Current Challenges of affordability of Healthcare in Ireland
advertisement

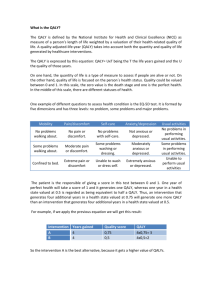
Current Challenges of affordability of Healthcare in Ireland Discuss the role of health economic evaluation in determining the therapeutic drugs, devices and treatment programmes that will be available in Ireland Economic evaluation and access to medicines in the Irish Healthcare setting. Michael Barry National Centre for Pharmacoeconomics November 2014 For discussion • HTA of pharmaceuticals in Ireland • Economic evaluation and cost-effectiveness threshold • Issues with negative HTAs and drivers of HTA recommendations • Recent challenges and opportunity cost • Affordability • Reference pricing • Medicines Management Programme When considering the pricing & reimbursement of a new medicine there are two questions Is it value for money ? When considering the pricing & reimbursement of a new medicine there are two questions Is it value for money ? & Can we afford it ? The NCPE conducts the health technology assessment (HTA) of pharmaceutical products for the Health Service Executive Recommendations on 192 medicines for 208 indications 3rd November 2014 Explicit cost-effectiveness analysis to inform decisions • • • • • • • Australia Canada Sweden The Netherlands United Kingdom Ireland New Zealand “New products need to be compared with existing treatments …generic measures of health outcome such as the quality adjusted life year (QALY) are useful as a common yardstick of health care benefit” Sculpher M. ISPOR Connections FRAMEWORK AGREEMENT BETWEEN THE IRISH PHARMACEUTICAL HEALTHCARE ASSOCIATION LTD AND THE DEPARTMENT OF HEALTH AND THE HEALTH SERVICE EXECUTIVE ON THE SUPPLY TERMS, CONDITIONS AND PRICES OF MEDICINES INTRODUCTION The Association and the Department of Health and the Health Service Executive have agreed on the terms set out below and this Agreement will come into effect on 1st November 2012. 4.3 Pharmacoeconomic Assessment prior to reimbursement “The HSE reserves the right to assess new and existing technologies (pharmaceuticals, diagnostics and devices) that may be high cost or have a significant budget impact on the Irish healthcare system, or to determine the cost-effectiveness of products from time to time.” www.ipha.ie The HTA process Rapid review to determine whether a full HTA is required The process begins with the price application by the manufacturer Full HTA with a 90 day time frame NCPE submission to the HSE – CPU. This report will be considered by the New Drugs Group Committee Number of products reviewed by the NCPE 2006 - 2014 Economic evaluations conducted in the Irish healthcare setting are usually in the form of CEA or CUA. Cost-effectiveness analysis (CEA) e.g. COST/LYG Cost-utility analysis (CUA) e.g. COST/QALY Cost-effectiveness threshold The line passing through the origin represents our ‘acceptable’ costeffectiveness ratio. That is our maximum (or threshold) willingness-to-pay for a unit of effect ( life year or QALY). Cost (€) Q4 Q1 Effect (QALY) Q3 Q2 The QALY threshold to be used in the HTA process is € 45,000 Information for the decision maker following pharmacoeconomic assessment The cost effectiveness in terms of the incremental costeffectiveness ratio (ICER) e.g. € 15,000/QALY The probability of cost-effectiveness e.g. 60% at the € 20,000/QALY threshold level Expected value of perfect information e.g. € 5,000,000 Products reviewed in 2014 • HTA not required = 24 (44%) • Reimbursement recommended = 5 (9%) • Full HTA required = 12 (22%) • Reimbursement not recommended = 13 (24%) 5 at the submitted price & 7 oncology products 1st November 2014 Modelling issues with negative HTAs • Incomplete modelling of the relevant clinical scenario (abiraterone) • Validity of the chosen health states (Sativex) • Uncertainty around the natural history of disease progression (teriflunomide, Sativex) • Time horizon (nab-paclitaxel, suboxone, regorafinib) • The choice of survival model used to extrapolate progression free survival (PFS) and overall survival (OS) beyond the trial period (brentuximab, regorafinib, trastuzumab emtansine, nab-paclitaxel) Modelling issues with negative HTA • Lack of comparative efficacy data for treatment arms (vismodegib, brentuximab) • Data synthesis, indirect treatment comparisons (enzalutamide, luxisenatide, canagliflozin) • Pricing issues (canagliflozin, regorafinib) • Utility values used in the modelling (nab-paclitaxel, Sativex, trastuzumab emtansine, regorafinib, suboxone, enzalutamide, abiraterone, vismodegib) Estimating revealed weights for a multi criteria decision analysis approach to Health Technology Assessments: A case study in Ireland The analysis confirms that recommendations for or against reimbursement of technologies are driven by the following: Cost-effectiveness (ICER) Quality of available evidence Safety & Tolerability Innovation Schmitz S. et al. 2013 Since September 2009 the cost-effectiveness of all medicines is considered prior to reimbursement under the Community Drugs Schemes Health Technology Assessment & the public Ipilimumab ‘Ippi’ “We believe the Company has failed to demonstrate the cost-effectiveness of ipilimumab for the treatment of advanced melanoma in adult patients who received prior therapy. We cannot recommend reimbursement at the submitted price”. Price: € 85,000/patient Budget impact: € 4,800,000 - € 7,400,000 per annum Δ median overall survival = 3.6 months Basecase ICER: € 147,899/QALY or € 92,443/LYG September 2011 The Ippi controversy ! Final ICER ~ € 116,000/QALY Opportunity cost ! Reimbursement of Ipilimumab (Yervoy®) – opportunity cost ! Original price – revised price: implications for the treatment of other patients with serious medical conditions such as hepatitis C & MS e.g. We could treat an additional 65 patients with Fingolimod (Gilenya) or We could treat an additional 60 patients with Telaprevir (Incivo) Recent challenges in reimbursement ‘new innovative medicines’ Cost effectiveness of crizotinib (Xalkori) for the treatment of adult patients with previously treated anaplastic lymphoma kinase (ALK) – positive advanced non-small cell lung cancer (NSCLC) € 6,242.00 Price: € 49,719/patient Budget impact: € 1,610,893 per annum Δ median progression free survival (PFS) = 4.7 months No evidence of an overall survival benefit ( 20.3 vs 22.8 months) Basecase ICER: € 165,616/QALY Probability that crizotinib is cost effective = 5% Cost effectiveness of crizotinib (Xalkori) for the treatment of adult patients with previously treated anaplastic lymphoma kinase (ALK) – positive advanced non-small cell lung cancer (NSCLC) “At its current price the NCPE cannot recommend crizotinib as a cost – effective treatment option. We do not consider the health benefit to be sufficient to justify the proposed price” NCPE 27th August 2013 (www.ncpe.ie) Innovation – definition ? “a new or existing medicine applied in a way which significantly improves healthcare at a price the HSE can afford” - does not have to be a new product - does have to significantly improve heath outcomes - does have to be affordable there must be added value Cost effectiveness of pertuzumab (Perjeta) in combination with trastuzumab and docetaxel in adults with HER2-positive metastatic or locally recurrent unresectable breast cancer who have not received previous anti-HER2 therapy or chemotherapy. € 2,761.65 Price: € 74,000/patient Budget impact: € 6,000,000 - € 9,800,000 per annum Δ median progression free survival (PFS) = 6.1 months An improvement in overall survival benefit has been seen Basecase ICER: € 203,028/QALY Probability that pertuzumab is cost effective = 2.5% Cost effectiveness of pertuzumab (Perjeta) in combination with trastuzumab and docetaxel in adults with HER2-positive metastatic or locally recurrent unresectable breast cancer who have not received previous anti-HER2 therapy or chemotherapy. “The NCPE believes that pertuzumab is not cost – effective at the submitted price and we cannot recommend reimbursement. A significant price reduction is required to ensure value for money” NCPE 28th August 2013 (www.ncpe.ie) Opportunity Cost ! € 203,028/QALY Pertuzumab BC ? Crizotinib NSCLC € 165,616/QALY Cabazitaxel PC € 110,032/QALY ? € 116,000/QALY Ipilimumab MM € 112,905/QALY Vemurafenib MM € 105,420/QALY Abiraterone PC Cost (€) € 16,023/QALY Telaprevir – Hep C € 11,411/QALY Boceprevir – Hep C Effect (QALY) Opportunity Cost ! € 203,028/QALY Pertuzumab BC ? Crizotinib NSCLC € 165,616/QALY Cabazitaxel PC € 110,032/QALY ? € 116,000/QALY Ipilimumab MM € 112,905/QALY Vemurafenib MM € 105,420/QALY Abiraterone PC Cost (€) € 16,023/QALY Telaprevir – Hep C € 11,411/QALY Boceprevir – Hep C Effect (QALY) Opportunity Cost ! € 203,028/QALY Pertuzumab BC ? Crizotinib NSCLC € 165,616/QALY Cabazitaxel PC € 110,032/QALY ? € 116,000/QALY Ipilimumab MM € 112,905/QALY Vemurafenib MM € 105,420/QALY Abiraterone PC Cost (€) What is the € and health outcome value of this ? Effect (QALY) € 16,023/QALY Telaprevir – Hep C € 11,411/QALY Boceprevir – Hep C Affordability ! Drug expenditure in Ireland The Community Drugs Schemes Ivacaftor for cystic fibrosis Price of CF drug may be health cuts elsewhere ‘About one-third of the entire budget for new drugs this year will go towards making new CF drug available’ nd Irish Times 2 February 2013 Affordability – treatment of Hepatitis C infection ? ‘Following recent developments in the treatment of Hepatitis C virus (HCV) infection we can predict that as early as 2015 sustained virologic response rates will exceed 90% for most HCV genotypes’ This effective ‘cure’ will be achieved through short, interferon free, fixed dose, single pill regimens with adverse effect profiles that are markedly better than those of past regimens Potential budget impact – anywhere between € 200,000,000 - € 700,000,000 What is being done to improve access to medicines ? Reference pricing • The HSE sets a price for the original branded product and its generics (phase 1 reference pricing) • Some 37 products have been deemed ‘interchangable’ and 22 products have been reference priced to date • Atorvastatin ( Lipitor ) was the first drug to be reference priced on 1st November 2013 • Esomeprazole ( Nexium ) followed on the 1st January 2014. Reference pricing and Statins Total expenditure on statins under the GMS scheme from Jan 12 - May 14 6,000,000 5,000,000 4,000,000 Simvastatin € 3,000,000 Atorvastatin reference priced on 1/11/2013 Pravastatin Fluvastatin Atorvastatin 2,000,000 Cerivastatin Rosuvastatin 1,000,000 € 7,087,354/month May-14 Apr-14 Mar-14 Feb-14 Jan-14 Dec-13 Nov-13 Oct-13 Sep-13 Aug-13 Jul-13 Jun-13 May-13 Apr-13 Mar-13 Feb-13 Jan-13 Dec-12 Nov-12 Oct-12 Sep-12 Aug-12 Jul-12 Jun-12 May-12 Apr-12 Mar-12 Feb-12 Jan-12 0 € 3,534,412/month Reference pricing and PPIs Esomeprazole reference priced 1/1/2014 € 6,551,397/month € 4,212,521/month The Medicines Management Programme (MMP) • Multi-disciplinary Team – including the National Medicines Information Centre (NMIC) and the National Centre for Pharmacoeconomics (NCPE) in collaboration with the HSE-Primary Care. • Aim - sustained national leadership relating to – Safe – Effective – Cost –effective prescribing Statins - SIMVASTATIN LANSOPRAZOLE ACE inhibitor - RAMIPRIL ARB - CANDESARTAN SSRI - CITALOPRAM SNRI - VENLAFAXINE Antimuscarinics - TOLTERODINE PPI - The Medicines Management Programme (MMP) HTA and the Medicines Management Programme • • • • • Pregabalin (Lyrica) Omalizumab (Xolair) Lignocaine 5% plasters (Versatis) Ezetimibe (Ezetrol) ?? Ezetimibe + Simvastatin (Inegy) ?? Affordability ! Delivering affordable cancer care in high-income countries “The cancer profession and industry should take responsibility and not accept a substandard evidence base and an ethos of very small benefit at whatever cost: rather, we need delivery of fair prices and real value from new technologies” Sullivan et al, Lancet Oncol 2011;12:933-980 Delivering affordable cancer care in high-income countries medical “The cancer profession and industry should take responsibility and not accept a substandard evidence base and an ethos of very small benefit at whatever cost: rather, we need delivery of fair prices and real value from new technologies” Sullivan et al, Lancet Oncol 2011;12:933-980 “fair prices and real value” Thank you NCPE www.ncpe.ie