Name _________________ Test 3 November 13, 2013
advertisement
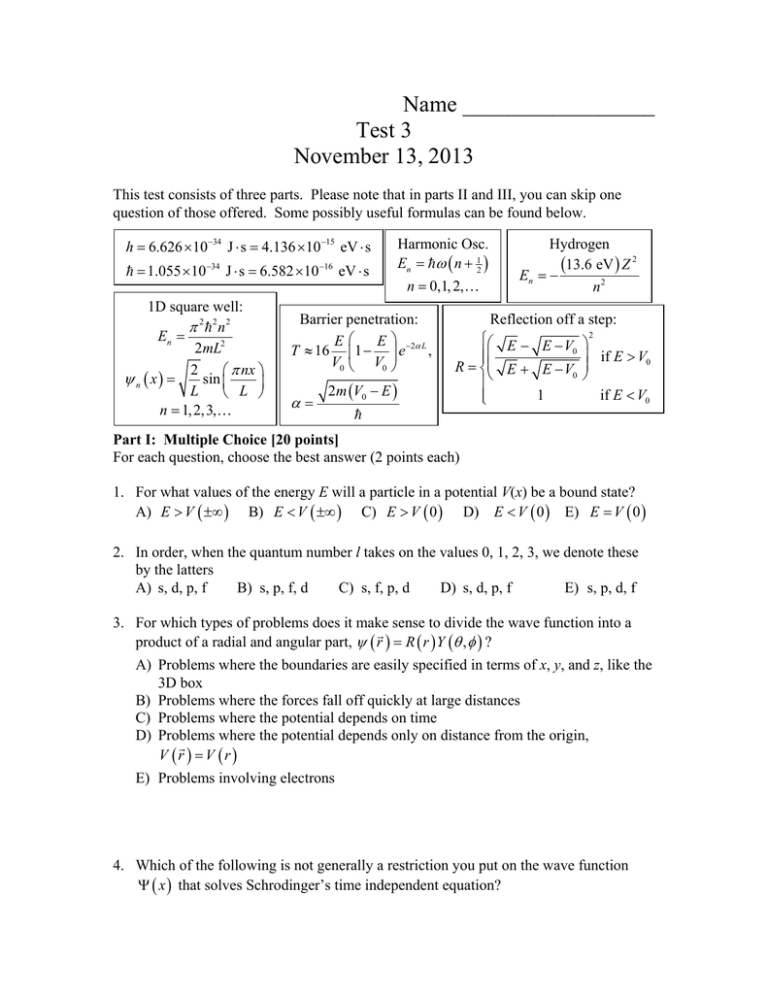
Name _________________ Test 3 November 13, 2013 This test consists of three parts. Please note that in parts II and III, you can skip one question of those offered. Some possibly useful formulas can be found below. h 6.626 1034 J s 4.136 1015 eV s 1.055 1034 J s 6.582 1016 eV s 1D square well: 2 2 n2 En 2mL2 2 nx sin n x L L n 1, 2,3, Harmonic Osc. En n 12 n 0,1, 2, Barrier penetration: E E T 16 1 e 2 L , V0 V0 2m V0 E Hydrogen 13.6 eV Z 2 En n2 Reflection off a step: E E V 0 R E E V0 1 2 if E V0 if E V0 Part I: Multiple Choice [20 points] For each question, choose the best answer (2 points each) 1. For what values of the energy E will a particle in a potential V(x) be a bound state? A) E V B) E V C) E V 0 D) E V 0 E) E V 0 2. In order, when the quantum number l takes on the values 0, 1, 2, 3, we denote these by the latters A) s, d, p, f B) s, p, f, d C) s, f, p, d D) s, d, p, f E) s, p, d, f 3. For which types of problems does it make sense to divide the wave function into a product of a radial and angular part, r R r Y , ? A) Problems where the boundaries are easily specified in terms of x, y, and z, like the 3D box B) Problems where the forces fall off quickly at large distances C) Problems where the potential depends on time D) Problems where the potential depends only on distance from the origin, V r V r E) Problems involving electrons 4. Which of the following is not generally a restriction you put on the wave function x that solves Schrodinger’s time independent equation? A) B) C) D) E) It must be normalized It must be continuous Its derivative must be continuous It must satisfy Schrodinger’s time independent equation It must be real 5. The solutions of the 1D infinite square well are given above as n x 2 L sin nx L . Why is that factor of 2 L there for? A) B) C) D) E) To normalize it correctly To make sure it vanishes at the boundaries x = 0 and x = L To make sure it satisfies Schrodinger’s equation To make the energy formula come out correctly To make it continuous at the boundaries 6. Suppose I have found a solution of Schrodinger’s time independent equation with energy E and wave function x . What would the solution x, t of Schrodinger’s time-dependent equation look like? D) x e i t E E) x e i t E A) x ei Et B) x e i Et C) x ei t E 7. If a single photon is passed through a half-silvered mirror, and two perfect detectors X and Y are then placed along both subsequent paths, what happens, according to the standard interpretation of quantum mechanics? A) The photon will always go to X, or always go to Y, depending on the exact position on the mirror it strikes B) The photon will go to X 50% of the time, and Y 50% of the time, but these are uncorrelated, so 25% of the time it goes to both C) The photon will go to X 50% of the time, and Y 50% of the time, but never both and never neither D) A half energy photon will go to each detector, every time E) A full energy photon will go to each detector, every time 8. Written in terms of the Hamiltonian H, Schrodinger’s time-dependent equation is simply A) i H t H t C) i H t D) i H t E) i H t B) i 9. In classical physics, you normally specify the initial conditions by stating a particle’s position and velocity. In addition to the initial wave function x, t 0 , what else must be specified in quantum mechanics? A) The initial position (only) B) The initial momentum (only) C) The initial position AND the initial momentum x, t 0 D) The time derivative of the wave function, E) Nothing; x, t 0 is sufficient 10. Which of the following is true about electrons if you have multiple electrons? A) There is one wave function for each electron B) Because of the repulsion between electrons, you can’t usually make more than one electron go into an atom C) Two electrons cannot go into the same quantum state D) You can only have one electron for each value of n in the atom E) Different electrons have different values of s, the total spin quantum number Part II: Short answer [20 points] Choose two of the following three questions and give a short answer (2-4 sentences) (10 points each). 11. Classically, if a particle with energy E reaches a barrier of height V0, it will continue through (100% of the time) if E > V0, and it will be reflected (100% of the time) if E < V0. What happens qualitatively quantum mechanically in these two cases? 12. When we first solved the infinite square well for a particle restricted to the region 0 x L , we initially tried functions like sin kx and cos kx , but eventually settled on sin kx and also restricted k. How did we narrow it down like this? 13. An electron in hydrogen is described by four numbers, n, l, m, and ms. Which of these does the radial function R(r) depend on? Which of these does the angular function (spherical harmonics) Y (,) depend on? Part III: Calculation: [60 points] Choose three of the following four questions and perform the indicated calculations (20 points each). 14. A harmonic oscillator has an electron in it. It is found that when the electron transitions from the n = 5 to the n = 4 state, a photon is emitted with energy E = 0.356 eV. (a) What is the angular frequency for this Harmonic oscillator? (b) What is the minimum energy (zero point energy) that this electron can have? (c) Visible light photons have energy ranging from about 1.65–3.26 eV. If this harmonic oscillator starts in the lowest energy state and absorbs one visible light photon, what are the possible states n that it could end up with (I want a list)? 15. A group of electrons with mass m 5.11105 eV / c 2 have kinetic energy E = 15.0 eV approach a barrier of height V0 = 20.0 eV and thickness L = 0.53 nm. (a) What is the probability that a given electron makes it through the barrier? (b) The thickness L is now increased to a new length, and now it is found that only 1.00×10–5 of the electrons make it through the barrier. What is L now? 16. A particle has wave function given by Nxe x a for x 0 , for x 0 . x 0 Some possibly useful integrals appear below. (a) What is the correct normalization factor N? (b) What are x and x 2 for this wave function? (c) What is the uncertainty x for this wave function? Useful integral: 0 x n e x dx 1 n n !, where n ! 1 2 3 n . 17. Sometimes it is useful to discuss the total angular momentum of an electron, which is the sum of the orbital angular momentum, and the spin angular momentum. For example, the z-component of the total angular momentum is given by J z Lz S z (a) Write a simple formula for the z-component of the total angular momentum Jz in terms of some combination of two of the standard quantum numbers, m, and ms. (b) Suppose that an electron in hydrogen has n = 5 and J z 52 . List all possible values for m and ms that would make this possible. (c) For each of the values m and ms that you found in part (b), work out explicitly all possible values of s and l, subject to the restrictions n = 5, and it is an electron. (d) For each of the values of s and l you found in part (c), work out the corresponding values of L2 and S 2 .






