of direct immunotoxicity in the rat. Toxicology 125:183–210 Thymus: A Mediator of T Cell Development and Potential Target of Toxicological Agents Michael Laiosa NIAID/NIH Bethesda, MO 20897 USA Allen Silverstone Upstate Medical University 166 Irving Ave. Syracuse, NY 13210 USA Synonyms T-cell development, thymocyte development, T-cell selection, thymocyte selection, thymocyte education, positive selection, negative selection, thymus, thymus atrophy, thymus involution Definition T-cell development is the process by which hematopoietic progenitor cells from the bone marrow home to the thymus and undergo a complex process of differentiation, proliferation and selection to become mature T-cells that will emigrate from the thymus to peripheral lymphoid organs such as the spleen and lymph nodes. Additional maturation and differentiation into T-helper (Th) type 1 and Th type 2 subsets occur in the periphery and are discussed elsewhere. Characteristics The thymus is the central organ for T-cell development in the body, and the principle function of the thymus is to regulate T-cell recognition of self antigens presented by the body to insure that useless or selfreactive T-cells do not mature. T-cell development is characterized by progenitor cells that originate in the fetal liver or bone marrow and enter the thymus through the blood stream (1). The thymocytes then undergo a highly regulated process of differentiation, proliferation, selection, and maturation to become Tcells. The stages of murine thymocyte differentiation can be distinguished by differentially expressed surface molecules stained with fluorochrome-labeled antibodies and detected using flow cytometry. The thymocyte subpopulation that appears earliest is identified by expression of the lymphoid homing receptor CD44 and cKit, the receptor for the stem cell factor, (CD44+CD25−, DN1) (1). Subsequently, the high affinity interleukin receptor IL-2α (CD25) and the heat stable antigen (HSA, CD24) are upregulated and the proliferation rate of this population also increases (DN2) (1). Following expression of CD25, CD44 is down modulated leading to the next stage of differentiation, CD44−CD25hi (DN3) (1). In the DN3 population, the αβ and γδ T-cell antigen receptor (TCR) lineages begin to diverge as recombination activating gene products 1 and 2 (RAG1, RAG2) begin somatic gene rearrangement of the TCR β locus (1). Successful rearrangement and surface expression of a functional TCR β chain in a complex with the pre-Tα protein results in a burst of proliferation and the gradual reduction of CD25 expression on the cell surface (DN4) (1). Subsequent to successful expression of TCR β, rearrangement of TCR α begins and the CD8 and CD4 molecules are expressed on the cell surface(1). It has been calculated that it takes 3– 4 days for a DN3 cell to differentiate into the DP stage of T-cell development (1). Once TCRα rearrangement is complete, the CD4+CD8+ double positive (DP) thymocytes begin a rigorous selection process by engaging their αβ TCR with complexes of self peptides bound to major histocompatibility complex (MHC) class I and II proteins (1), expressed by epithelial, myeloid, and dendritic antigen presenting cells (APCs) in the cortex of the thymus (2). The TCR-MHC interaction leads to one of three possible outcomes depending on the nature of 3 642 Thymus: A Mediator of T Cell Development and Potential Target of Toxicological A which can interrupt or inhibit various stages of T-cell development, which ultimately leads to atrophy of the thymus. Agents that have been shown to cause thymic atrophy in vivo include corticosteroids, estrogens and estrogen-like compounds, polychlorinated biphenyls (PCBs), and polychlorinated dibenzodioxins and dibenzofurans (PCDD and PCDF). Representative agents that are known to induce thymic atrophy and possible mechanisms by which they can induce atrophy are listed in Table 1 (4,5). Evidence of thymic atrophy after toxicant exposure has a relatively strong correlation to predicting if an agent will be immunotoxic as defined by classic immunotoxicity assays such as delayed-type hypersensitivity (DTH), and the sheep red blood cell (SRBC) challenge assay (6). However, linking immunotoxicant-induced defects in thymic development to deficiencies in a functional response has been a major obstacle in the field of immunotoxicology. Relating thymic atrophy to alterations of functional responses have suffered from a lack of data and agreement on the type of assays, kinetics, and dosing protocols to be used. Relevance to Humans The thymus has been shown to be essential for development of T-dependent immune responses. Indeed, DiGeorge syndrome who patients with the rare lack a thymus present with a severe immunodeficiency associated with a complete lack of T-cells. The DiGeorge T-deficiency can be completely restored by the transplantation of an allogeneic thymus graft (7). The essential role for the thymus in T-cell development has been further appreciated in recent clinical studies. These studies show that despite the longstanding observation of thymus atrophy with increasing age, the adult thymus is fully capable of producing and selecting new T-cells following periods of systemic Tcell depletion. Following chemotherapy, production of new thymic-derived naive T-cells has been observed (7). Additionally, infection with HIV has been shown to cause a dramatic thymic pathology characterized by thymic atrophy and a block in T-cell development at the CD3−CD4−CD8− stage of development. However, thymopoieis can be restored in some HIV patients undergoing highly active antiretroviral therapy ( HAART) (7). Finally, evidence of TCR gene rearrangement in recent thymus emigrants has been observed in normal adults of at least 60 years of age (7). These data strongly support an active and dynamic role for the thymus organ in mediating new T-cell development throughout an individual’s life. The effect of immunotoxicants as mediators of thymic atrophy in humans has been controversial and difficult to assess for some time. The lack of consensus on whether a particular toxicant can cause thymic at3 3 3 3 the interaction. TCRs with no or weak affinity for MHC will die by neglect. In comparison, potentially self-reactive TCRs with too high or strong affinity for the peptide MHC complex undergo negative selection. Only TCRs with the appropriate affinity for peptide MHC complexes will undergo maturation, CD4 (class II MHC) or CD8 (class I MHC) lineage commitment and positive selection (1). The signal transduction that results in positive selection begins with phosphorylation of the intracellular portion of the TCRζ chain by the src kinase Lck. Phosphorylation of TCRζ results in the subsequent recruitment of Zap70, which becomes activated and phosphorylates the linker of activated T-cells (LAT). The phosphorylated LAT acts as a docking complex, which recruits and activates a number of molecules involved in TCR signal transduction and calcium ion (Ca2+) flux (3). The generation of a Ca2+ flux has been shown to depend on phospholipase C γ (PLCγ), which generates inositol-3-phosphate (IP3) and diacylglycerol (DAG) (1). IP3 is responsible for the increase in intracellular Ca2+ and leads to the activation of the calcineurin pathway and the NFAT family of transcription factors (1). In contrast DAG is involved in activating protein kinase C (PKC) family members and can be a mediator in activation of the Ras pathway. In DP thymocytes it is thought that DAG activates the guanine nucleotide exchange factor RasGRP1 leading to activation of the extracellular signal-related kinase (ERK) (1). ERK activation in thymocytes undergoing positive selection is thought to be involved in activating the early growth response-1 (EgR-1) nuclear transcription factor (1). The positively selected DP thymocytes then upregulate Bcl-2 and mature to become either class II restricted (CD4+; T helper) or class I restricted (CD8+; T cytotoxic) single positive thymocytes. Additional selection occurs in the medulla of the thymus before final maturation and emigration of the SP T-cells into the periphery (1). Although negative selection results in a profoundly different outcome (cell death rather than maturation) many of the signaling pathways utilized are the same or similar. Most current data on thymocyte selection favor a model where the affinity between a TCR and self peptide-MHC complexes determines whether a thymocyte will be positively selected or deleted. High affinity interactions with TCR and self peptideMHC may activate additional signaling pathways such as the Jnk pathway, which ultimately lead to apoptosis (1). In comparison, TCRs with weak or no affinity for self peptide-MHC complexes will die by neglect in the thymus within 1–3 days (1). Only thymocytes possessing the appropriate affinity and duration of binding between a TCR and self peptide-MHC complexes can be positively selected (1). A number of toxicological agents have been identified 643 T 3 3 644 Thymus: A Mediator of T Cell Development and Potential Target of Toxicological A Thymus: A Mediator of T Cell Development and Potential Target of Toxicological Agents. Table 1 Agents known to cause thymic atrophy and mechanism of atrophy induction1 Agent Mechanism Androgens Loss of DP thymocytes; mediated by androgen receptor Cisplatin Apoptosis in proliferating thymocytes Cyclosporin A Prevents Ca++ mobilization; inhibits positive selection; delayed negative selection Dexamethasone (and other corticosteroids) Apoptosis in DP thymocytes Dibutyl and tributyltin Possible apoptosis; inhibition of proliferation of DN thymocytes Diethylstilbestrol (DES), estradiol, estrogens and estrogen-like chemicals No evidence of apoptosis, possible effects on progenitors and cell cycle; estrogen receptor-mediated Ethylene glycol monomethyl ether Reduction in DP thymocytes, but no evidence of apoptosis Reduction of lymphocyte progenitor capacity Ethanol Apoptosis; increase in CD4+ mature cells, loss of CD25+ DN cells; evidence of Ca++ increase and protein kinase C activation Malnutrition, vitamin deficiency Increase of glucocorticoid levels; apoptosis of DP thymocytes 2,3,7,8, tetrachlorodibenzo-p-dioxin No evidence of apoptosis in vivo; inhibition of bone marrow progenitors; inhibition of cell proliferation in thymic DN cells; all effects mediated by the aryl hydrocarbon receptor T-2 toxin and other mycotoxins Elimination of putative lymphocyte progenitor cells in fetal liver; no evidence of apoptosis induction. 1 Adapted from Luster et al. (4) and Silverstone (5) rophy is due in part to the obvious ethical considerations with human studies. Moreover, the vast majority of immunotoxicity assays that have been developed are in rodent models that possess inherent flaws when attempting to determine dose, pharmacokinetic, and risk assessment comparison models to humans. The challenges of relating risk assessment models to humans should be overcome in the future as immunotoxicologists begin to develop nonhuman primate models, novel in vitro models and comparative toxicogenomic studies to fill in the gaps in knowledge about particular toxicants as related to T-cell development and immunotoxicity (6,8,9). 3 Regulatory Environment Regulatory agencies in the USA have recently started to stress the importance of understanding how immunotoxicants affect the developing immune system in children. The need to understand the effects of immunotoxicants in children is particularly important because of the possibility that during the period when the immune system is most actively developing, it may be especially sensitive to the effects of an immunotoxicant. Moreover, immunotoxicant exposure in children may lead to more severe effects and/or a higher risk for long-term deleterious outcomes when compared to doses determined for adults (9). Although there are currently limited data comparing adult and child re- sponses to immunotoxicants on the developing immune system, several possibilities for differences exist. An immunotoxicant may affect the developing immune system of a child but not an adult. Furthermore, an immunotoxicant may affect the developing immune system of a child at a lower dose than in an adult (9). In an attempt to get the full picture about childhood exposure to immunotoxicants and the effect of exposure on the developing immune system of children, several EPA sponsored workshops have listed the need for expanding exposure studies in very young animals as a high priority. These workshops include the EPA sponsored workshop on endocrine disruptors held in 1995, and the EPA sponsored workshop by the Risk Science Institute of the International Life Sciences Institute held in 1996. More recently, the EPA added a recommendation to the two-generation reproductive study (OPPTS 870-3800), stating: for F1 and F2 weanlings that are examined macroscopically, the following organs should be weighed for one randomly selected pup per sex per litter: brain, spleen and thymus (9). The recommendation to use thymus and spleen weights was made because numerous studies have concluded that thymic and splenic weight may be immunotoxicant predictors (6). In 2001 the EPA created a developmental immunotox- Tight Junctions Thymus-dependent antigens (TD) are protein antigens which only can induced an antibody response with the help of thymus-derived T helper cells. This T cell help is also essential for the class switch observed during TD immune responses. Idiotype Network Thymus Gland The thymus is a primary lymphoid organ, the site of Tcell development. It is situated in the anterior superior mediastenum, behind the breastbone. The organ, in particular its epithelial cells and connective tissue provide the microenvironment wherein thymocytes proliferate, rearrange their T-cell receptor genes, and undergo positive and negative selection. The thymus slowly atrophies after puberty, but can become fully functional again in clinical situations like radiation therapy and stem cell transplantation. Thymus Dioxins and the Immune System Thymus: A Mediator of T Cell Development and Potential Target of Toxicological Agents Systemic Autoimmunity 3 3 3 Thymus Involution T 3 1. Starr TK, Jameson SC, Hogquist KA (2003) Positive and negative selection of T cells. Ann Rev Immunol 21:139– 176 2. Anderson G, Jenkinson EJ (2001) Lymphostromal interactions in thymic development and function. Nat Rev Immunol 1:31–40 3. Germain RN, Stefanova I (1999) The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Ann Rev Immunol 17:467–522 4. Luster MI, Dean JH, Germolec DR (2003) Consensus workshop on methods to evaluate developmental immunotoxicity. Environ Health Persp 111:579–583 5. Silverstone AE (1997) T cell development. In: Sipes G, McQueen CA, Gandolfi AJ (eds) Comprehensive Toxicology, 1st edn. Elsevier Science, New York, pp 39 ff 6. Holladay SD, Blaylock BL (2002) The mouse as a model for developmental immunotoxicology. Hum Exp Toxicol 21:525–531 7. Spits H (2002) Development of alpha-beta T cells in the human thymus. Nat Rev Immunol 2:760–772 8. Buse E, Habermann G, Osterburg I, Korte R, Weinbauer GF (2003) Reproductive/developmental toxicity and immunotoxicity assessment in the nonhuman primate model. Toxicology 185:221–227 9. Holsapple MP (2003) Developmental immunotoxicity testing: a review. Toxicology 185:193–203 Thymus-Dependent Antigen 3 References Loss of thymocyte weight and cellularity after exposure to an immunotoxicant. Thymus: A Mediator of T-Cell Development and Potential Target of Toxicological Agents 3 Lastly, in 2003, the National Institute of Environmental Health Sciences (NIEHS) and National Institute for Occupational Safety and Health (NIOSH) cosponsored a consensus workshop on methods to evaluate developmental immunotoxicity. This workshop made several recommendations for immunotoxicant screening assays as well as assays that needed further validation and assays for research development (4). The recommended screening assays for developmental immunotoxicants were the primary antibody response (T-dependent), delayed-type hypersensitivity response, complete blood count (CBC), and weights of thymus, spleen and lymph nodes. Assays that require additional validation include phenotypic analyses, macrophage function and natural killer cell activity. Finally, stem cell functional assays were listed as assays that require additional research and development (4). Thymus Atrophy 3 icology working group. The mission of the this group is to determine: * the state of science to support the creation of a guideline for developmental immunotoxicology * what should be included in such a guideline * how this guideline would be validated * when a developmental immunotoxicology guideline would be used (9). 645 Thymus: A Mediator of T-Cell Development and Potential Target of Toxicological Agents Tight Junctions An intercellular junctional structure, typically found in epithelia and endothelia. In the tight junction the two membranes of neighboring cells are brought into close proximity through binding of specific transmembrane proteins. This results in a selectivity barrier that seals the apical lumen from the basolateral intercellular 646 Time-Resolve Fluorometry space and also establishes cellular polarity by preventing membrane-linked molecules from freely diffusing between the apical and the basolateral cell surface. Cell Adhesion Molecules 3 Time-Resolve Fluorometry An instrumental design to collect emission at a certain time interval after the pulsed excitation and to improve the detection sensitivity by means of a temporal rejection of background. Cytotoxicity Assays 3 Tissue Factor This cellular receptor for factor VII/VIIa is constitutively expressed on cells of the media and adventitia of the vessel wall. When it is exposed to plasma clotting factors at sites of vascular injury it serves as a potent (extrinsic) cofactor for the activation of factor X. Tissue factor is also associated with platelets and microparticles and is responsible for intravascular activation of blood clotting in the absence of tissue damage. Blood Coagulation 3 Tm Mouse Knockout, Genetic 3 TNF-α Tumor Necrosis Factor-α 3 Tolerance Anke Kretz-Rommel Principal Scientist Alexion Antibody Technologies Suite A, 3958 Sorronto Valley Rd San Diego, CA 92121 USA Synonyms Immunological unresponsiveness Definition The primary function of the immune system is to protect the host from foreign materials while at the same time ensuring that no attack against self proteins occurs. Immunological tolerance is the absence of immunological responsiveness to specific antigens, encompassing unresponsiveness to self antigens, but also tolerance to therapeutics such as antibodies, recombinant proteins and conventional drugs. Breakdown of immune tolerance is defined by the appearance of Tcells or antibodies to self antigen or the therapeutic entity. The result may be autoimmune disease or allergic or anaphylactic reactions. Furthermore, an immune response to a drug may reduce its efficacy. Immune tolerance is an active process at both the B cell and T-cell level, involving processes taking place in central lymphoid organs (thymus and bone marrow) and peripheral lymphoid organs (blood, spleen, lymph node, mucosal immune system). The underlying mechanisms are subject to a continuous debate involving clonal deletion, anergy, regulatory T cells and regulatory dendritic cells. In this chapter these concepts will be outlined with reference to drugs affecting various tolerance mechanisms, and the interested reader is referred to more in depths reviews. Characteristics of T Cell Tolerance Central Mechanisms T cells develop in the thymus. Recombination of gene segments creates the two chains that make up the T cell receptor (TCR) resulting in a large repertoire of receptor specificities. To ensure the export to the periphery of T cells that recognize peptides in the context of self major histocompatiblity complex (MHC), but do not strongly react to self antigens, the cells have to undergo positive and negative selection processes as outlined in Figure 1. Selection is a rigorous process that results in the death of approximately 95% of T cells. T cells first have to undergo positive selection on self peptide presented in the context of self MHC. Successful signaling through the TCR has been suggested to raise the threshold of activation of these T cells possibly through the production of negative regulators (1). If the T cells still can be activated in a subsequent encounter of self peptide presented by MHC the T cell will undergo clonal deletion by apoptosis, a process termed negative selection. This leaves only T cells to be exported to the periphery with a threshold of activation that can not normally achieved by self peptides. Interference with negative selection in the thymus has been proposed as a mechanism for the induction of autoimmunity. TCDD and cyclosporine have been evoked to affect both positive and negative selection processes. The reactive metabolite of the antiarrhythmic procainamide hydroxylamine (PAHA) has been shown to interfere Tolerance 647 Tolerance. Figure 1 Central tolerance mechanisms. After migration from the bone marrow to the thymus, T cells first undergo selection on self peptides presented by thymic epithelial cells. Cells productively interacting with the presented peptide proceed to negative selection resulting in deletion of cells with high affinity for self peptide. TEC=thymic epithelial cell; APC=antigen presenting cell. with positive selection in the thymus, resulting in the export to the periphery of autoreactive T cells and autoantibody production similar to that observed in patients with drug-induced lupus. Peripheral Mechanisms T cells leaving the thymus still might respond to self antigens if the antigens are present in such high concentration that they can bind to “weak” receptors or if they did not encounter the self peptide in the thymus which might be the case for certain tissue-specific antigens. A number of peripheral mechanisms can control these potentially self-reactive cells (2) as summarized in Figure 2. Lack of Costimulation Activation of T cells not only requires interaction of the TCR with peptide presented by MHC on antigenpresenting cells (APC), but also a second signal (costimulation). Among the most important of these costi- mulatory molecules are members of the B7 family, interacting with CD28 on the T cell. Ligation of CD28 by either B7-1 or B7-2 lowers the threshold of TCR signaling needed to induce T-cell activation and increases the effect of that signal by promoting T cell expansion and proliferation. Recently, additional members of the B7-CD28 family involved in the development or maintenance of immune tolerance have been identified such as ICOS which is expressed by activated T cells. Ligation of ICOSL by ICOS prolongs T cell activation. If a T cell receives a signal through the TCR in the absence of costimulation, cells are unresponsive to subsequent stimulation by the peptide in context of MHC in the presence of costimulation—a process termed anergy. While this phenomenon has only been demonstrated in vitro, it recently has been recognized that naive T cells (T cells that have not been stimulated before) in the periphery require frequent interaction with peptide presented by MHC in order to survive. This has been suggested to T 648 Tolerance Tolerance. Figure 2 Peripheral tolerance mechanisms. A: Only T cells with high affinity for the antigen presented by antigen presenting cells (APC) will proliferate. Since thymic emigrants have been tuned to have a threshold of activation generally above that achieved by most self peptides, T cell interaction with self peptide presented by major histocompatability complex (MHC) does not result in proliferation. B: If a T cell sees antigen in the context of MHC in the absence of costimulatory signals, anergy can be induced. The T cell is subsequently unresponsive to challenge with the cognate antigen by APCs, even in the presence of costimulatory molecules. C: Death molecules such as fatty acid synthetase (FAS) and tumor necrosis factor (TNF) get upregulated in the course of a T cell response to limit proliferation and cytokine production. T cells involved in the response to antigen will undergo activation-induced cell death (AICD) by apoptosis. D: A number of immunoreceptors downregulate the T cell response. Some of them are upregulated during the T cell response to limit it, and some of them are constitutively expressed on tissues to prevent damage by T cells. E: Tolerogenic dendritic cells can induce Treg which control the response of other T cells. be an important mechanism of peripheral tolerance, maintaining a high activation threshold of T cells which can only be overcome by foreign antigen. Drugs could potentially provide a “danger” signal to the immune system resulting in upregulation of costimulatory molecules and activation of self-reactive T cells. However, clearly not all drugs inducing cell stress or cell death result in an activation of the im- mune system. Evidence is emerging though that some compounds can alter dendritic cells resulting in upregulation of the costimulatory molecule CD86 or provoking migration of dendritic cells by upregulating the CCR7 receptor. Failure to Encounter Self Antigens (Immune privilege) Under normal conditions, the cells in nonlymphoid Tolerance organs throughout the body are not in contact with T cells and are thus sequestered from the immune system. This lowers the probability of a low affinity self-reactive T cell encountering a specific self antigen. Only in the presence of “danger” signals such as provided by bacteria can T cells enter non-lymphoid organs. Certain tissues are particularly protected from the entry of T cells, such as the interior of the eye, brain and testes. Constitutive expression of immunosuppressive receptors and cytokines ensures protection of these organs from immune-mediated damage. Receipt of Death Signals An important mechanism of maintaining immune homeostasis is the downregulation of the immune response after activation. Activation of antigen-presenting cells by bacteria or viruses results in upregulation of costimulatory molecules and production of proinflammatory cytokines such as tumor necrosis factor (TNF)-α. Persistence of an inflammatory environment increases the risk of activating T cells by self peptides by providing costimulation to these cells. Also, there is a risk of cross-reactivity of T cells activated by pathogens with self antigen, because activated T cells require less costimulation. Therefore, most activated T cells ultimately undergo a process of programmed death or apoptosis. Apoptosis of activated cells (activation-induced cell death, AICD) occurs by cytokine withdrawal and by induction through fatty acid synthetase (FAS) and TNF-α. FAS acts on FAS ligand expressed on activated T cells. These cells therefore can kill themselves as well as activated B cells and macrophages. Also, expression of other receptors mediating immune suppression play an important role in downregulating the immune response as discussed in the following section. Immunosuppressive Receptors The immune response can be terminated by upregulation of the T cell surface molecule CTLA-4. While CTLA-4 is present at very low levels on resting T cells, it is markedly upregulated after T cell activation. Similar to the positive costimulatory molecule CD28, CTLA-4 binds to B7.1 and B7.2. Due to its substantially higher affinity for these molecules, CTLA-4 outcompetes CD28, thereby transducing inhibitory signals to the activated T cell. More inhibitory molecules have recently been identified. PD-1 is expressed on activated T cells, B cells and myeloid cells, and engagement by its ligands PD-L1 and PDL-2 inhibits T cell proliferation and cytokine production. Expression of immunosuppressive receptors on nonlymphoid organs is another safeguard mechanism against self attack of T cells. 649 Regulatory Cells and Cytokine Milieu A minor population of T cells known as regulatory T cells (Treg) suppresses the proliferative response and production of inflammatory cytokines of other T cells. They may constitute a specialized T cell subset to reduce the activity of autoreactive T cells. Treg constitutively express CTLA-4 and secrete transforming growth factor(TGF)-β and interleukin(IL)-10. Mechanisms of action are still under debate, but they seem to require direct cell-cell contact. In addition to regulatory T cells, dendritic cells and macrophages play a major role in immune tolerance. The functional activities of dendritic cells are mainly dependent on their state of activation and differentiation. Terminally differentiated mature dendritic cells can efficiently induce the development of T effector cells, whereas immature dendritic cells are involved in maintenance of peripheral tolerance. The means by which immature dendritic cells maintain peripheral tolerance are not entirely clear, however, their functions include the induction of anergic T cells, T cells with regulatory properties as well as the generation of T cells that secrete immunomodulatory cytokines. Depending on the cytokines produced by the macrophage/dendritic cell, the immune response can be steered towards a Th1 or Th2 response. Th1 cells produce IFN-γ, IL-2 and TNF-α and regulate classical delayed (type IV) hypersensitivity. Th2 cells secrete IL-4, IL-5, IL-6 and IL-10 and participate in immediate (type I) hypersensitivity reactions and B cell antibody-mediated immunity. The effect of drugs on cytokine production and the importance of the cytokine milieu resulting in drug-induced autoimmunity are being studied extensively. Characteristics: B cell Tolerance Similar to T cells, B cells are constantly being tolerized to self antigens. For a thorough discussion of B cell tolerance the reader might refer to Jacquemin et al. (3). Central Mechanisms B cells mature and undergo selection on self peptides in the bone marrow. A large population of B cells with different specificities is created by genetic recombination within the immunoglobulin locus generating a broad range of heavy- and light-chain sequences that rearrange to form a B cell receptor (BCR). If the immature B cell encounters extracellular antigen capable of crosslinking its BCR, a signal is created that will block further development of this autoreactive cell. The B cell will initiate the receptor editing process to produce BCR with new antigen specificities. If it cannot alter its BCR effectively, the immature B cell will be deleted by apoptosis. Some autoreactive T 650 Tolerance B cells escape deletion and enter the peripheral circulation in an anergic state. Peripheral Mechanisms After recognition and uptake of antigen in the periphery, these partially activated B cells migrate through the lymphoid tissue. If an activated B cell encounters a T cell that has been activated by the same antigen, antibodies against that antigen are produced. B cells cannot respond to most antigens without receiving help from T helper cells. Therefore, ensuring self tolerance of T cells is an important mechanism of keeping B cells from producing autoantibodies. However, drugs affecting B cell tolerance can ultimately result in autoimmunity when the individual has other predisposing factors, as might be the case for pristane. Additional Mechanism for Drugs to Break Immune Tolerance The most common hypothesis of how drugs result in immune stimulation is the formation of drug-protein conjugates by reactive drug metabolites with self antigens. The resulting haptens might be recognized as foreign by the immune system. Although formation of haptens has been demonstrated for a number of drugs associated with idiosyncratic immune adverse reactions (e.g. phenytoin, carbamazepine, halothane, tielinic acid, procainamide and diclofenac) these adducts are not a predictive factor for adverse immune reactions indicating that additional factors are required to induce the immune response. It has been demonstrated that binding of halothane to CF3CO proteins mimics very closely the structure of the E2 subunit proteins of the 2-oxoacid dehydrogenase complexes and protein X—autoantigens associated with halothane hepatitis. Furthermore, binding of drugs to protein can alter their cleavage and presentation after cell death. Exposure of macrophages to mercuric chloride has been show to alter fibrillarin processing, resulting in the appearance of self epitopes not normally encountered by the immune system. In addition to covalent drug binding to proteins, noncovalent interactions of drugs such as sulfamethoxazole with MHC-peptide complexes have been implicated in immunological adverse reactions. While disruption of immune tolerance by classical chemical drugs leaves many unanswered questions, immune responses after administration of bioengineered drugs is far more straightforward. The importance of antibodies in therapeutics gains increasing recognition. Often, these antibodies are of mouse origin and certain residues are recognized as foreign by the human immune system. Engineering methods known as “humanization” and pegylation decrease the risk of an immune response against the therapeutic. Preclinical Relevance Adverse drug reactions affecting immune tolerance are difficult to address in the preclinical setting. However, a number of assays have been developed to address the potential of drugs to sensitize the immune system, such as the popliteal lymph node assay that assesses the effects of drugs on macrophages, or assays looking for altered cytokine profiles. Few animal models demonstrating chemically induced autoimmunity are available, but are specific for the compound used. As far as immunogenicity of biotherapeutics is concerned, some animal models have proved to be useful. For example, transgenic mice were developed to produce and secrete human tissue plasminogen activator to which they developed immune tolerance. These mice were capable of producing antibodies to a form of human tissue plasminogen activator that had been modified by a single amino acid substitution. Furthermore, nonhuman primates have been used successfully in predicting the relative immunogenicity of different forms of human growth hormone. Also, computer modeling methods are used in predicting the immunogenicity of proteins. Relevance to Humans Adverse drug reactions account for 2%–5% of all hospital admissions, a portion of which is based on immune-mediated reactions. With more than 80 recombinant proteins in clinical use and more than 400 therapeutic antibodies in clinical trials, immune tolerance to these proteins is a major issue and predicting immunogenicity is crucial (4). Regulatory Environment Regulatory issues for drug-induced autoimmunity and allergy are covered in their respective chapters. For clinical trials of recombinant proteins, patients are screened for the development of antidrug antibodies. References 1. Grossman Z, Singer A (1996) Tuning of activation thresholds explains flexibility in the selection and development of T cells in the thymus. Proc Natl Acad Sci USA 93:14747–14752 2. Sharpe AH, Freeman GJ (2002) The B7-CD28 superfamily. Nat Rev Immunol 2:116–126 3. Jacquemin MG, Vanzieleghem B, Saint-Remy JM (2001) Mechanisms of B-cell tolerance. Adv Exp Med Biol 489:99–108 4. Pendley C, Schantz A, Wagner C (2003) Immunogenicity of therapeutic monoclonal antibodies. Curr Opin Mol Ther 2:172–179 Toxicogenomics (Microarray Technology) An illness associated with the ingestion of adulterated rapeseed oil in Spain in 1981. The most distinctive lesion is a non-necrotizing vasculitis involving different types and sizes of blood vessels in every organ. Systemic Autoimmunity Toxicogenetics The genetic basis for individual differences in susceptibility to toxicity, with single nucleotide polymorphisms (SNPs) being the prime source of variability in the genome. Toxicogenomics (Microarray Technology) 3 Unresponsiveness to antigenic stimulation that is either mediated by genetics, or is acquired by special conditions of antigenic exposure. The immune system has established several mechanisms that prevent immune reactions against self antigens. Of central importance is the tolerance of the immune regulatory helper T cells. Activation of helper T cells can be controlled by tolerance induction in the thymus, by sequestration of antigens in immune privileged sites (brain, testis, cornea) and by active suppression of immune responses by regulatory T cells. Antigen Presentation via MHC Class II Molecules Graft-Versus-Host Reaction Autoantigens Autoimmune Disease, Animal Models Antinuclear Antibodies Lymphocytes Transforming Growth Factor β1; Control of T cell Responses to Antigens Toxic Oil Syndrome (TOS) 3 Tolerance and the Immune System 651 3 3 3 3 3 3 3 Toll-Like Receptors Toxicogenomic Studies Studies in toxicology which screen for global changes in gene expression following exposure to a toxicological agent. Thymus: A Mediator of T Cell Development and Potential Target of Toxicological Agents 3 A family of receptors expressed by cells of the innate immune system and directed against conserved structures present on many micro-organisms. Ten members of this receptor family are present in humans (e.g. TLR4 specific for lipopolysaccharide; TLR2 for peptidoglycan; TLR5 for flagellin). They are named after the Drosophila protein Toll which is involved in the antibacterial defense of the fruit fly. B Cell Maturation and Immunological Memory Toxicogenomics The measurement of altered gene expression upon exposure to a compound or drug, thereby identifying the toxicant and characterising its mechanism of action. Toxicogenomics (Microarray Technology) 3 3 Toxic Epidermal Necrolysis (TEN) Toxic epidermal necrolysis (TEN) represents the most serious extreme of the febrile mucocutaneous syndrome in which there is a full-thickness sloughing of the epidermis. According to the criteria, TEN is defined as detachment affecting about 30% of the body surface area. Stevens-Johnson syndrome is similar to TEN in terms of the histopathology and the responsible drugs, indicating that these two conditions are part of the same spectrum. Fas-Fas L interactions appear to be involved in the epidermal necrolysis. Drugs, Allergy to Toxicogenomics (Microarray Technology) Rob J Vandebriel Laboratory for Toxicology, Pathology and Genetics National Institute for Public Health and the Environment 3720 BA Bilthoven The Netherlands Synonyms Gene profiling, expression profiling, global gene expression analysis T 3 652 Toxicogenomics (Microarray Technology) Definition Microarray technology is the simultaneous individual measurement of the mRNA expression level of thousands of genes in a given sample by means of hybridization. Toxicogenomics is the measurement of altered gene expression upon exposure to a compound or drug, thereby identifying the toxicant and characterizing its mechanism of action. 3 3 Characteristics Although individual differences exist, the basic principle of microarray technology is the same for different platforms (1). The term platforms means types of arrays or array suppliers, in the latter case combined with dedicated hardware and software. First, per gene, a single probe or a few different probes are generated, using either polymerase chain reaction(PCR)-amplified complementary DNA (cDNA), or synthetic DNA segments (oligonucleotides or oligos) devised on the basis of these cDNA sequences. Usually they are spotted onto a glass surface in a regular array. This process is called spotting or arraying, and requires dedicated machinery. Some companies manufacture oligos in situ, either using photolithography (Affymetrix) or chemical coupling (Agilent). Several options exist to obtain arrays: * ready-made arrays (e.g. Affymetrix, Agilent) * custom-made arrays (e.g. Affymetrix, Agilent) * in-house spotting of a PCR-amplified clone collection (e.g. Invitrogen) or of an oligo collection (MWG, Operon, Sigma). Other manufacturers of ready-made arrays include Operon, MWG, and Phase-1, but this list is by no means exhaustive. A clone collection is a collection of bacteria, each containing a plasmid consisting of a different cDNA insert. Care has to be taken that the individual clones indeed contain the correct insert; verifying clone sets by sequencing the inserts is not uncommon. Second, RNA or mRNA is isolated from cells or tissues and cDNA is synthesized. This cDNA is labeled using a fluorescent label, either during or after synthesis. The labeled cDNAs are then hybridized to the array. The Affymetrix platform uses a single labeled cDNA (Cy3) per hybridization, whereas other platforms rely on two labeled cDNAs (Cy3 and Cy5; most often test and control). The array is then read using a scanner (with fitted laser (s)) that measures for each spot the fluorescence intensity. These data are then transferred to a personal computer. This process is outlined in Figure 1. During and after this process a number of controls have to be performed to assure that the results obtained are correct. For the arrays these controls include the shape of the spots and the amount of DNA spotted (e. g. by hybridization of labeled random hexamers). After hybridization these controls include a similar average staining intensity over the entire array, and plotting the intensity ratio of both labels against the intensity of the label for the control sample. This ratio should be independent of the intensity for most of the genes interrogated. To exclude artifacts caused by differential incorporation of the two labels into the cDNAs a dye swab is useful. If replicate samples are tested, statistics can be performed. Ratios of test vs control of > 2 are generally considered significant. If several time points, dose groups, or organs are analyzed, more advanced statistics can be done, such as cluster analysis and/or principal component analysis (2). To this end several algorithms have been written, most of them being freely available on the internet. Commercial software packages have the advantage of easier data handling, compared to the tedious process of uploading data-sets to algorithms on the web (see Baxevanis and Francis Ouelette for a primer on the subject) (3). The number of genes to be analyzed is of interest. Obviously, for mechanistic studies as well as for seeding databases that are ultimately aimed at identifying toxic profiles of compounds, the number of genes should be maximal, nowadays meaning virtually all genes. With statistics aiding in the process of gene selection, signatures of toxicity (such as peroxisome proliferators) or pathology (such as liver necrosis) may eventually be addressed by interrogating a small number of genes. Preclinical Relevance A first important issue of toxicogenomics is to establish specific types of toxicity, or even compounds on the basis of signature expression profiles. A proof-ofprinciple approach to obtain such signature profiles proved to be successful (4,5). A first step towards preclinical relevance is to obtain a database consisting of gene profiles for a range of model compounds. Since studies aimed at seeding such a database are usually divided between different laboratories and the outcome has to be useful also for laboratories outside the study group, care has to be taken that results from these laboratories can be compared, or used back and forth. With the current state of technology, various methodologies and platforms exist for assessing gene expression, making it difficult to compare and compile data across laboratories. An important initiative in this respect is the “minimum information about a microarray experiment” (MIAME) document (6), produced by the microarray gene expression database (MGED) society (http:// www.mged.org). This set of guidelines is in the process of extension for toxicogenomics (MIAME/Tox), aiming to define the core that is common to most Toxicogenomics (Microarray Technology) 653 Toxicogenomics (Microarray Technology). Figure 1 Schematic illustration of microarray analysis. In this particular example PCR amplified cDNAs are dotted. toxicogenomic experiments. The major objective of MIAME/Tox is to guide the development of toxicogenomics databases and data management software. The draft document can be found at http://hesi.ilsi.org. Efforts to build international public toxicogenomics databases are underway at the National Center for Toxicogenomics, National Institute of Environmental Health Sciences, USA (http://www.niehs.nih.gov/nct) and at the EMBL European Bioinformatics Institute (http://www.ebi.ac.uk/microarray/index.html) in conjunction with the International Life Sciences Institute Health and Environmental Sciences Institute (http:// hesi.ilsi.org). This database will be made public in late 2003 or early 2004. A provisional conclusion from experiments conducted so far is that multiple sources of variability exist, including expected sources of biological variability, isolation and labeling of mRNA samples, hardware and software settings, microarray lot numbers and gene coverage, and annotation. Nevertheless, the gene expression profiles relating to biological pathways are robust enough to allow insight into mechanism, strong information on topographic specificity is provided, dose-dependent changes are observed, and concerns of over sensitivity may be unfounded (http://hesi.ilsi. org). Relevance to Humans A second important issue of toxicogenomics is the genetic basis for individual differences in susceptibil- ity to toxicity. Much of the variability in the genome stems from single nucleotide polymorphisms or SNPs, that occur roughly every 1000 nucleotides. A map describing over 1.4 million SNPs (7) is available (http://snp.cshl.org). The next step is then to find an association of a particular SNP and a disease trait. Generally, two approaches can be taken to find such associations: one is a candidate gene approach, where genes in key biochemical pathways are investigated for SNPs, and in the second approach SNPs and thereby target genes are identified by whole genome approaches. Mixed approaches can of course also be taken. An example of a successful candidate gene approach is the SNP mapping of the hypersensitivity response (HSR) to the drug abacavir. Over 100 SNPs were tested on the basis of candidate genes. Polymorphisms from two of the candidate genes (tumor necrosis factor(TNF)-α and human leukocyte antigen (HLA)-B57) were found to be highly associated with the hypersensitivity response to abacavir (8). Similar to gene profiling, creating a database that describes associations between SNPs and disease is an important goal. Using high-density SNP mapping it should be feasible to study the genetic basis for several common diseases simultaneously. For drug adverse effects this will surely be more difficult since only few patients with a certain drug prescribed will show adverse effects. A recent development comes from the finding that the human genome can be parsed into haplotype blocks, T TR1 Cells Regulatory Environment Regulations that rely on genomics are not yet in place but there is little doubt that within the next 5–10 years gene expression profiles will be used for safety as well as efficacy assessment. This requires a firm database of expression profiles that can be directly related to well characterized toxicological and pathological endpoints. Second, risk assessment has traditionally been performed across whole populations with widely varying responses. The goal is that by genetically identifying sensitive subpopulations, the accuracy of risk assessment can be improved. Possibly, this may eventually lead to personalized risk profiles. References 1. Duggan DJ, Bittner M, Chen Y, Meltzer P, Trent JM (1999) Expression profiling using cDNA microarrays. Nature Genet 21S:10–14 2. Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression analysis. Proc Natl Acad Sci USA 95:14863–14868 3. Baxevanis AD, Francis Ouelette BF (eds) (2001) Bioinformatics. John Wiley & Sons, New York 4. Hamadeh HK, Bushel PB, Jayadev S et al. (2002) Gene expression analysis reveals chemical-specific profiles. Tox Sci 67:219–231 5. Hamadeh HK, Bushel PB, Jayadev S et al. (2002) Prediction of compound signature using high density gene expression profiling. Tox Sci 67:232–240 6. Brazma A, Hingamp P, Quackenbush J et al. (2001) Minimum information about a microarray experiment (MIAME)—toward standards for microarray data. Nature Genet 29:365–371 7. Sachidanandam R, Weissman D, Schmidt SC et al. (2001) A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature 409:928–933 8. Roses AD (2002) Genome-based pharmacogenetics and the pharmaceutical industry. Nature Rev Drug Disc 1:541–549 9. Gabriel SB, Schaffner SF, Nguyen H et al. (2002) The structure of haplotype blocks in the human genome. Science 296:2225–2229 TR1 Cells Suppressor Cells Trace Metals Those metals commonly found in minute amounts in the organism. Trace Metals and the Immune System Trace Metals and the Immune System Judith T Zelikoff Depart. of Environmental Medicine New York University School of Medicine 57 Old Forge Road Tuxedo, NY 10987-5007 USA Synonyms CD4+, T helper lymphocyte, CD4+/CD8−, T helper lymphocyte, CD8+, T suppressor lymphocyte, COPD, chronic obstructive pulmonary disease, asthma, bronchitis, emphysema. Definition Trace metals are normally present in minute quantities in the body. Many of them are also transition elements, essential for life due to their ability to control metabolic and signaling functions, such as zinc (Zn), manganese (Mn), and copper (copper). However, these same essential metals can also be toxic because of their ability to evade established controls for cellular uptake, transport, and compartmentalization. Aluminum (Al) is a toxic trace element, unavoidable by the general population because of its widespread environmental distribution. The immunotoxicity of trace metals other than Al, copper , Mn, and zinc can be found in a number of review articles (1–3). Molecular Characteristics Aluminum Aluminum is the third most prevalent element in the Earth's crust. It is an A-type metal, or hard acid, that strongly prefers oxygen-donor ligands; hydroxide, citrate, phosphate, and nucleoside phosphate groups are probably the most important low-molecular-mass bioligands for the predominant trivalent cation (Al3+). It also binds readily to the two high-affinity iron-binding sites of the serum transport protein, transferrin (TF). There is a wide variation in the ability of different ligands to solubilize and transport the Al3+ ion to critical target sites. 3 being regions over which there is little evidence for historical recombination and within which only a few haplotypes are observed (9). Markers for these haplotype blocks are now available, which makes it possible to identify the genetic control of responses to toxicants without the necessity to identify the specific SNP responsible. 3 654 Copper Copper is a Group II (or IB) element, the third most 3 Trace Metals and the Immune System Manganese Manganese is the only Group VIIB element commonly found in biological environments. Although the inorganic chemistry of manganese displays a range of stable oxidation states, its biological chemistry is dominated by the divalent form (Mn2+). Because Mn2+ is very similar in size and charge density to magnesium (Mg2+) and Zn2+ and also prefers to assume thetrahedryl and octahedral geometric structures, Mn2+ can replace Mg2+ in the enzyme pyruvate carboxylase and Zn2+ in superoxide dismutase (SOD) with only negligible effects on enzyme activities. Zinc Zinc is found in large quantities in the vertebrate body (second only to iron); it is the first member of Group IIB elements and forms stable complexes with sulfur, phosphate, and carbon atoms. Biological complexes contain zinc only in the divalent oxidation state (Zn2+). Since Zn2+ is the only stable oxidation state of the metal, it does not play a redox-active role in biological processes. However, Zn2+ can actively participate in enzymatic reactions as a Lewis acid or as a structural cofactor. Zinc is part of, or a cofactor for, such enzymes as carbonic anhydrase, carboxypeptidase, SOD, lactate dehydrogenase, phosphatase, and glutamate dehydrogenase. Zinc also displays a structural role in biological systems, as exemplified by its role in maintaining the integrity of zinc finger transcription factors that bind to DNA and regulate the transcription of genetic information. Relevance to Humans Aluminum While some daily exposure to aluminum is unavoid- able, inhalation by the general population is usually considered negligible (i.e. 0.14 mg aluminum dust per day). However, smelters, miners, welders and other workers involved in various metal industries are often acutely exposed to localized atmospheres containing 2–4 mg/m3 of aluminum, resulting in timeweighted-average (TWA) intakes of > 23 mg per 8hour shift. Increases in pneumonia, bronchitis, asthma, pneumoconiosis, lung cancers, and pulmonary fibrosis have been described in occupationally exposed workers. In addition, there is little doubt that aluminum can cause encephalopathy, osteopathies, and anemia in kidney dialysis patients. Although early studies set 100 μg/l plasma as the level of aluminum below which neurotoxicity failed to occur, recent studies have demonstrated subtle neurocognitive and/ or psychomotor effects, as well as EEG abnormalities in dialysis patients expressed at levels well below this limit. Infants are a particularly susceptible subgroup for aluminum toxicity partly due to their rapidly growing and immature brain and skeleton and their developing blood-brain barrier; preterm infants are generally recognized to be at risk for aluminum loading due to their immature kidney function. While the reference range for blood aluminum levels in healthy individuals is < 10 µg/l, studies in infants have demonstrated plasma aluminum levels > 50 μg/l after oral intake of aluminum-containing antacids. Copper As an essential element, copper promotes iron absorption from the gastrointestinal system, it is involved in the transport of iron from tissues into plasma, it helps maintain myelin in the nervous system, it is necessary for hemoglobin synthesis, and it is important in the formation of bone and brain tissue. Apart from occupational exposure, daily copper intake averages ∼ 0.02 mg. The fine balance required for copper in humans is evident in genetically inherited inborn errors of copper metabolism. For example, in Wilson's disease there is failure to excrete copper from the liver to the bile, resulting in copper overload in the liver, brain, kidneys, and cornea; and in Menkes disease, which is characterized by severe copper deficiency due to an error in copper transport from the intestines. Copper, usually in the form of cuprous oxide and cupric hydroxide (which converts to cupric oxide), is generally encountered in high concentrations in the air of metallurgical processing plants, iron and steel mills, and around coal-burning power plants. In contrast to airborne copper concentrations in rural/suburban areas that average 0.01–0.26 μg/m3, particulate copper levels in workplace sites be 50–900 μg/m3. Inhalation of such levels can result in an immunologically-based condition called “copper fever”. 3 abundant transition metal found in living things. It exists in one of two stable oxidation states: as cuprous (Cu1+) and cupric (Cu2+) ions. Consequently, its biological chemistry is dominated by participation in redox reactions. Copper is necessary in the diet for iron utilization and as a cofactor in enzymes associated with oxidative metabolism. It is transported in serum bound initially to albumin and later more firmly to αceruloplasmin where it is exchanged in the cupric form; normal copper serum level is 120–145 μg/l. At elevated levels, copper is toxic to cells, presumably by binding indiscriminately to thiol moieties or by catalyzing a Fenton-type reaction to produce reactive hydroxyl radicals. Binding of copper by biological ligands such as small peptides, large proteins, and enzymes is required to minimize potential deleterious effects. Most stored copper is usually bound to metallotheinein (MT), a ubiquitous class of proteins that is well suited to the role of metal sequestration. 655 T Trace Metals and the Immune System Zinc Zinc is ubiquitous in the environment and present in most foodstuffs, water, and air. It is a nutritionally essential element that serves as a cofactor for more than 70 metalloenzymes. Daily dietary intake of zinc is usually 12–15 mg/day and ~ 20%–30% of ingested zinc is absorbed; zinc deficiency results in a wide spectrum of clinical effects depending upon age, stage of development and deficiencies of related metals (i.e. zinc deficiency can exacerbate impaired copper nutrition and exacerbate cadmium and lead toxicity). Airborne concentrations of zinc are usually < 1 μg/m3, with the majority of zinc being derived from automobile exhaust, soil erosion, and local commercial, industrial or construction activities. In urban areas, atmospheric zinc concentrations are in the range 0.02–0.50 μg/m3; rural air contains 0.01–0.06 μg/m3. Because zinc also contaminates certain workplace environments, national guidelines of 1.0 mg, 5– 10 mg, and 0.1 mg/m3 have been established for soluble zinc, insoluble zinc oxide, and carcinogenic zinc chromate, respectively. Putative Interactions with the Immune System Aluminum Although limited in number, immunotoxicologic studies using a variety of animal models have demonstrated that injection of soluble aluminum compounds increases mononuclear cell mitotic index; injection of the metal or insoluble aluminum agents alter monocyte/macrophage numbers and immune function (1). Dietary exposure of rodents to soluble aluminum reduces cytokine production, T helper (Th) and T suppressor (Ts) cell numbers, and host resistance to Listeria monocytogenes infection. Repeated inhalation exposure of rabbits and hamsters to soluble aluminum increases lung immune cell numbers; similar effects were not seen in aluminum-exposed workers. Effects of inhaled aluminum on host resistance are inconsistent, showing decreased resistance to subsequent bacterial challenge in some studies and no effect in others. Differences between the studies are thought to be due to intratracheal versus inhalation exposure routes. In vitro studies employing soluble aluminum salts demonstrate a range of effects on immune cells derived from a variety of animal species including humans. For example, aluminum chloride treatment of rat alveolar macrophage reduced reactive oxygen intermediate production. Copper Much the same as for manganese and zinc, studies of copper immunotoxicity are complicated by the fact that copper is essential to maintenance of immunocompetence and, thus, most immunotoxicity occurs as a result of copper insufficiency. Splenomegaly and thymic atrophy are consistent findings in copper-deficient mice. Alterations in antibody response and B-lymphocyte function are also well documented with experimentally induced copper deficiency. In contrast, serum antibody levels in humans with nutritional or genetic copper-deficiency are reported to be normal. B-lymphocytes are increased in number in copper-deficient animals, but they respond poorly to mitogen stimulation. Although the effects of copper deficiency on T-lymphocyte populations are well characterized, the overall effect on cell-mediated immunity is unclear. Except in female rats, CD4+ and CD8+ subsets are decreased in the peripheral blood and spleen of copper-deficient rodents. Though no gender effect has been observed in mice, the immune system of male rats appears more susceptible to copper deficiency than that of females. Clinical studies involving healthy men on low copper diets fail to support the animal studies with respect to circulating T-lympho3 Manganese Manganese, an essential trace element for all living organisms, is necessary for bone formation, cholesterol and fatty acids synthesis, and as a dissociable cofactor for several enzymes including SOD. Despite its essentialness, the toxic effects of manganese are well known, particularly those associated with the nervous system (4). Manganese is widely employed in many industries: in alloy steel manufacture for deoxidation and to promote hardenability; in the electric industry for production of dry cells; in the chemical industry, where they are used as oxidants, for the manufacture of fertilizers, paints and varnishes, and in the production of glass and glazes (3). Apart from the direct release of manganese into the air by several types of mining industries and alloy and steel production facilities, manganese is introduced into the ambient environment by the combustion of manganese-containing fossil fuels (used as anti-knock additives and combustion improvers). Manganese (whose toxicity in many cases depends upon compound solubility) has been found at measurable levels in the majority of suspended particulate matter (including coal flyash) in urban environments. While air levels of manganese in many metropolitan areas containing steel or alloy plants can range from 0.5–3.3 μg/m3, the majority have levels ≤ 0.1 μg/m3; average air levels in the absence of any contributing point sources are in the range 0.03–0.07 μg/m3. Alternatively, occupational airborne levels of manganese are usually in the range 1–≥ 100 μg/m3 (although levels as high as 1 mg/m3 have been measured); workplace permissible exposure limits (PEL) of 300 (TWA) and 500 μg/m3 have been recommended by the World Health Organization and OSHA (Occupation Safety and Health Association), respectively. 3 656 Transendothelial Migration cytes and CD4+ and CD8+ subsets. While effects of copper-deficiency on innate immunity are inconclusive in animal studies, reduced neutrophil numbers and functionality are well defined in clinical studies. The most consistent immune defect associated with copper deficiency in epidemiologic, clinical, and toxicological studies is impaired host resistance due primarily to suppressed antibody-mediated responses and phagocyte antimicrobicidal activities (2). Manganese While relatively few studies have investigated the effects of manganese on the immune system, immunotoxicity appears dependent (like many of the other metals discussed herein) upon compound solubility. Immune responses of the lung appear particularly sensitive to the immunomodulating effects of manganese. Inhalation of insoluble manganese compounds reduces the ability of the lungs to resist and clear subsequent bacterial/viral infections and exacerbates ongoing viral infections (3). Inhalation studies examining the effects of soluble manganese reveal little effect on lung immune cell-related functionality. In contrast, studies wherein rabbit alveolar macrophages were exposed in vitro to manganese chloride demonstrated decreased cell viability and number, increased incidence of cell lysis, and reduced phagocytic activity. 657 cific immune cell types, effects of zinc on innate immunity are conflicting. References 1. Zelikoff JT, Cohen MD (1997) Metal immunotoxicology. In: Massaro EJ (ed) Handbook of Human Toxicology. CRC Press, New York, pp 811–852 2. Omara FO, Brousseau P, Blakley BR, Fournier M (1998) Iron, zinc, and copper. In: Zelikoff JT, Thomas PT (eds) Immunotoxicology of Environmental and Occupational Metals. Taylor and Francis, London, pp 231–262 3. Cohen MD (2000) Other metals: aluminum, copper, manganese, selenium, vanadium, and zinc. In: Cohen M, Zelikoff JT, Schlesinger RB (eds) Pulmonary Immunotoxicology. Kluwer, Boston, pp 267–299 4. Inoue N, Makita Y (1996) Neurological aspects of human exposures to manganese. In: Chang LW (ed) Toxicology of Metals. CRC Lewis, New York, pp 415–421 5. Zelikoff JT, Chen LC, Cohen MD et al. (2003) Effects of inhaled ambient particulate matter (PM) on pulmonary anti-microbial immune defense. Inhal Toxicol 15:101– 120 Trans-Signaling 3 Transcription Factors Proteins (enzymes) that bind to regulatory sequences (response elements) in the promoter region of a gene, forming a complex to which RNA polymerase binds. The process of transcription converts the genetic information contained in DNA into an RNA message for synthesis of a specific protein. Glucocorticoids Signal Transduction During Lymphocyte Activation 3 3 Zinc deficiency (like copper) can impair humoral and cell-mediated host immunocompetence. Zinc-deficient children and laboratory animals consistently present with thymic hypoplasia; oral administration of zinc supplements appear to reverse this effect. In zinc-deficient animals, secondary antibody responses to T-dependent antigens are suppressed in conjunction with accelerated thymic hypoplasia and a decreased number of CD4+ cells. Administration of zinc to immunosuppressed human populations appears to increase numbers of CD4+ and CD8+ thymocytes which, in turn, give rise to increased numbers of Th-cells important for the activation of cytotoxic T- and B-lymphocytes. In contrast, zinc suppresses concanavalin A-induced T lymphocyte proliferation by in vitro-exposed human immune cells (2) and compromises pulmonary host resistance against bacterial infection (5); suppressive effects of inhaled zinc on pulmonary antimicrobial activity are most likely due to zinc-induced reductions in macrophage phagocytic activity. While the main immunological effect of occupational zinc exposure is metal fume fever, inhalation of particulate zinc by occupationally exposed workers also alters pyrogenic, chemotactic, and anti-inflammatory cytokines. While zinc appears to play an important regulatory role in membrane-associated events of certain nonspe- 3 Zinc The soluble Interleukin-6 receptor α-chain binds interleukin-6 and can then interact with the transmembrane receptor subunit glycoprotein 130 (gp130) and induce signal transduction. Thus a cell lacking an endogenous binding subunit of the interleukin-6 receptor can respond to Interleukin-6 in the presence of the soluble receptor-derived from distant producer cells, hence trans-signaling. Cytokine Receptors Transendothelial Migration This is the exit of circulating leukocytes from blood into tissue by means of traversing the microvascular endothelium. This process involves loose interactions of blood leukocytes with the luminal side of blood T 3 Transferrin vessels (mostly mediated by selectins), and this is followed by firm adhesion and leukocyte transmigration. This latter step critically depends on the rapid and transient modulation of integrin function, which itself is controlled by chemokine receptor signaling. Only those leukocytes firmly arrest that bear the appropriate set of chemokine receptors and, therefore, the chemokines present on the luminal side of microvessels are viewed as key controllers of leukocyte extravasation. Immune Cells, Recruitment and Localization of 3 Transferrin A protein that combines with and competes for iron with bacteria. Trace Metals and the Immune System 3 Transferrin Receptor These are cell membrane receptors for transferrin. They play a role in iron uptake by the cell, and are highly expressed in proliferating cells. Interferon-γ 3 Transforming Growth Factor β1; Control of T Cell Responses to Antigens Susan C McKarns Laboratory of Cellular and Molecular Immunology NIAID/NIH Building 4, Room 111, MSC 0420, 4 Center Drive Bethesda, MD 20892 USA Synonyms TGF-β1(the nomenclature is used worldwide with the number designating the isoform) Definition The transforming growth factor-β (TGF-β) superfamily consists of more than 40 structurally related secreted proteins (1). Three members (TGF-β1, 2, 3) are expressed in mammals; despite a 70%–76% sequence homology, these isoforms have expression pattern and functional differences. Whereas, TGF-β2 and TGF-β3 are important for cellular differentiation, development, and embryogenesis, the effects of TGF-β1 are predominantly—albeit not exclusively—immunologic. Lymphoid cells selectively produce TGF-β1. The name 'transforming' is something of a misnomer because this factor is not always associated with oncogenesis. TGF-β1 is possibly the most pleotropic of all the cytokines and growth factors, and its activity is cell-type- and context-dependent. The ability of TGF-β1 to suppress cell growth distinguishes it from most other cytokines/growth factors. Mechanistically, it converts receptor ligation at the cell surface into an enzymatic signaling cascade within the cell to change the level of expression of target genes. In this manner, it is able to target a vast array of immune cell lineages to modulate their ability to proliferate, differentiate, survive, perform effector functions, and migrate to sites of antigen presentation and/or inflammation. These events are vital to the initiation, progression, and resolution of inflammatory responses. Dysregulated expression or function of TGF-β1 is implicated in autoimmune disease, chronic inflammation, and tumor progression. The driving force behind TGF-β1 seems to be maintaining homeostasis of controlled immune responses, and it achieves its goal by orchestrating a network of intracellular signaling crosstalk that enables cells to rapidly respond to changes in their environment. 3 658 Characteristics Cellular sources TGF-β1 expression is present at the four-cell embryo stage and persists, in most tissues, during morphogenesis and into adulthood. Most all mature cells have been shown to produce this factor. Likewise, nearly all cell types have functional TGF-β1 receptors. Although controversial, it has been postulated that the primary effector function of a small cohort of regulatory T cells (e.g. Th3 and CD4+CD25+) is to secrete TGF-β1. Regulation of activity It is well established that the bioavailability and activity of TGF-β1 are influenced by the environment (Table 2), and it generally is accepted that some of Transforming Growth Factor β1; Control of T Cell Responses to Antigens. Table 1 GenBank accession numbers for transforming growth factor-β1 (TGF-β1) Accession numbers (partial listing only) Species Gene Protein Human (Homo sapiens) J04431, J05114 PO1137 Mouse (Mus musculus) AH003562 P04202 Transforming Growth Factor β1; Control of T Cell Responses to Antigens TGF-β1 signaling The predominant mechanism by which TGF-β1 elicits its activity is through modulation of gene transcription. TGF-β1 mediates the association of transmembrane type II (TβRII) and type I (TβRI) receptors. Ligand binding propagates signaling through phosphorylation of multiple effector proteins. The only known direct TGF-β1 signaling effectors are a class of structurally similar Smad proteins (2). Once the ligand has bound to the serine-threonine kinase receptor a signaling complex is formed, leading to the phosphorylation 3 Transforming Growth Factor β1; Control of T Cell Responses to Antigens. Table 2 Factors that modulate bioactivity of transforming growth factor-β1 (TGF-β1) Enhance TGF-β synthesis and secretion Liver hepatotoxicants (carbon tetrachloride, acetaminophen, alcohol) Tissue injury (liver, renal, and lung) Hypoxia Stress Viral infection Parasitic infection Steroid hormones (retinoids, vitamin D, and tamoxifen) Activate extracellular latent TGF-β1 Mannose 6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2R) of Smad 2 and Smad 3 and their subsequent trafficking to the nucleus, where they bind well-defined Smad response elements and function as transcriptional modulators to regulate transcription of TGF-β1 target genes (Fig. 1). Smads can also positively regulate gene expression by recruiting coactivators such as CBP/p300 or negatively by forming complexes with histone deacetylases (HDACs) or corepressors (such as c-ski and SnoN) which themselves associate with HDACs. One key negative regulation of Smad signaling is the expression of the inhibitory Smad, Smad7. Smad7 blocks TGF-β signaling by competing with R-Smads for association with TβRI, or by targeting receptors for ubiquitin-mediated degradation. Potent inducers of Smad7 are interferon-γ (IFN-γ), tumor necrosis factor (TNF)-α, and interleukins IL-1β, and IL-7. Induction of Smad7 represents an important regulatory interplay between TGF-β1 and cytokines in immune cell function. It is noteworthy that type TβRI and TβRII receptors distinguish themselves from other cytokine/ growth factor receptors by their specificity for serine/ threonine, rather than tyrosine kinase, activity. Smad-independent signaling pathways also regulate TGF-β1 signaling. For instance, TGF-β1 activates mitogen-activated protein (MAP) kinases including the extracellular regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs) and p38 kinases. TGF-β1 has also been shown to activate Rho-like GTPases and phosphatidulinostiol-3-kinase (P13K) and signal through protein phosphatase 2A (PP2A). One key point of cross-talk among signaling intermediates is MAP kinase activation that occurs downstream of growth factors, integrins, and chemokine receptors. In most cases, activated MAP kinases promote the actions of TGF-β1 to enhance cell migration. Thus, activation of growth factor receptor and the pattern of cytokine/ chemokine signaling have a tremendous impact on the response of cells to TGF-β1 (Fig. 1). Finally, TGF-β1 3 these changes increase human susceptibility to immunologic-related diseases. TGF-β1 predominantly is secreted as a biologically inert complex consisting of mature TGF-β1, latency associate protein (LAP), and latent TGF-β1-binding protein (LTBP). Prior to binding to TGF-β receptors, the latent complex must be cleaved into the 25 kDa active TGF-β1 homodimer. 659 Transglutaminase T Plasmin/plasminogen activator Apoptotic T cells Reactive oxygen species αvβ6 Integrin receptor Suppress activation of extracellular latent TGF-β1 α2-Macroglobulin Decorin Endoglobin Mucosal mast cell protease (MMCP) Antagonize TGF-β1 signaling Cytokines: tumor necrosis factor-α, interferon-γ, interleukins IL-1β, IL-6, IL-2 Transforming Growth Factor β1; Control of T Cell Responses to Antigens. Figure 1 660 Transforming Growth Factor β1; Control of T Cell Responses to Antigens signaling can also modulate protein stability. For example, it enhances degradation of TβRI. Immunological activities A loss-of-function mutation in TGF-β1 results in the rapid onset of lethal multiorgan inflammation and autoimmune phenotype. These transgenic mouse models clearly establish the critical role of this factor in maintaining immune homeostasis for the prevention of disease and chronic inflammation (Fig. 2). A role for TGF-β1 has been implicated, in several different mouse models, including tolerance, and particularly in mucosal immunity. While these studies clearly demonstrate the onset of inflammation in the absence of TGF-β1 signaling, more recent data suggest that the role for TGF-β1 in controlling T cell homeostasis may be restricted to preventing inappropriate responses to self- or environmental antigens, rather than regulating T cell responses to low-avidity self-ligands (3). In addition to its immunosuppressive and anti-inflammatory properties, TGF-β1 is capable of promoting inflammation (4). For example, at the early stages of inflammation, it enhances lymphoid, neutrophil, monocyte, and macrophage migration, presumably to enhance the localization of these cells at the site inflammation. Probably TGF3 also prolongs the inflammation associated with numerous autoimmune disorders by actively sequestering activated T cells at the site of inflammation. TGF-β1 also exerts numerous suppressive effects on T and B lymphoid effector and antigen-presenting cells and many of these effects are summarized in Table 3. 3 Preclinical Relevance Implications for disease Dysregulated expression of TGF-β1 or response of immune cells to TGF-β1 signaling have been implicated in the pathogenesis of many human diseases, including hypersensitivity reactions such as asthma and food allergies, as well as autoimmune disorders, including encephalomyelitis, arthritis, systemic lupus erythematosus, and allograft rejection. While it is com- Transforming Growth Factor β1; Control of T Cell Responses to Antigens. Figure 2 monly accepted that both environmental and genetic factors contribute to the incidence of these immune responses, it remains unclear why some individuals are susceptible to these disorders while others are not. Appropriate levels of TGF-β1 have been shown to be essential for maintaining immunologic balance, to prevent the pathogenesis of hypersensitivity reactions/chronic inflammation and autoimmune disorders. Perhaps a better mechanistic understanding of how it modulates cellular and molecular pathways will provide important insights that will enhance our understanding of susceptibility to these diseases. Outlined below are three prevalent immune disorders that occur in response to common environmental exposure; which, TGF-β1 is key to regulation of the ensuing pathological immune responses. Asthma The development of asthma in response to environmental antigens affects up to 20% of the population in developed countries. Asthma is a chronic inflammatory disease of the airways that is characterized by mononuclear infiltration, eosinophil degranulation, and bronchoconstriction. TGF-β1 is constitutively expressed by airway epithelial cells, eosinophils, T lymphocytes, macrophages, and fibroblasts, and stored in the extracellular matrix of the airways. Rodent models of asthma suggest that it mediates both anti-inflammatory and profibrotic effects (5). Prior to allergen exposure, it is thought to play a critical protective role against the onset of asthma by suppressing airway inflammation and hyper-responsiveness through the suppression of T lymphocytes, dendritic cells, eosinophils, mast cells, and IgE production. Notably, mononuclear cell infiltration into the lungs is prevalent in TGF-β1 null mice. Additionally, TGF-β1 may further suppress airway CD4+ T cell allergen exposure by enhancing activity of T regulatory cells. However, repeated long injury is accompanied by a profound TGF-β1-mediated recruitment of fibroblasts into the airways, a progressive deposition of extracellular matrix, and subsequent fibrosis and bronchoconstriction. Food allergy Food allergy is characterized by an adverse hypersensitive response to food consumption. A normal healthy gastrointestinal immune response must discriminate between harmful pathogens and harmless dietary antigens and commensal bacterial flora. The mucosal immune system has generated two adaptive immune responses to meet this challenge: induction of a local secretory IgA response, which is propagated in the absence of a measurable systemic immune response, to clear potentially dangerous antigens; and induction of oral tolerance, a state of non-responsiveness or Transforming Growth Factor β1; Control of T Cell Responses to Antigens 661 Transforming Growth Factor β1; Control of T Cell Responses to Antigens. Table 3 Biological activities of transforming growth factor-β1 on the immune system Parameter TGF-β1-mediated effect T lymphocytes TCR-induced CD4+ and CD8+ T cell Suppress; memory CD4+ resistant to G1 cell cycle arrest proliferation IL-2-induced CD4+ and CD8+ T cell proliferation Suppress, dependent upon IL-2 concentration Th1 differentiation Suppress, but dependent upon strength of T cell stimulation; inhibits T-bet and INF-γ expression Th2 differentiation Suppress; inhibits GATA-3 and IL-4 expression Th1 effector function Suppress; inhibits INF-γ and IL-2 production; inhibits IL-12 signaling Th2 effector function Suppress; inhibits IL-4 and IL-5 production CD8+ cytotoxic T cell effector function Suppress CD4+ and CD8+ migration/adhesion Enhance; increases CXCR4 and α4β7 expression CD4+CD25+ regulatory T cell function Enhance; increases Foxp3, GITR, CD103, CTLA-4 expression IL-12 signaling Suppress; downregulates IL-12 receptor β2 chain T cell apoptosis Suppresse or enhance, dependent on microenvironment B lymphocytes Proliferation Suppress Effector function Enhance IgA and IgG2b and suppressed most other isotopes Antigen-presenting cells MHC class I and class II molecules Suppress Monocytes and macrophages Monocyte chemotaxis Enhance or suppress Macrophage chemotaxis Suppress Neutrophils Neutrophil chemotaxis Enhance or suppress Chemokine and receptor expression Suppress or enhance: chemokine, receptor, and cell type-dependent CTLA=cytotoxic T-lymphocyte-associated protein 4; GITR= glucocorticoid induced TNF receptor; Ig=immunoglobulin; IL=interleukin; INF=interferon; MHC=major histocompatibility complex; TCR=T cell receptor; TGF=transforming growth factor; Th=T helper cell. hypo-responsiveness which minimizes unnecessary immune reactions against harmless antigens. A failure to induce or an inability to maintain oral tolerance may leads to a food allergy. TGF-β1 is abundant throughout the mucosa and has been shown in several experimental models to play a profound role in maintaining oral tolerance (5). It is well documented that a population of TGF-β1-secreting T helper cells is generated when low doses of antigen are consumed. These TGF-β1-secreting regulatory T cells also produce various amounts of IL-4 and/or IL-10. Secreted TGF-β1 suppresses T cell proliferation and promotes class switching of B cell IgA isotypes to modulate the adaptive immune response. Moreover, it also enhances the preservation of the epithelial barrier between environmental antigens in the gut flora and T lymphocytes in the mucosa to add yet another level of regulatory control over adaptive T cell responses. Childhood food allergies have been associated with a reduction in the number of mucosal TGF-β1-producing lymphocytes. Aberrant levels of mucosal TGF-β1 and associated dysregulated responses to the normal gut flora have also been implicated in the pathogenesis of inflammatory bowel disease. Collectively, these data implicate a role for TGF-β1 to maintain oral tolerance in humans. T 662 Transforming Growth Factor β1; Control of T Cell Responses to Antigens Autoimmunity Discordance in incidence of autoimmune disease in monozygotic twins demonstrates a role for environmental exposure in regulating immune homeostasis. Although numerous environmental factors have been implicated, the underlying mechanisms remain relatively undefined. The autoimmune phenotype of the TGF-β1 knockout mouse, characterized by circulating antinuclear antibodies and glomerular deposit s of immune complexes, probably best defines the role of this factor in the disease process. 100% of TGF-β1 knockout mice succumb to a massive multiorgan inflammation involving the heart, lung, liver, gut, salivary glands, eyes, brains, and other tissues. The inflammatory infiltrates are predominantly perivascular and vary from neutrophilic in the stomach to lymphocytic in the brain. In agreement, systemic administration of exogenous TGF-β1 or adoptive transfer of TGF-β1 −producing T cells protect against autoimmune diseases in several experimental models, including diabetes, encephalomyelitis, inflammatory bowel disease, arthritis, systemic lupus erythematosus, and allograft rejection. Targeted deletion of TGF-β signaling in T cells alone has been demonstrated to be sufficient to induce an autoimmune phenotype. It remains to be determined whether other non-T cell TGF-β1-producing cells (e.g. macrophages) contribute to the disease process as well. The precise mechanisms of actions underlying the ability of this factor to regulate autoimmune disorders remains speculative. Recent evidence implicates a significant role for regulatory T cells. CD4+ CD25+ regulatory T cells, also called suppressor T cells, can be delineated into two subsets of CD4+ CD25+ T cells with inherent activity to suppress autoreactive T cells: these are ‘natural’ regulatory CD4+ CD25+ T cells that emerge from the thymus, and adaptive regulatory CD4+ CD25+ T cells that are induced in the periphery. TGF-β1 has been shown to positively regulate both subsets, and TGF-β1mediated expansion of CD4+ CD25+ T cells protects against autoimmune diabetes (7). However, in view of the diversity of pathology associated with autoimmune disorders, it is highly likely that TGF-β1 also utilizes other critical mechanisms of action. For instance, modulation of Th2/Th1 cytokine balance, cell survival, migration, effector function, and Th3-mediated tolerance represent likely alternative mechanistic routes (8). Relevance to Humans The phenotype of the TGF-β1 mouse resembles human SLE, Sjögren syndrome, graft-versus-host disease, and polymyositis, suggesting that TGF-β1 may play a similar regulatory role in human immunologic disorders. The levels of TGF-β1in serum and of its mRNA in tissue can be measured and have been used as diagnostic or prognostic markers for other human diseases. For example, high levels of the factor in RNA in tissues are associated with gastric cancer. High serum levels also correlate with the development of fibrosis in patients with breast cancer who have received radiation therapy. Understanding the mechanisms of action of environment-induced immune disorders in experimental models will potentiate the development of better predictive risk assessment assays to prevent disease as well as more specific therapeutic regimens aimed at increasing effectiveness and diminishing deleterious side effects. Regulatory Environment Although interaction of chemicals with cytokines or chemokines may have an important impact on the function and regulation of the immune system they are not regulated by any specific immunotoxicity guideline. The cytokine network is mentioned in different guidelines or guideline drafts but exclusively in connection with extended 'case-by-case' investigations. The regulation of the immune system is complex, and identification of the mechanism, of action of chemicalinduced immune toxicity is critical for the understanding of the disease process. The regulatory cytokine TGF-β1 may be of special interest for such investigations. A better understanding of the disease process will provide the basis for the development of more sensitive and predictable assays for risk assessment. Chemical-induced immunotoxicity may be indirectly mediated via the soluble potent immune modulator, TGF-β1. TGF-β1 may be a useful biomarker of chemical-induced and/or environmental-induced immunotoxicity. A critical challenge is to determine the appropriate therapeutic level of active TGF-β1 or signaling pathways that positively influence cell responsiveness to ameliorate disease while minimizing deleterious side effects. References 1. Flanders CF, Roberts AB (2000) TGF-β. In: Oppenheim JJ, Feldman M, Durum SK, Hirano T, Vilcek J, Nicola NA (eds) Cytokine reference: A compendium of cytokines and other mediators of host defense. Academic Press, New York, pp 719–746 2. Shi Y, Massague J (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 13;113:685–700 3. Gorelik L, Flavell RA (2002) Transforming growth factor-β in T-cell biology. Nat Rev Immunol 2:46–53 4. McCartney-Francis NL, Frazier-Jessen M, Wahl SM (1998) TGF-β: A balancing act. Int Rev Immunol 16:553–580 5. Duvernelle C, Freund V, Frossard N (2003) Transforming growth factor-β and its role in asthma. Pulmon Pharmacol Ther 16:181–196 Transgenic Animals 6. Weiner HL (2001) Oral tolerance: immune mechanisms and the generation of Th3-type TGF-β-secreting regulatory cells. Microbes Infect 11:947–954 7. Peng Y, Laouar Y, Li MO, Green EA, Flavell RA (2004) TGF-β regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci USA 101:4572– 4577 8. Prud'homme GJ, Piccirillo CA (2000) The inhibitory effects of transforming growth factor-beta-1 (TGF-β1) in autoimmune diseases. J Autoimmun 1:23–24 663 neoplastic and cardiovascular disease), in immunology, mutagenesis and carcinogenesis research, and in novel target evaluation and drug discovery. In immunotoxicology, although the potential usefulness of transgenic mice is widely recognized, practical application has been limited. Characteristics Generation of Transgenic Animals (1) There are two principal methods by which transgenic mice are created: microinjection of genetic material into the pronucleus of a fertilized ova; or gene transfection of embryonic stem cells (ES cells) cells followed by injection of the transgenic cells into a blastocyst. In either case, the resulting transgenic embryo is implanted into a recipient female prepared for pregnancy. The principal difference between the techniques is that, when successful, microinjection of the pronucleus results in homozygous offspring, while transfection of ES cells results in chimerae (see chimera) that must be selectively bred to yield homozygous animals. There are two principal types of transgenic events, those with one or more random insertions of the transgene and those where homologous recombination results in targeted insertion. Either type of transgenic event can result in a knock-in (KI) that will express the coding sequences of the transgene. Although random insertion will by definition result in a mutation in the recipient genome, as most DNA is noncoding these mutations are generally silent. Homologous recombination is used to selectively disrupt expression of the homologous gene, resulting in a knock-out (KO). This requires design of the inserted DNA so as to contain sequences homologous to the desired host species’ gene. A third type of transgenic animal—the knock-inknock-out (KI-KO) mouse—has also been created. If the inserted DNA has a sequence homologous to a murine gene and also codes for a foreign protein, a KI-KO mouse can be created in a single step. Alternatively, these two types of transgenic events can be combined in a two-step process to result in a KI-KO strain. 3 3 3 Peter J Bugelski Experimental Pathology Centocor, Inc. R-4-2, 200 Great Valley, Parkway Malvern, PA 19355 USA Synonyms Knock-out, knock-in, genetically modified, recombinant Definition Animals whose genome has been modified using recombinant DNA technology so as to have a foreign gene expressed (knock-in) or a native gene suppressed (knock-out) in a heritable fashion. A number of transgenic species have been created: mice, rats, pigs, goats, cattle, sheep and fish. Currently, the vast majority of transgenic animals are mice. Transgenic mice have applications in numerous areas of biomedical research (e.g. neurologic, inflammatory, autoimmune, Transgene Expression In some cases, expression of the transgene by the host is not important. For example in mutagenesis research the endpoint can be a mutation in the transgene that will be expressed and detected ex vivo. In most cases however, expression of the transgene is desired. Insertion of multiple copies of the gene and linkage to a potent promoter (e.g. simian virus 40 promoter, will likely ensure widespread and high-level gene expression. Depending on the experiments to be conducted, however, it may be important that the site (s) 3 3 Transgenic Animals 3 TGF-β1 is the prototype for a superfamily of secreted proteins that control many aspects of growth and development. It was named transforming growth factor because upon its discovery it was shown to induce a transformed or tumor cell phenotype in normal cells. TGF-β1 is now known to regulate a diverse array of cellular functions unrelated to cell transformation. Within the immune system, TGF-β1 is critical for cell growth, differentiation, effector cell function, survival, and migration. Transforming Growth Factor β1; Control of T cell Responses to Antigens Mucosa-Associated Lymphoid Tissue 3 Transforming Growth Factor β1 (TGF-β1) T Transgenic Animals of expression, the magnitude of expression and timing of expression be controlled. This can be accomplished, but is no means guaranteed by selecting the gene promoter sequences included in the transgene. Techniques are now reasonably well established for controlling the sites and magnitude of expression and “conditional” gene expression in transgenic animals is an active field of research. 3 Preclinical Relevance Transgenes Transgenic mice have been created which express a wide and ever increasing range of genes (as of June 2003 the database maintained by BioMed Net lists 2300 transgenic mice (2)). These genes include reporter constructs (e.g. β-galactosidase or green fluorescent protein (GFP)) and viruses (e.g. hepatitis C), and a wide variety of human proteins. Of greatest relevance to immunotoxicology is the expression of human cytokines, cell surface markers and immunoglobulins. Mice transgenic for mutant reporter genes have application in genotoxicity, and mice transgenic for mutant oncogenes have application as shortterm replacements for 2-year cancer bioassays. In efforts to facilitate xenotransplantation, transgenic pigs have been created that express a small number of human genes. There have also been reports on a model of colitis in rats expressing a human major histocompatibility antigen (HLA)-B27. Function Expression, however, is not sufficient for the transgenic strain to have preclinical relevance. The transgene gene product (i.e. the protein) must be functionally active in the transgenic animal. In the case of immunotoxicology, this will generally require that the human protein binds, and will result in signal transduction in its respective murine receptor (e.g. CCR and FcR or binding proteins such as major histocompatability complex (MHC) class II). Genetic Background Two strains of inbred mice are widely used for creating transgenic mice; 129 and C57 black. Once a transgenic strain has been created however, it may be possible to “move” the transgene into an alternate genetic background (e.g. BALB/c, by selective breeding. This can be of critical importance in application of transgenic mice in immuntoxicology where the genetic background of the mice can have a significant impact on the experiment (e.g. delayed-type hypersensitivity, transplantation, immunogenicity and host defense against infection or neoplasia). Fecundity Fecundity can also be an important factor in determin- ing the success of application of transgenic mice in toxicology. Many strains of transgenic mice show low fecundity as determined by fertility and number of offspring. As we must have sufficient numbers of animals for study, low fecundity can have a serious impact on our ability to conduct a given experiment. Simple animal husbandry (e.g. selection of proven breeders) may be sufficient to solve this issue. Maintaining the breeding colony as heterozygotes may be required. However, as the offspring of heterozygotes will be a mix of transgenic and nontransgenic, the offspring must be genotyped or phenotyped prior to enrolment in studies. Application of Transgenic Rodents in Immunotoxicology Transgenic mice have been used extensively for studying the immune system. As of June 2003 the National Institutes of Health Medline lists over 2800 papers describing the use of transgenic mice to study immunology. Obviously, there are far too many examples to list here. However, applications of direct relevance to immunotoxicology are much rarer. Some selected examples are listed in Table 1. Relevance to Humans As with any animal system, the relevance of transgenic animals to humans is somewhat limited. Some of the factors which lead to this limited relevance are listed in Table 2. One must also keep in mind that in most cases while the transgenic animal may be transgenic for one human protein (and therefore immunotolerant to that human protein) it will likely not be inherently tolerant to any administered human therapeutic protein. With these caveats in mind, a priori, transgenic mice should have a much relevance to humans as any murine system. Regulatory Environment Use of transgenic animals for demonstrating pharmacologic activity and safety are gaining increasing acceptance by regulatory authorities. They are specifically dealt with in the following guidance documents: * FDA Guidance for Industry. Clinical Development Programs for Drugs, Devices, and Biological Products for the Treatment of Rheumatoid Arthritis (RA) http://www.fda.gov/cder/guidance/1208fnl. pdf * FDA Guidance for Industry. Immunotoxicology Evaluation of Investigational New Drugs. http:// www.fda.gov/cder/guidance/4945fnl.doc * ICH Guidance for Industry. S1B Testing for Carcinogenicity of Pharmaceuticals http://www.fda.gov/ cder/guidance/1854fnl.pdf 3 664 Animal Models of Immunodeficiency Transgenic Animals 665 Transgenic Animals. Table 1 Examples of application of transgenic rodents in immunotoxicology (KI, knock-in; KO, knock-out) Transgenic system Application Reference Various cytokine KI and KO mice Drug hypersensitivity 3 TNF-α receptor KO Mechanism of toluene diisocyanate asthma 4 Human CD4 KI-murine CD General and immunotoxicity of a chimeric antihuman CD4 monoclonal 5 KO antibody Human CD4 KI-murine CD Embryo–fetal and immunotoxicologic development study of a chimeric 6 KO antihuman CD4 monoclonal antibody Human growth hormone KI rats Immunogenicity 7 Human interferon-α KI Breaking immune tolerance to interferon-α Human carcinoembryonic Safety of human carcinoembryonic antigen tumor vaccine antigen KI mice 8 9 Transgenic Animals. Table 2 Examples of sources of limitation of the relevance of transgenic mice to human immunotoxicity Physiology Kinetics Generally more rapid clearance of xenobiotics and therapeutic proteins in mice Metabolism Differences between murine and human P450 usage and inducibility, substrate specificity and metabolite profile Immunology Ontogeny Differences in timing of cytogenesis, histogenesis and organogenesis of the immune system Receptors Differences in binding affinity and signal transduction of human proteins for murine receptors and binding proteins Immunogenicity and tolerance Differences between human and murine antigen processing and MHC restrictions T cells Differences in T helper 1 and 2 usage and switching B cells Differences in immunoglobulin class switching Macrophages Differences in Fc receptor utilization References 1. Hofker MH, Van Deursen J (eds) (2002) Transgenic Mouse: Methods and Protocols. Methods Molecular Biology, Vol. 209. Humana Press, Clifton NJ 2. BioMed Net. http://www.biomednet.com/db/mkmd (accessed June 2003) 3. Moser R, Quesniaux V, Ryffel B (2001) Use of transgenic animals to investigate drug hypersensitivity. Toxicology 158:75–83 4. Matheson JM, Lemus R, Lange RW, Karol MH, Luster MI (2002) Role of tumor necrosis factor in toluene diisocyanate asthma. Am J Respir Cell Mol Biol 27:396– 405 5. Bugelski PJ, Herzyk DJ, Rehm S et al. (2000) Preclinical development of keliximab, a Primatized anti-CD4 monoclonal antibody, in human CD4 transgenic mice: characterization of the model and safety studies. Hum Exp Toxicol 19:230–243 6. Herzyk DJ, Bugelski PJ, Hart TK, Wier PJ (2002) Practical aspects of including functional endpoints in developmental toxicity studies. Case study: immune function in HuCD4 transgenic mice exposed to antiCD4 MAb in utero. Hum Exp Toxicol 21:507–512 7. Takahashi R, Ueda M (2001) The milk protein promoter is a useful tool for developing a rat with tolerance to a human protein. Transgenic Res 10:571–575 8. Braun A, Kwee L, Labow MA, Alsenz J (1997) Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon alpha (IFNalpha) in normal and transgenic mice. Pharm Res 14:1472–1478 9. Francini G, Scardino A, Kosmatopoulos K et al. (2002) High-affinity HLA-A(*)02.01 peptides from parathyroid hormone-related protein generate in vitro and in vivo antitumor CTL response without autoimmune side effects. J Immunol 169:4840–4849 T Transgenic Mouse Transgenic Mouse Transgenic mice are genetically engineered mice that over-express foreign DNA and are typically referred to as transgenic, while those in which foreign DNA has replaced an endogenous gene are termed gene targeted (or knockout). In the strict sense, however, both these procedures yield a transgenic mouse (i.e. one with added genetic material). Knockout, Genetic Trichinella spiralis A helminthic parasite, invading the gut mucosa and residing as larvae in striated muscle tissues. Host Resistance Assays 3 666 Triglycerides 3 Tricglycerides are molecules that consist of a glycerol backbone esterified to three fatty acids. Fatty Acids and the Immune System 3 Transglutaminase Trivalent Chromium The ionic form of chromium when three outer shell electrons (one from 4s and two from 3d orbitals) have been shed, thereby giving the atom an overall charge of +3. Chromium and the Immune System 3 The epidermal keratinocyte transglutaminase I is a calcium-dependent enzyme that plays a central role in keratinocyte cornification. It catalyzes the cross-linking between glutamine and lysine residues of isopeptides at the inner surface of keratinocyte cell membranes, which is an essential step for the stabilization of their cornified cell envelope (CCE). Three-Dimensional Human Skin/Epidermal Models and Organotypic Human and Murine Skin Explant Systems 3 Transition Element Elements that occupy the middle portions (the dblock) of the periodic table, have valence electrons in two or more shells instead of only one, and are characterized in most cases by variable oxidation states and magnetic properties. Chromium and the Immune System Vanadium and the Immune System Trypanosomes, Infection and Immunity Ronald Kaminsky Centre de Recherche Santé Animale Novartis CH-1566 St-Aubin Switzerland Synonyms hemoflagellates 3 3 Definition Transporter Associated with Antigen Processing (TAP) TAP is composed of two subunits, TAP1 and TAP2. This heterodimer, which belongs to the ABC (ATPbinding cassette) transporter family is responsible for the shuttling of peptides from the cytosol into the lumen of the endoplasmic reticulum. MHC Class I Antigen Presentation Trypanosomes are protozoan parasites of the family of Trypanosomatidae, belonging to the order of Kinetoplastida of the class of Zoomastigopohora. Three species are pathogenic to man—Trypanosoma brucei gambiense and T brucei rhodesiense cause African human sleeping sickness in sub-Saharan Africa, while T. cruzi causes Chagas disease in South America (Table 1). Characteristics Characteristics of the parasites The prominent morphological feature of the unicellular protozoan parasites is the kinetoplast, an organelle which contains about 15% of the cells DNA. The kinetoplast can be visualized by Giemsa staining or flu- 3 Trypanosomes, Infection and Immunity 667 Trypanosomes, Infection and Immunity. Table 1 Characteristics of human pathogenic trypanosomes Trypanosoma species Disease T brucei gambiense Sleeping sickness T. brucei rhodesiense T. cruzi Transmission Mode of transmission Animal reservoirs Geographic distribution Tsetse flies (Glossina spp.) Bite Mainly dogs, pigs, and certain game animals West and Central Africa Sleeping sickness Tsetse flies (Glossina spp.) Bite All major domestic animals and various game animals East Africa Chagas disease Reduviid bugs (Triatoma spp., Rhodnius spp., Panstrongylus spp.) Contamination by bug feces Southern and Domestic (dogs, cats, guinea-pigs), rodents and Central wild animals (opossums America etc.) vector orescent dies like DAPI. Movement of trypanosomes is via a flagellum which originates at the basal body near the kinetoplast and which is attached to the body of the parasite by an undulating membrane. The African trypanosomes are extracellular parasites (16–30 μm long) which move within the blood (hence their designation as hemoflagellates) or within the cerebral spinal fluid. T. cruzi occurs in man in both as extracellular and intracellular form. After introduction into the blood T. cruzi invades various cell types including macrophages and muscle cells. The intracellular form (3 μm in diameter) is much smaller than the extracellular form and does not posses a flagellum, but still contains the kinetoplast. All bloodstream forms of African trypanosomes are coated with variable surface glycoproteins (VSGs). The VSGs are anchored through a glycosyl phosphatidyl inositol lipid to the body of the parasite. These highly immunogenic VSGs have, at any one point of time, the same structure resulting in a specific variant antigen type (VAT). However, the VSGs are periodically removed and replaced with the result that the parasite population bearing one VSG are killed by an antibody response and are replaced by a new population with another variant antigen type. This antigenic variation is a mechanism that plays a key role in the escape of trypanosomes from total destruction by the immune response of their mammalian hosts (1). 'kissing' bugs, the family of Reduviidae (Table 1), not by direct inoculation when the vector is feeding but by contamination through parasites in feces. Tri- Trypanosomes, Infection and Immunity. Figure 1 Trypanosoma brucei brucei bloodstream forms. 3 T Cyclical transmission African trypanosomes are cyclically transmitted by tsetse flies (various Glossina species) (Table 1). After a fly has taken a blood meal from an infected host, the trypanosomes undergo various changes and multiplication within the fly. They finally mature in the salivary glands of the tsetse fly, to infectious metacyclic forms which are transmitted to a naive host. The American T. cruzi is transmitted cyclically by 3 Trypanosomes, Infection and Immunity. Figure 2 Trypanosoma cruzi: in vitro cultured amastigote forms in mammalian feeder cells. Trypanosomes, Infection and Immunity atoma infestans is the major transmitting species, but various others species including Triatoma spp., Rhodnius spp., and Panstrongylus spp. are capable of transmitting T. cruzi. Characteristics of the diseases Sleeping sickness is 100% fatal if left untreated. There are two disease stages for human African trypanosomiasis. A chancre, a primary lesion at the site of the bite, it is not observed frequently. The first stage, when trypanosomes are in the blood, is characterized by non-specific symptoms, such as fever, severe headache, joint or muscle aches. The second stage of the disease (also called late stage) starts with the invasion of the central nervous system by trypanosomes, which cross the blood-brain barrier 3– 6 month post infectionem. It is in the late stage of the disease that the characteristic symptoms of sleeping sickness occur, such as sleep disturbances, alteration of mental state, muscle tone disorders, abnormal movements, and sensory and coordination disorders, up to a final general apathy. The acute form of Chagas disease is characterized by general malaise with a variety of clinical manifestations. Symptoms can be very mild and atypical. At the site of entry of T. cruzi a local inflammation called a chagoma may develop; this is known as a Romana sign if it occurs at the eyelid. The acute form is followed by a period of an indeterminate form without any clinical symptoms. It is estimated that 20%–50% of persons with the indeterminate form of the infections will suffer from cardiac, digestive, or neurological damage 10–20 years after infection (2). 3 Preclinical Relevance African and American trypanosomes can be manipulated in vitro and in various animal models. T. brucei gambiense appears to be the most difficult species for laboratory work. In vitro assays and in vivo models are being used to identify new active compounds, nonvariant vaccine targets, and to monitor drug resistance. Some forms play an important role in host resistance models for immunotoxicity screenings. Relevance to Humans Infection Sixty Million people in 36 countries of sub-Saharan Africa live at risk of acquiring sleeping sickness. In 1999 around 45 000 cases were reported, but the number of people thought to have the disease at any one time is between 300 000 and 500 000. Chagas disease affects 16–18 million people, and about 100 million (25% of the population of Latin America) are at risk of acquiring Chagas disease. Due to the chronic character (indeterminate stage) of Chagas disease, transmission occurs not only via insect vectors, but also by congen- ital transmission, and from transfusions with contaminated blood, and organ transplantations. Immunity Due to antigenic variation of the African trypanosomes, which can express approximately 1000 different variant antigen types (1), immunity to the parasites develops only to specific VATs but does not provide protection against infection. Treatment Two drugs, pentamidine and suramin, are used in the first stage of sleeping sickness prior to CNS involvement. The first-line treatment for late-stage cases, when trypanosomes are established in the CNS, is the arsenic-based drug melarsoprol (3). The drug has been in use since 1949. However, up to 5% of treated patients may die because of lethal encephalopathy due to the drug. Recently a new treatment schedule (4) was designed, but the number of patients with encephalopathy syndromes was the same as before. Nevertheless, the new 10-day schedule is a useful alternative to the present standard 26-day treatment schedule. Eflornithine (DFMO) is used mainly as a back-up in instances of melarsoprol-refractory T. brucei gambiense. Its efficacy against East African sleeping sickness is limited due to an innate lack of susceptibility of T. brucei rhodesiense based on higher ornithine decarboxylase turnover. The unsatisfactory treatment situation for sleeping sickness is hampered further by the occurrence of melarsoprol-resistant trypanosomes (5) in several regions of sub-Saharan Africa. A molecular mechanism in the resistant isolates was identified: the majority of individual resistant isolates from geographically distant localities contained the same set of point mutations in their TbAT1 genes (6), which codes for an adenosine transporter (7). The drug of choice for treatment of Chagas disease is nifurtimox, with benznidazole as a back-up. However, these drugs are associated with side effects (2). Nifurtimox and benznidazoles were introduced at the beginning of the 1970s. Treatment success varies according to the phase of Chagas disease, the period of treatment, and the dose, the age, and geographical origin of the patients. Good results have been achieved in the acute phase, in recent chronic infection, and congenital infections. However, there is still controversy about their use in chronic cases (2). 3 668 Regulatory Environment At present there is only one new antitrypanosomal drug on clinical trial in Africa—the diamidine derivative DB289. Identification of novel compounds and their development to drugs is pursued by various private-public initiatives. Registration of new drugs Tumor-Associated Antigens TSK An acronym for tight skin which is associated with thickened skin and fibrosis due to mutations in the fibrillin gene. Systemic Autoimmunity 3 might be facilitated when these drugs are classified as orphan drugs. As mentioned above, trypanosomes are indirectly regulated by different immunotoxicology guidelines by the recommendation for infection models using these parasites in host-resistance assays. More detailed information is given in the relevant entries in this book. 669 References 3 Tsetse Fly Tsetse flies (Glossinidae; more than 30 species) are sub-Saharan bloodsucking flies (Diptera). The females do not lay eggs but give birth to living larvae. Both sexes feed on the blood of humans, livestock, and wild animals. Tsetse flies transmit human and animal pathogenic trypanosomes. Ingested trypanosomes of an infested host undergo a development cycle in the tsetse fly to mature to metacyclic forms which are infective for the next host. Trypanosomes, Infection and Immunity Tuberculin-Type Reaction A classical example of a delayed-type hypersensitivity (DTH) is the tuberculin-type reaction. In sensitized individuals, it is induced by an intradermal injection of tuberculin, an extract of Mycobacterium tubercolosis. This particular example of DTH was first described by R. Koch. He who observed that patients with tubercolosis reacted with fever and shock after the subcutaneous injection of tuberculin. Typically, the T cell mediated local immune reaction appears one or two days after the application. The tuberculin test, however, is not an allergic reaction. It is a diagnostic proof for the previous infection with M. tubercolosis and also other pathogens such as M. leprae or Leishmania tropica. Delayed-Type Hypersensitivity Tumor Antigen Any molecule leading to immune recognition of tumor. A generic term that encompasses tumor-specific antigens, antigens shared by normal and neoplastic cells, and specificities recognized by xenogeneic antibodies (e.g. human molecules bound by mouse monoclonal antibodies) that can be non-antigenic in the species of origin. Tumor, Immune Response to 3 α-Amino-β-indole-propionic acid; a component of proteins; it is chromogenic, producing a violet color with chlorine or bromine solution. Serotonin Mixture of antigens obtained from the culture of Mycobacterium tuberculosis. Mitogen-Stimulated Lymphocyte Response 3 Tryptophan Tuberculin 3 1. Borst P (2002) Antigenic variation and allelic exclusion. Cell 109:5–8 2. Coura JR, de Castro SL (2002) A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz 97:3–24 3. Legros D, Ollivier G, Gastellu-Etchegorry M et al. (2002) Treatment of human African trypanosomiasis—present situation and needs for research and development. Lancet Infect Dis 2:437–440 4. Burri C, Nkunku S, Merolle A, Smith T, Blum J, Brun R (2000) Efficacy of new, concise schedule for melarsoprol in treatment of sleeping sickness caused by Trypanonosoma brucei gambiense: a randomized trial. Lancet 355:1419–1425 5. Kaminsky R, Mäser P (2000) Drug resistance in African trypanosomes. Curr Opin Anti-infect Invest Drugs 2:76– 82 6. Matovu E, Geiser F, Schneider V et al. (2001) Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Molec Biochem Parasitol 117:71–81 7. Mäser P, Sütterlin C, Kralli A, Kaminsky R (1999) A nucleoside transporter from Trypansoma brucei involved in drug resistance. Science 285:242–244 Tumor-Associated Antigens Tumor-associated antigens (TAA) are tumor-specific proteins that can be recognized by immune effector cells of the host. To date, a variety of TAA are T 3 Tumor, Immune Response to known. These are derivatives of either (i) physiological self-antigens or tissue specific differentiation antigens that are dramatically overexpressed by tumor cells in comparison to other cells, (ii) mutated selfproteins or specific oncogenic antigens inappropriately expressed by tumor cells, or (iii) those derived from virally encoded antigens. The recognition pattern induced by TAA allows the immune system to distinguish the transformed neoplastic cells from surrounding normal tissue cells and triggers the immune cascade against them. Cancer and the Immune System 3 Tumor, Immune Response to Pier-Luigi Lollini Cancer Research Section Department of Experimental Pathalogy, University of Bologna Viale Filopanti 22 I-40126 Bologna Italy Synonyms Immune response to cancer, anti-tumor immunity. Definition The immune system of the host responds to tumor growth as it does to infectious agents, with specific (e.g. T cells and antibodies) and non-specific (e.g. natural killer cells and cytokines) effector and regulatory mechanisms. The immune response reduces the number of tumors arising in the host, but is no longer effective against established tumors. Tumor immunotherapy is the attempt to elicit a therapeutic immune response in cancer patients. Characteristics The immune response against tumors was formally demonstrated in the late 1940s and early 1950s using transplantable tumors induced with chemical carcinogens or retroviruses in inbred mice (1). The experiments showed that mice vaccinated with a given tumor reject a subsequent challenge with the same tumor (immune memory), but fail to reject an unrelated tumor (specificity). The immunization-challenge system was extensively used to characterize the effector and regulatory mechanisms of the immune response against tumors using two strategies: * cellular and molecular analysis of local and systemic components elicited by immunization and/or involved in rejection * use of mice with selective immune deficiencies of genetic origin (spontaneous mutation or genetically modified mice) or induced by exogenous treatments like monoclonal antibodies or drugs. Specific immune responses against tumors are mainly due to T cells. Cytotoxic T cells (CTL) expressing the CD8 surface molecule are the final effectors capable of tumor cell lysis. Helper T cells (Th) expressing CD4 play a fundamental positive or negative regulatory role. Tumor immunologists tend to downplay the importance of B cells, antibodies and complement because solid tumors are resistant to complementmediated cytotoxicity (tumor cells express complement inhibitors like CD55 and CD59) and in immunization-challenge systems B cells can even favor tumor growth (“enhancement”). Most cells of the innate (also called natural or nonadaptive) immune system directly affect tumor growth, and are required for the generation of T cell immunity. Professional and non-professional phagocytes destroy tumor cells and generate antigenic material that is subsequently picked up by antigen-presenting cells (APC) like dendritic cells, that are indispensable to activate T cell responses. Natural killer (NK) cells can kill tumor cells in tissues and in the bloodstream, thus are important in the control of systemic metastatic spread. In the course of the immune response many cytokines released by various cell types have regulatory and effector activities. Interferons IFN-α, IFN-β, and IFN-γ and tumor necrosis factors TNF-α and TNF-β, in addition to their roles as internal mediators of the immune system, directly inhibit tumor cell proliferation, trigger apoptosis, and induce the secretion of anti-angiogenic chemokines like MIG and IP-10 (2). Immune Surveillance The immune surveillance hypothesis, originally proposed in the late 1950s, postulates that the immune system protects the host not only from infectious agents, but also from tumor onset (1). Two predictions can be derived from the theory: * tumors that grow despite the immune system have found a way to escape surveillance, thus must be poorly immunogenic * tumor incidence should be higher in immunodepressed than in immunocompetent individuals. 3 670 The low immunogenicity of spontaneous (as opposed to carcinogen-induced or viral-induced) tumors in mice was easily verified, and is also a property of human tumors. Demonstration of the second prediction has been more controversial, because the degree and duration of immunodepression in experimental systems and in human conditions is highly variable Tumor, Immune Response to 3 Tumor Antigens The search for tumor antigens in human tumors was conducted for many years by means of antisera and monoclonal antibodies obtained after immunization of rodents with human cells or tissues. This endeavor led to the discovery of a wealth of molecules expressed by human tumors that are recognized by xenogeneic antibodies. However some molecules detected by rodent antibodies display little or no antigenicity in the human species, or data on recognition by the human immune system are not available. Application of the term “tumor antigens” to molecules that are not recognized as such in the species of origin is inappropriate, whereas “tumor markers” is more appropriate. Even though the immunological import of tumor markers is dubious, they have a great clinical relevance in tumor diagnosis, prognosis, and follow-up. Some examples are lactate dehydrogenase (used to monitor treatment of testicular cancer, Ewing’s sarcoma and other human tumors), neuron-specific enolase (neuroblastoma and small cell lung cancer), and DU-PAN-2 (pancreatic carcinoma). To distinguish “true” tumor antigens, that can induce a specific immune response in the species of origin, leading to tumor rejection, the terms “tumor rejection antigens” or “tumor specific transplantation antigens” are sometimes used. Having clearly established the distinction between tumor markers and tumor antigens, here we will simply use the latter term. Molecular cloning of tumor antigens became possible in the 1980s thanks to technologies based on T cell recognition under syngeneic or autologous conditions. Identification of tumor antigens and measure of specific responses are currently based on T cell clones with helper or cytotoxic activity in vitro, identification of peptides bound to major histocompatibility complex (MHC) molecules on the surface of tumor cells, molecular cloning of T cell receptor (TCR) genes from tumor-infiltrating lymphocytes (TIL), soluble MHC tetramers produced in vitro and bound to synthetic peptides, and screening of DNA libraries with patient’s sera ( SEREX) (2). It can be noted that SEREX is antibody-based, however it makes use of high affinity human IgGs that derive from a Th-induced immunoglobulin class switch, thus SEREX can be viewed as a T-B hybrid technology. The main groups of tumor antigens are shown in Table 1 (3). One major fact is that most tumor antigens are not tumor specific. The protein expressed by tumor cells, and the antigenic peptides derived from it are identical to those of normal cells, thus leading to the conclusion that the immune response to tumors is actually an autoimmune response. Experimental and clinical proofs of the autoimmune nature of antitumor immune responses were obtained in melanoma-bearing individuals, who develop autoimmune vitiligo as a consequence of vaccination with tumor antigens (2). The autologous nature of many tumor antigens is one of the reasons why tumors are poorly immunogenic, suggesting that a break of immune tolerance is a prerequisite to an effective anti-tumor immune response. In a few cases immune tolerance does not operate, either because normal cells expressing the antigen are in immunologically privileged sites (e.g. cancer-testis antigens), or because the antigen is involved in a physiological network of immune responses (idiotypes of T and B cell neoplasms). The only truly tumor-specific antigens are those that derive from mutations of oncogenes (RAS, CDK4) or tumor suppressor genes (p53), from chimeric proteins encoded by chromosomal translocations (BCR-ABL), or from tumor-specific alternative splicing (MUC-1, possibly HER-2). Experimental evidence shows that, even when tumor antigens are not shared by normal cells and are tumor-specific, spontaneous immune responses are quite low and ineffective in the tumorbearing host. 3 and rarely complete. Only recently, with the advent of knockout mice, has it been clearly demonstrated that aging immunodepressed mice develop significantly more tumors than immunocompetent mice (1). Tumors arising in such immunodepressed mice are more immunogenic than tumors of immunocompetent mice, thus providing a further demonstration of the hypothesis. In long-term immunodepressed adult humans (e. g. transplant recipients or HIV-infected patients) the incidence of virus-induced tumors (such as Kaposi sarcoma or cervical carcinoma) is increased, but many other tumor types display an incidence similar to that of the immunocompetent population. 671 Low Immunogenicity of Tumors A complete understanding of the reasons why tumors are poorly immunogenic is of paramount importance to devise immunotherapeutic strategies to induce a protective response (1). Basically tumors are tolerated by the immune system because their antigenic profile is almost identical to that of normal cells. In addition, genetic instability of tumor cells generates a large array of phenotypes that can escape immune recognition using a variety of passive and active strategies. Down-regulation of antigen expression is an obvious alternative that has been incompletely investigated. The most common defect in human tumors (80%– 90% of all solid tumors) is a partial down-regulation of MHC class I molecules required for peptide binding and T cell recognition (4). Active strategies may include the induction of regulatory (i.e. suppressive) cells of myeloid (CD11b+/Gr1+) or lymphoid (CD4+/ CD25+) origin, the secretion of suppressive cytokines like TGF-β or IL-10, or the expression of pro-apopto- T 3 3 3 3 672 Tumor, Immune Response to Tumor, Immune Response to. Table 1 Examples of tumor antigens Tumor antigen group Examples* Cancer-testis antigens MAGE-A1–A12, B1–B4, C1, C2 BAGE GAGE-1–8 NY-ESO-1 Differentiation or lineage-specific tumor antigens gp100 Melan-A (MART-1) Prostate specific antigen (PSA) Tyrosinase Tyrosinase-related proteins (TRP) Shared tumor antigens Carcinoembryonic antigen (CEA) HER-2/neu MUC-1 Telomerase catalytic unit (TERT) Mutated antigens RAS β-catenin Cyclin-dependent kinase 4 (CDK4) MUM-1 p53 Fusion proteins BCR-ABL PML-RARα PAX3-FKHR SYT-SSX1/2 EWS-WT1, EWS-FLI1 * Complete listing is given in reference 3. 3 From Tumor Immunology to Immunotherapy Spontaneous immune responses are incapable of eradicating established tumors (spontaneous regression has been rarely described in human melanoma and renal cell carcinoma). Preclinical evidence demonstrates that the immune response, if properly activated, can cure tumors. Analogous conclusions can be drawn from some successful clinical approaches. A convincing clinical example is the ability of allogeneic T cell transplants to reduce the risk of leukemic relapse by 30%–40%, a phenomenon known as “graft versus leukemia” (GvL). The main strategies to induce a therapeutic immune response in human patients are based on the administration of preformed immunologic “drugs” ( passive immunotherapy) or of therapeutic vaccines ( active immunotherapy). Passive immunotherapy is currently the most successful way to target human tumors (2). A small number of monoclonal antibodies with significant activity against human tumors emerged from clinical trials and is approved for clinical use. The best known examples are trastuzumab (Herceptin), a humanized monoclonal an- tibody against HER-2 for breast cancer, and rituximab, a monoclonal antibody against CD20 for non-Hodgkin’s lymphoma (NHL). Several other monoclonal antibodies against similar, or different, target antigens are being developed. It is interesting to note that the therapeutic activity of monoclonal antibodies is only partly mediated by classical immune functions such as complement-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC). Therapeutic effect is also attributable to the activity of monoclonal antibodies as “receptor antagonists”, through the inhibition of receptor dimerization and signaling, and the induction of receptor internalization and degradation. Immunotherapy with cytokines (2) received a considerable attention throughout the 1980s and 1990s. The major clinical drawback has been the high toxicity of cytokines. Immune cytokines physiologically reach high local concentrations, but high systemic dosages are usually associated with severe toxicity. Toxicity hampered the clinical development of promising molecules such as IL-2, TNF-α, and IL-12. IFN-α is a good example of a cytokine that can be administered systemically at active dosages to cancer patients with tolerable toxicity. IFN-α initially showed therapeutic activity against hairy cell leukemia, and has signifi3 tic surface molecules (1). On top of all immune regulations, an expanding tumor could overcome the immune response by sheer cell kinetics. 3 3 Tumor, Immune Response to cantly prolonged survival in chronic myeloid leukemia (CML). It is also used for some solid tumors, such as melanoma, with a significantly lower activity than against hematologic malignancies. As previously noted for therapeutic monoclonal antibodies, IFN-α owes its anti-tumor activity to a combination of immune and non-immune effects. The latter include inhibition of tumor cell proliferation, induction of cell differentiation, and inhibition of neo-angiogenesis. Molecular definition of tumor antigens prompted a large number of vaccination trials based on a variety of immunological approaches to cancer vaccines (2). One possibility is to vaccinate with the DNA encoding a tumor antigen that is picked up and translated by host cells (DNA vaccination). Alternatively, vaccines are made of whole cells, recombinant proteins or synthetic peptides admixed with adjuvants. Dendritic cellbased vaccines exploit the pivotal role of antigen presentation in the generation of T cell responses. Dendritic cells cultured in vitro are fed (“pulsed”) with tumor antigens and then injected in vivo. Promising results were obtained in small phase I/II clinical trials, but definitive evidence of a marked clinical benefit from therapeutic cancer vaccines is still lacking. 3 Preclinical Relevance Study of the immune response to tumors was largely conducted in preclinical model systems. The results were mostly confirmed by human studies, when possible, thus it is generally assumed that preclinical evidence and features of the immune response to tumors apply to human and clinical situations. One area that requires caution is the toxicity of cytokines endowed with anti-tumor activity. Because of species specificity, human cytokines are inactive, or partially active in rodents, therefore mouse cytokines must be used for mouse studies. This situation is quite different from the development and testing of conventional anticancer drugs, in which the same molecule is used in preclinical and in clinical studies. Some cytokines like TNF-α that display a potent anti-tumor activity in mice are clinically useless because in humans the maximum tolerated dose is much lower than the effective dose (2). A major stumbling block for cancer vaccines, as well as for other forms of tumor immunotherapy, is the fact that most clinical trials recruit advanced patients that are heavily immunosuppressed and poorly responsive to vaccines, whereas preclinical data clearly show that the ideal use of vaccines would be for cancer prevention in healthy individuals at risk, or for adjuvant therapy against micrometastatic foci, rather than for therapy of bulky and advanced lesions (5). In conclusion it is conceivable that active immunotherapy will demonstrate its anti-tumor potential only when clinical stu- 673 dies will follow the path clearly marked by preclinical data. Relevance to Humans Cancer patients treated with monoclonal antibodies respond to therapy only if the tumor expresses high levels of the target antigen, thus demonstration of high antigen levels in tumor lesions is a prerequisite for therapy. Some indications are available for specific antigens (e.g. HER-2), for which clinical benefit of antibody therapy at intermediate antigen levels is dubious. Assessment of the anti-tumor immune response in cancer patients is not routinely performed outside clinical trials of immunotherapy. Most immune tests applied in patients receiving immunotherapy are not standardized, and in some instances are of questionable value. For example it is not clear if tests performed on peripheral blood lymphocytes (the easiest sampling route) correlate with the immune response at tumor sites. Correlation of positive or negative clinical results with the immune status of patients and with modifications of the immune response induced by immunotherapy is a major open issue (2). Regulatory Environment Preclinical data are required to design clinical trials, but analysis of human immune responses to tumors is confined to in vitro systems, thus there is no need for guidelines concerning animal testing. Within clinical trials, study of the immune response is highly dependent on treatment (e.g. type of vaccine or cytokines used) and in most instances there are no gold standards or guidelines pertinent to immune testing of cancer patients for what concerns antibody responses, cytokine release or T cell cytotoxicity against autologous or non-autologous tumor cells. Skin tests (cf. delayedtype hypersensitivity) are used to detect responses elicited by anti-tumor vaccines. Immunotherapy trials are being conducted with a wide range of approaches that span practically all classes of therapeutic agents and therapies. The range of adverse or unwanted effects that can affect patients is correspondingly wide. In addition to general toxicity of the therapeutic agent, and to the presence of contaminants in the preparation, a specific type of potential adverse effect of cancer immunotherapy is the induction of autoimmunity. In practice all treatments aimed at inducing an immune response against tumor antigens shared by normal cells involve an autoimmune response. It must be underlined that regulatory requirements for prophylactic vaccines to be administered to healthy individuals in the general population are quite different from those applying to cancer patients. In fact some relatively mild forms of autoimmunity induced by immunotherapy in cancer patients, such as vitiligo T Tumor Immunology * * * * * * * * European Agency for the Evaluation of Medicinal Products (EMEA) http://www.emea.eu.int European portal to the pharmaceutical regulatory sector (EudraPORTAL) http://www.eudra.org US Food and Drug Administration (FDA) http:// www.fda.gov FDA Center for Biologics Evaluation and Research (CBER) http://www.fda.gov/cber FDA Center for Drug Evaluation and Research (CDER) http://www.fda.gov/cder Japanese Ministry for Health, Labour and Welfare http://www.mhlw.go.jp Organization for Economic Co-operation and Development (OECD) http://www.oecd.org International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) http://www.ich. org References 1. Pardoll D (2003) Does the immune system see tumors as foreign or self? Ann Rev Immunol 21:807–839 2. Rosenberg SA (2000) Principles and practice of the biologic therapy of cancer. Lippincott Williams & Wilkins, Philadelphia 3. Renkvist N, Castelli C, Robbins PF, Parmiani G (2001) A listing of human tumor antigens recognized by T cells. Cancer Immunology Immunotherapy 50:3–15 4. Garrido F, Algarra I (2001) MHC antigens and tumor escape from immune surveillance. Adv Cancer Res 83:117–158 5. Lollini PL, Forni G (2002) Antitumor vaccines: is it possible to prevent a tumor? Cancer Immunol Immunother 51:409–416 Cancer and the Immune System Tumor-Infiltrating Lymphocytes Lymphocytes (usually T cells) isolated from tumor specimens. Tumor-infiltrating lymphocytes (TILs) can be cultured in vitro to analyze their functional and molecular features, and can be also injected in vivo for therapeutic purposes. Tumor, Immune Response to Tumor Necrosis Factor (TNF) TNF-α and TNF-β lymphotoxin are produced by macrophages and T lymphocytes. First described as cytotoxins for tumor cells, later as important cytokines for the inflammatory response, cooperation with other leukocytes, induction of fever and interference with fat metabolism; therefore TNF is also named cachectin. Cytokines 3 Regulatory Bodies and Agencies Tumor Immunology 3 in melanoma, are regarded as surrogate markers of anti-tumor immune response (2). In various instances a single therapeutic rationale can be implemented with different treatment modalities that depend on different regulatory environments. For example, to enhance tumor antigen recognition cytokines can be administered systemically to the patient (drug therapy), or tumor cells can be genetically modified to secrete the cytokine (gene therapy), or be fused with autologous or allogeneic dendritic cells (adoptive cell therapy). A complete listing of all the guidances, guidelines, and regulations pertaining to each and every immunotherapeutic approach goes beyond the scope of this article. The reader is referred to the web sites of the regulatory bodies for comprehensive listings and full text of documents. 3 674 Tumor Necrosis Factor-α Victor J Johnson Toxicology and Molecular Biology Branch National Institute for Occupational Safety and Health 1095 Willowdale Road Morgantown, WV 26505 USA Synonyms tumor necrosis factor-α, lymphotoxin, cachectin, TNF-α Definition Tumor necrosis factor-α (TNF-α) is a pleiotropic proinflammatory cytokine that mediates key roles in homeostasis, cell growth and proliferation, tissue damage, repair and chronic diseases. TNF-α production is induced by a plethora of stimuli including bacterial products, oxidative stress, other cytokines, and general tissue damage. As such, this cytokine has a central role in orchestrating many injury and disease states including immunotoxicity. Tumor Necrosis Factor-α Human TNF-α is synthesized as a 26-kDa pro cytokine destined for expression on the plasma membrane. Proteolytic processing by members of the matrix metalloproteinase family of enzymes results in the extracellular release of the mature soluble 17-kDa form of TNF-α. Both the membrane-bound and soluble forms are biologically active and play important roles in overlapping and distinct signaling processes. Signaling is achieved through ligation of two structurally distinct receptor subtypes, TNF-receptor 1 (TNF-R1) and TNF-R2. TNF-R1 is constitutively expressed on most nucleated cells whereas TNF-R2 has a more restricted expression, mainly on cells of the immune system and is inducible. A schematic of the major signaling pathways and molecular mediators is shown in Figure 1. Membrane and soluble TNF-α form trimers that induce the trimerization of the TNF- receptor upon binding. Activation of the receptor initiates the formation of unique signaling complexes that are distinct for each receptor subtype. A death-inducing signaling complex is formed at the intracellular domain of TNF-R1 involving the recruitment and binding of a number of accessory proteins, including TNF-R1-associated death-domain-containing factor (TRADD), Fas-associated death-domain-containing protein (FADD) and TNF-R-associated factor-2 (TRAF2). Binding is achieved through mutual death domains (DD) present on TNF-R1 and the accessory proteins, and the DD sequence is unique to the intracellular portion of TNF-R1. The resulting complex recruits 3 Molecular Characteristics and Mediators of Signaling 675 T Tumor Necrosis Factor-α. Figure 1 Death and survival pathways in tumor necrosis factor (TNF)-α signaling. TNF-α exerts its biological effects through ligation of two distinct receptors, TNF-R1 and TNF-R2. Activation of TNF-R1 results in the formation of a death-inducing signaling complex, consisting of TNF-R1 intracellular DD, TRADD, FADD and TRAF2. This complex recruits other intracellular signaling molecules that activate pathways culminating in cell death (caspase and JNK pathways) and cell survival (NFκB activation pathway). Additionally, activation of MAP kinases and NFκB can result in upregulation of genes involved in inflammation, including TNF-α itself. On the other hand, activation of TNF-R2 leads to the formation of a signaling complex via mutual TRAF domains. This complex is known to lead to NFκB activation and anti-apoptotic signaling. Increasing evidence suggests a role for TNF-R2 in apoptosis, possibly through potentiation of TNF-R1 pro-apoptotic signaling. Overall, these signaling pathways can contribute to immunotoxicity by directly inducing cell death and tissue damage, initiating and contributing to inflammation, and/or altering the proliferative capacity of cells and tissues. 676 Tumor Necrosis Factor-α other proteins with enzymatic activity culminating in the induction of several major signaling pathways, including the caspase pathway, mitogen-activated protein (MAP) kinase pathways and pathways that lead to nuclear factor κB (NFκB) activation. The cell death and tumor regression properties of TNF-α are attributed to TNF-R1-mediated activation of caspases and c-Jun NH2-terminal kinase (JNK). JNK activation has been shown to cleave the Bcl-2 interacting domain (Bid) resulting in jBid translocation to the mitochondria. This induces the release of Smac/DIABLO from the mitochondria, which then sequesters inhibitor of apoptosis (cIAP) proteins leading to FADD-induced activation of caspase 8. Caspase 8 activates effector caspases 3 and 7 leading to the cleavage of several intracellular proteins and apoptotic cell death. Apoptosis is not the predominant outcome in most cell types in vivo and can be prevented through the activation of the NFκB survival pathway. Activation of NFκB results from the coordinated action of receptor interacting protein (RIP), NFκB inducing kinase (NIK) and inhibitor of κB (IκB) kinases. The outcome is phosphorylation-dependent ubiquitination and degradation of IκB, which results in the release of NFκB into the cytoplasm which then translocates to the nucleus via a nuclear localization sequence. Heterodimers and homodimers of the NFκB/Rel family drive the transcription of many survival and inflammation genes containing NFκB response elements. Therefore, this pathway functions to prevent cell death in normal healthy cells and upregulates the production of proteins involved in inflammatory processes. The intracellular domain of TNF-R2 lacks the DD and instead has TRAF domains responsible for the recruitment of TRAF1, TRAF2, and TRAF3. This complex recruits cIAP and NIK both responsible for NFκBmediated anti-apoptotic signaling. However, several investigators have reported that TNF-R2 may be important in the regulation and potentiation of TNF-R1induced apoptosis. Several mechanisms have been proposed, including TNF-R2, acting as a high-affinity trap/ ligand passer and TNF-R2-induced upregulation of endogenous TNF-α production, both leading to autocrine and paracrine activation of TNF-R1mediated apoptosis. A dual role in cell survival and death has been shown such that in the absence of TNFR1 signaling, TNF-R2 promotes not only proliferation of naïve T lymphocytes but also apoptosis in activated CD8+ T lymphocytes. Additionally, the affinity of TNF-R2 for membrane-bound TNF-α is much greater than that for the soluble form (affinities are equal for TNF-R1), suggesting that TNF-R2 is important in direct cell to cell regulation and localized immune responses. TNF-R2 can also be shed from the plasma membrane resulting in soluble TNF-R and downregulation of TNF-α signaling. Relevance to Immunotoxicity TNF-α is produced by many cell types including immune cells (macrophages, monocytes, dendritic cells, T lymphocytes, B lymphocytes), endothelial cells, epithelial cells, and fibroblasts following activation by appropriate stimuli. Therefore, this cytokine can be envisioned to play a role in immunotoxicity in many target organs. Indeed, TNF-α, through its ability to influence inflammatory processes, has been demonstrated in response to immuntoxicants targeting the liver, kidney, lung, muscle, eye, skin and brain, to name a few sites. Gene knockout of TNF-α, its receptors, or neutralizing-antibody studies have been used to investigate the role of TNF-α in immunotoxicity. For example, mice deficient in TNF-R1 and TNF-R2 show reduced lung inflammation and cytokine changes in response to toluene diisocyanante, an occupational asthmogen. Removal of TNF- signaling almost completely abrogated inflammation and fibrosis in the liver following treatment with the known immunotoxicant, carbon tertrachloride. TNF-α plays an important role in the immunotoxicity of many mycotoxins such as vomitoxin and fumonisin B1. Kidney TNF-α levels increase in adriamycin-induced nephropathy and may play a direct role in the associated proteinuria. Immediate production of TNF-α in the skin is evident following exposure to agents that cause allergic and irritant contact dermatitis and is positively correlated with the inflammatory response. Significantly, mice deficient in TNF-Rs show reduced development of contact dermatitis. In addition to its role in chemical-mediated immunotoxicity, TNF-α also mediates critical events in the pathogenesis of iodiopathic diseases involving the immune system, including bacterial infection and sepsis, chronic inflammatory lung diseases, cancer, and autoimmunity. The vastness of TNF-α involvement in chemical and idiopathic immunotoxicity stems from its central role in many cytokine/chemokine networks. TNF-α signaling (see Figure 1) can modulate the expression of other important mediators of inflammation required for the recruitment and activation of effector cells (macrophages, lymphocytes, neutrophils, eosinophils) that can contribute to tissue injury. Therefore, enhanced synthesis and release of TNF-α following immunotoxicant exposure or during disease can initiate and exacerbate acute and chronic inflammation—known contributors to tissue injury, repair and remodeling (fibrosis). Relevance to Humans Examining the association between genetics and disease prevalence and disease severity provides valuable insight into the mechanisms of disease. As such, greater than 1000 scientific studies have been conducted investigating the association between polymorphisms 3 Type 1 or Type 2 T Cell Responses Tumor-Specific Antigen Antigen expressed by tumor cells, but not by normal cells. Truly specific tumor antigens are generated by oncogenic genetic lesions, such as mutations in oncogenes and tumor suppressor genes, or chromosomal rearrangements leading to the synthesis of fusion proteins. The idiotype of T and B cell receptor in lymphoid malignancies is considered a tumor-specific antigen (but there might be non-neoplastic clones sharing the same idiotype). Cancer-testis antigens are also considered tumor specific because male germ line cells (the only normal cell type sharing such antigens) lack MHC expression, thus cannot present the antigen to the immune system. Tumor, Immune Response to 3 Type I Error A decision error in which a true null hypothesis is incorrectly rejected. Statistics in Immunotoxicology 3 in the TNF family and diverse human diseases. Genetic variation in TNF-α has been associated with chemical toxicities and idiopathic diseases of the immune system and diseases with immune involvement. Examples include occupational lung diseases, such as silicosis and coal workers' pneumoconiosis, chemotherapy-induced pulmonary fibrosis, adverse drug reactions, response to hepatitis B vaccination, asthma, diabetes, and rheumatoid arthritis. The strong association with human disease has prompted research into potential therapies related to inhibition of TNF-α signaling. To date, several US FDA approved proteinbased injectable inhibitors that block TNF-TNF-R interactions have been used to successfully treat human disease, including rheumatoid arthritis and juvenile chronic arthritis. Ongoing clinical trials show promise for these therapeutics in other diseases like psoriasis, psoriatic arthritis, ankylosing spondylitis, and Crohn’s disease. Second-generation small-molecule inhibitors of TNF are now undergoing clinical trials and function through blocking specific mediators in the TNF signaling cascade. Caution must be exercised in the use of these treatments as side effects including potential exacerbation of congestive heart failure, activation of latent tuberculosis infection, development of antinuclear antibodies, and systemic lupus erythematosis have been reported. Nevertheless, TNF-α is a pinnacle cytokine in acute and chronic inflammatory disease and toxicity and represents a promising therapeutic target. 677 Type I Reactions According to Gell and Coombs IgE-Mediated Allergies 3 References Type I–IV Reactions Gell and Coombs described antibody and T cellmediated reactions with distinct clinical pathology and underlying pathomechanism. The type IV reactions can be subdivided in type IVa–IVd reactions, which reflect the involvement of distinct effector cells. Lymphocyte Transformation Test Hypersensitivity Reactions 3 3 1. Luster MI, Simeonova PP, Gallucci R, Matheson J (1999) Tumor necrosis factor alpha and toxicology. Crit Rev Toxicol 29:491–511 2. Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL (2003) Anti-TNF-α therapies: the next generation. Nature Rev Drug Discov 2:736–746 3. Gupta S (2002) A decision between life and death during TNF-α-induced signaling. J Clin Immunol 22:185–194 4. Chen G, Goeddel DV (2002) TNF-R1 signaling: a beautiful pathway. Science 31:1634–1635 5. Lui Z-G (2004) Adding facets to TNF signaling: the JNK angle. Mol Cell 12:795–796 6. Schook L, Laskin D (eds) (1994) Xenobiotics and Inflammation. Academic Press, San Diego CA Type 1 or Type 2 T Cell Responses Tumor Necrosis Factor ReceptorAssociated Factor-6 TRAF-6 induces multiple signals from TOLL-like receptors that sense infection. Interleukin-1β (IL-1β) Subset of T lymphocytes called T helper (Th) cells can respond to different stimuli by secreting different cytokine patterns. Two well characterized patterns are categorized as type 1 (Th1) and type 2 (Th2) responses. Type 1 responses promote inflammation and cell mediated immunity primarily through the production of IFN-γ. Type 2 responses promote allergies T 3 678 Type II Activation and antibody mediated responses primarily through production of IL-4 and IL-5. A type 1 response is antagonistic to a type 2 response and vice versa. Cytokine Assays Type II Error A decision error in which a false null hypothesis is not rejected. Statistics in Immunotoxicology 3 3 Type II Activation Type II Interferon 3 Macrophage Activation Interferon-γ 3