Name _________________ Test 3 November 14, 2012
advertisement
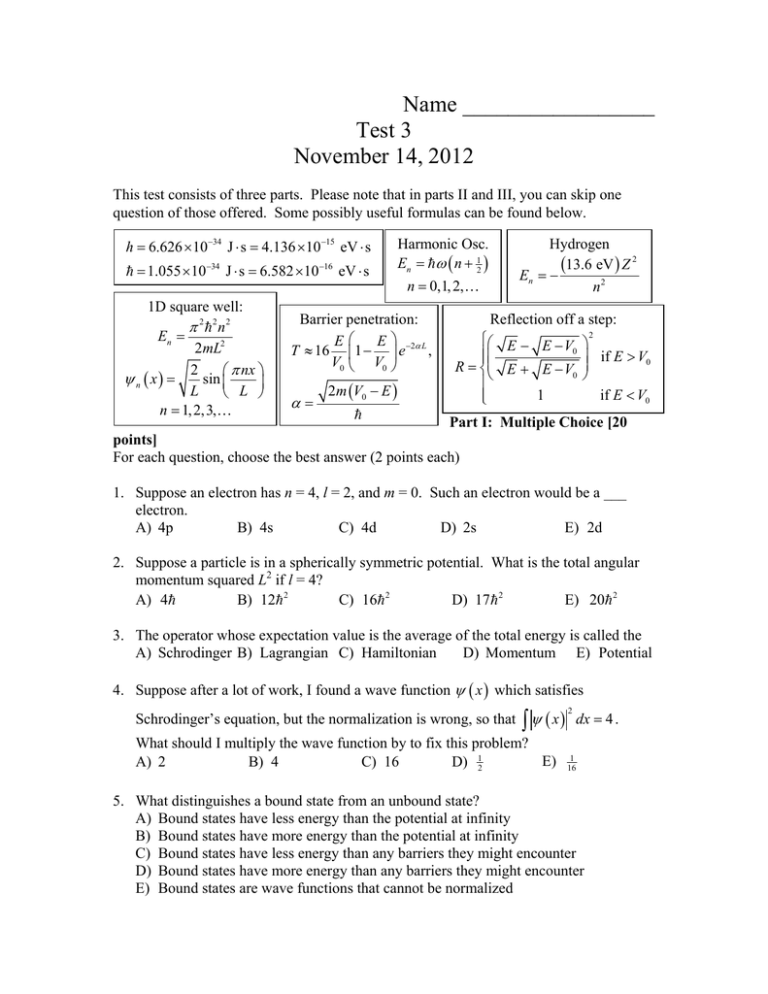
Name _________________ Test 3 November 14, 2012 This test consists of three parts. Please note that in parts II and III, you can skip one question of those offered. Some possibly useful formulas can be found below. h 6.626 1034 J s 4.136 1015 eV s 1.055 1034 J s 6.582 1016 eV s 1D square well: 2 2 n2 En 2mL2 2 nx sin n x L L n 1, 2,3, Harmonic Osc. En n 12 n 0,1, 2, Barrier penetration: E E T 16 1 e 2 L , V0 V0 2m V0 E Hydrogen 13.6 eV Z 2 En n2 Reflection off a step: E E V 0 R E E V0 1 2 if E V0 if E V0 Part I: Multiple Choice [20 points] For each question, choose the best answer (2 points each) 1. Suppose an electron has n = 4, l = 2, and m = 0. Such an electron would be a ___ electron. A) 4p B) 4s C) 4d D) 2s E) 2d 2. Suppose a particle is in a spherically symmetric potential. What is the total angular momentum squared L2 if l = 4? B) 12 2 C) 16 2 D) 17 2 E) 20 2 A) 4 3. The operator whose expectation value is the average of the total energy is called the A) Schrodinger B) Lagrangian C) Hamiltonian D) Momentum E) Potential 4. Suppose after a lot of work, I found a wave function x which satisfies Schrodinger’s equation, but the normalization is wrong, so that x dx 4 . 2 What should I multiply the wave function by to fix this problem? E) A) 2 B) 4 C) 16 D) 12 1 16 5. What distinguishes a bound state from an unbound state? A) Bound states have less energy than the potential at infinity B) Bound states have more energy than the potential at infinity C) Bound states have less energy than any barriers they might encounter D) Bound states have more energy than any barriers they might encounter E) Bound states are wave functions that cannot be normalized 6. In a complicated atom like Uranium, with 92 electrons, the reason you only get two electrons in the 1s state is because A) The Pauli Exclusion Principle says you can only have one particle per state, and there are only two states (spin up and spin down) in the 1s orbital B) The Pauli Exclusion Principle says you can only have two particles per state C) Electrical repulsion makes it unfavorable for more than two electrons to be in this state D) Putting multiple electrons into this small space means they have enormous momentum, according to the uncertainty principle E) The US constitution states that just as you can only have two Senators from each state, you can also only put two electrons in each quantum state. It wasn’t true before 1791 7. If I send photons one at a time through a half-silvered mirror, and then put ideal perfect detectors to see which way the photon went, what will the detectors see? A) Each detector will register half a photon each time B) Half the time one detector will see it, half the time the other will see it, but never both and never neither, and the results will be random C) Photons will alternate, one going one way and the next always going the other way D) Each detector sees the photon 50% of the time, so 25% of the time they will both see it and 25% of the time neither sees it. E) No photons will go either way, since quantum mechanical effects only happen if you do many photons at once 8. Which of the quantum numbers l, m, and ms for an electron in a hydrogen atom can sometimes be negative? A) All of them can be negative B) l and ms can be negative, but not m C) l and m can be negative, but not ms D) m and ms can be negative, but not l E) None of them can be negative 9. When we calculate the expectation value of the position x , what does it tell us? A) B) C) D) E) Where a particle will be found The average value of the position if you perform many measurements The most likely place to find the particle The uncertainty in the position The root-mean-square value of the position of the particle 10. The radial wave function R(r) for an electron in hydrogen depends on which quantum numbers? A) n only B) l only C) m only D) n and m E) n and l Part II: Short answer [20 points] Choose two of the following three questions and give a short answer (2-4 sentences) (10 points each). 11. When your professor was in high school, his chemistry teacher taught him that Schrodinger’s equation was E H , and he wondered why you couldn’t just simplify this to E H . Explain why this doesn’t make any sense. 12. A scanning tunneling microscope, to function, requires that electrons cross a gap between the tip of the microscope and some object that they are studying. How can the electrons cross that gap? 13. What is the electronic configuration for Cobalt, with Z = 27 electrons? Your answer should look like 1s2 2s2 … Part III: Calculation: [60 points] Choose three of the following four questions and perform the indicated calculations (20 points each). 14. A group of quantum mechanical congressmen with kinetic energy E = 400. J are approaching a fiscal cliff with potential V0 = –9,600 J. (a) Find the probability that a congressman is reflected from the fiscal cliff. (b) The potential V0 is now raised until it takes on a positive value, but less than E. It is nonetheless found that the same fraction of congressmen are reflected from the fiscal step. Find the new potential V0. 15. A proton of mass m 1.6726 1027 kg is trapped in a one-dimensional infinite square well of unknown size L . A physicist studying this discovers that he can make the electron jump from the ground state n = 1 to the state n = 4 when he bathes the system with microwaves with frequency f 6.13 1010 Hz 6.13 1010 s 1 . (a) What is the energy of one photon with this frequency? (b) What is the size L of the infinite square well? (c) The atom then transitions from n = 4 to n = 3. What is the energy of the resulting photon that is emitted? a 16. A particle has wave function given by 30 ax x 2 a 5/ 2 x 0 for 0 x a , otherwise . This wave function has already been properly normalized. It has expectation values x 12 a and x 2 72 a 2 . (a) Find the expectation values p and x a p 2 for this wave function. (b) What are the uncertainties x and p for this particle? (c) Does this wave function satisfy the uncertainty principle? 17. The wave function for the lowest energy (ground state) of the harmonic oscillator is 0 x 4 m x 2 m exp 2 (a) What is the probability density of the particle being at an arbitrary position x? (b) What is the probability density (in m-1 or nm-1) of an electron with mass m 1.6726 1027 kg being at the origin x = 0 if it is in a harmonic oscillator potential with angular frequency 1.50 1013 s 1 ? (c) If the wave function of a particle at t = 0 is given by x, t 0 0 x , what is x, t at other times?