Objective/Introduction ChE 4520/5520: Electrode Kinetics
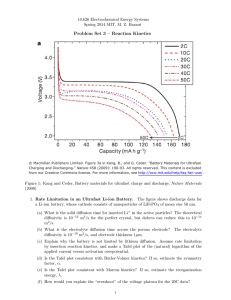
advertisement

ChE 4520/5520: Electrode Kinetics Gerardine G. Botte Objective/Introduction • In previous chapters we have calculated the thermodynamics feasibility of an electrochemical reaction • In this chapter we will learn how to evaluate the kinetics feasibility • We will learn about different models that are used to simulate electrochemical reaction rates • Electrochemical reactions rates offer advantages to chemical reactions – e.g., a 1 V change could represent a different of 10 order of magnitude in temperature – They can be controlled easier than chemical reactions by adjusting the potential difference • One of the disadvantages of electrochemical reactions is that their mechanisms are much more complicated than for chemical reactions • We will neglect any mass transfer limitations in this chapter (which means we will have plenty of reactants at the surface of the electrodes) 2 Outline • • Interface Role Electric Double Layer • Electrode Kinetics Models • Reference Electrodes – Helmholtz – Butler-Volmer – Tafel • Interface Role • Electric Double Layer – Helmholtz model • Electrode Kinetics Models – Butler-Volmer Equation – Tafel Equation • Reference Electrodes 3 1 Interface Role • • Interface Role Electric Double Layer • Electrode Kinetics Models • Reference Electrodes – Helmholtz – Butler-Volmer – Tafel • Electrode kinetics are governed by the potential difference across a thin (order 10 A) layer adjacent to the electrode surface • This layer is called the double-layer • Potential difference across the thin layer is about 0.1 V • Large magnitude of electric field (106 V/ cm) 4 Interface Role • • Interface Role Electric Double Layer • Electrode Kinetics Models • Reference Electrodes – Helmholtz – Butler-Volmer – Tafel • Large driving force for the electrode reaction • Because of the large electric field we will have charge separation in the double layer • Electroneutrality condition does not apply in the double layer region 5 Interface Role • • Interface Role Electric Double Layer • Electrode Kinetics Models – Helmholtz – Butler-Volmer – Tafel • Reference Electrodes • At equilibrium (thermodynamics relationships are used) there’s no current applied • When current is applied the potential will deviate from equilibrium • The difference between the potential and the equilibrium potential is called the overpotential (or surface overpotential) 6 2 Interface Role • The surface overpotential is given by: • • Interface Role Electric Double Layer ηs = φ − φ 0 – Helmholtz • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel Where: hs: surface overpotential, V f: potential due to the current, V f0: equilibrium potential, also called E or U (obtained from equilibrium relationships), V 7 Issues with Electrode Kinetics • • Interface Role Electric Double Layer • Electrode Kinetics Models • Reference Electrodes – Helmholtz – Butler-Volmer – Tafel • Electrode reactions are heterogeneous. This implies that a conductive surface must be in contact with the electrolyte • This arrangement produces a number of issues (we need a clean surface to evaluate pure kinetics): – Film formation – Changes in electrode microstructure – Electrolyte contamination – All of them cause variations in currentpotential measurements 8 Experimental Solutions • • Interface Role Electric Double Layer • Electrode Kinetics Models • Reference Electrodes – Helmholtz – Butler-Volmer – Tafel • Careful electrode surface preparation (e.g., polish the surface, in corrosion we need a rough surface instead) • Electrolyte purification (e.g., deoxygenation of the electrolyte) • Control of mass transport to the electrode surface (mixing) 9 3 Typical Kinetics Experimental Set-ups • • 1-reference electrode Interface Role Electric Double Layer 2-gas in 3-gas out – Helmholtz • 4-Luggin capillary Electrode Kinetics Models 5-platinum counter electrode – Butler-Volmer – Tafel • 6-rotating electrode Reference Electrodes 7-temperature probe 8-pH electrode 9-working electrode. ChE 455/555 10 Typical Kinetics Experimental Set-ups • • Interface Role Electric Double Layer • Electrode Kinetics Models • Reference Electrodes – Helmholtz – Butler-Volmer – Tafel • The electrode rotates to have control of the mass transfer limitations • The flow profile is known in this type of systems • Classical arrangements: – Rotating disk electrode (use for laminar flows) – Rotating cylinder electrode (use for turbulent flows) 11 More Issues with Electrode Kinetics • • Interface Role Electric Double Layer • Electrode Kinetics Models • Reference Electrodes – Helmholtz – Butler-Volmer – Tafel • Accurate measurement of the potential is difficult • Because we can’t measure an absolute value for the potential, we are forced to use a reference electrode • Reference electrodes are chosen based on: reversibility, stability, and convenience (cost) • Suitable placement of the reference electrode is another issue (need to correct for ohmic differences) 12 4 Electric Double Layer • • Interface Role Electric Double Layer • Electrode Kinetics Models • Reference Electrodes – Helmholtz – Butler-Volmer – Tafel • When we apply a potential to an electrode the charges that accumulate at the surface of the electrode attract opposite charges from the electrolyte • We expect to have a distribution of charges in order to balance the charges at the surface with the charges from the electrolyte 13 Electric Double Layer • • Interface Role Electric Double Layer • Electrode Kinetics Models – Helmholtz – Butler-Volmer – Tafel • Reference Electrodes • There are different models to determine the effect (or to simulate the effect) of the double layer: – Helmholtz model – Gouy and Chapman model – Stern model 14 Helmholtz Model • • Interface Role Electric Double Layer • Electrode Kinetics Models • Reference Electrodes – Helmholtz – Butler-Volmer – Tafel • It was developed in Electrode Electrolyte 1879 + S • It’s the simplest model S for double layer + S • Two parallel layers of S + S charges are separated S by solvent molecules S + • The distance (d) S S represents the outer + Helmholtz plane d • Fixed distribution of layer S: solvent (charges) 15 5 Helmholtz Model • • Interface Role Electric Double Layer • Electrode Kinetics Models • Reference Electrodes • Double layer model as a simple parallel plate capacitor CH = – Helmholtz – Butler-Volmer – Tafel Dε 0 d Eq. 1 C: capacitance per unit area (F/m2 or mF/cm2) D: dielectric constant (or relative permittivity) D: Separation between charges, cm e0: Permittivity of free space, 8.8542x10-14 F/cm 16 Helmholtz Model • • Interface Role Electric Double Layer • Electrode Kinetics Models • The potential distribution is a linear function between the two layers of charge – Helmholtz – Butler-Volmer – Tafel • Reference Electrodes φ= q C Eq. 2 q: charge • Because the distribution of the charges does not change the potential is constant, also C is constant 17 Helmholtz Model • • Interface Role Electric Double Layer • Electrode Kinetics Models • Reference Electrodes – Helmholtz – Butler-Volmer – Tafel • For water D≈10, d≈10 A, then: C≈ 10 (8.9 x10−14 F / cm ) 10−7 cm C ≈ 10µ F / cm2 ≈ 8.9 x10−6 F / cm 2 18 6 Helmholtz Model • Experimental measurements use a NaF solution in a mercury electrode • Mercury offers a uniform surface and NaF is not adsorbed • Capacitance in the right order of magnitude but it is not constant Fig. 5.2 Capacitance vs. potential relative to the point of zero charge for a NaF solution on • Only for high concentrations a mercury electrode at 25oC (experimental the capacitance tends to be data) constant 19 Gouy-Chapman Model • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • It was developed in 1910 • It’s analogous to the DebyeHückel theory Electrode + • The thickness of the double layer represents a compromise between electrical forces (tending to maintain the ordering) and thermal forces (tending to make the arrangement random) • No fixed charges • Significant deviation from electroneutrality occurs on the Debye length Electrolyte - + - + + - + l 20 Gouy-Chapman • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes • The capacitance is given by cG −C = ε ⎛ zFφ0 ⎞ cosh ⎜ ⎟ λ ⎝ 2 RT ⎠ Eq 3 – Butler-Volmer – Tafel 21 7 Gouy-Chapman Model Higher electrolyte concentration Lower electrolyte concentration Fig. 5.4 Capacitance vs. potential relative to the point of zero charge for a NaF solution, calculated using Gouy-Chapman model (Eq. 3) • It’s good at potentials near the zero charge region • At potentials more than 0.5 V in either direction the observed flattering of Fig. 5.2 Capacitance vs. the capacitance is potential relative to the not predicted point of zero charge for a NaF solution on a mercury electrode at 25oC (experimental data) 22 Stern Model • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Combines the Helmholtz model and the GouyChapman model Electrode + • Some of the charge is fixed (d region) and some of the charge is diffuse or spread out • The total length of the boundary layer is given by the fixed region plus the diffuse region Electrolyte S + S + S + S + S - S S - S S - d S: solvent 23 Stern Model • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • Because the capacitances are in series the capacitance of the double layer is given by: 1 1 1 = + Eq. 4 CS CH CG −C 24 8 Stern Model • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • • The smallest capacitance is the one that governs the behavior of the system: – If CH>>CG-C then CS≈CG-C – If CH<<CG-C then CS≈CH Eq. 5 Reference Electrodes 25 Stern Model Stern Model Experimental data Fig. 5.6 Capacitance of 0.001 M NaF vs. potential relative to the point of zero charge at 25oC. The experimental data (circles) agrees very well with the model (Stern Model, Eq. 4) • Assuming that CH is constant • The Stern model predicts the experimental data very well 26 Consequences of the DoubleLayer • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • Species outside the Helmholtz region are too distant to react • The driving force for the reaction is the potential drop across the Helmholtz region rather than the potential drop across the whole double layer 27 9 Consequences of the DoubleLayer • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Concentration at the bulk is different to the concentration at the surface of the electrode • When we study kinetics we need only the intrinsic effect of kinetics (need to eliminate the effect of the double layer) – Add a non-reacting supporting electrolyte to the solution – This increases the CG-C, then the overall capacitance is approximated by the CH 28 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • In ordinary kinetics we express the progress of a reaction by plotting the reaction coordinate vs. the energy of the species (assuming transition state theory) Ea 29 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Let us consider one elementary step electrochemical reaction: k c O+ + e− R k a Eq. 6 Where O+: oxidized species R: reduced species kc: cathodic reaction rate constant ka: anodic reaction rate constant 30 10 Electrode Kinetics • A more negative potential (more positive energy) tends to promote reduction • At progressively more negative potential, the energy of the oxidized species is increased • f3: reduction is favored • f1: oxidation is favored • f2: equilibrium potential, no net reaction takes place Eac2 Eac1 31 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • Consider the case where we start an experiment at the potential f1 and we reduce it to f2 • The activation energy for the first process (Eac1) is higher than for the second process (Eac2) 32 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • We can express the activation energy for the second process as a function of the first process by: Gc 2 = Gc1 + β nF (φ2 − φ1 ) Eq. 7 • Where – b is the symmetry factor (transfer coefficient) represents the fraction of energy that has been used to reduce the activation energy of the reaction 33 11 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Similarly the activation energy for the anodic process (which increases) can be expressed by: Ga 2 = Ga1 − (1 − β ) nF (φ2 − φ1 ) Eq. 8 n: is the number of electrons transferred in the reaction. For elementary steps n is most of the time 1, it is unusual to have more than 1 electron involved in an elementary step 34 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman • The form of our kinetic expression is the same as that for chemical reactions (using an Arrhenius dependence of temperature): ⎛ −G ⎞ k = k ' exp ⎜ ⎟ ⎝ RT ⎠ – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes Eq. 9 Where k’: is the a constant, cm/s G: is the free energy of activation 35 Electrode Kinetics • • Interface Role Electric Double Layer • The rate of electrochemical reaction is directly proportional to the current density: – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes r= Where i ⎛ −G ⎞ = k 'c exp ⎜ ⎟ nF ⎝ RT ⎠ Eq. 10 r: reaction rate, mol/s cm2 i: current density, A/cm2 (the area is the electrode surface area) c: is the reactant concentration (mol/cm3) 36 12 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • For the general anodic reaction given in Eq.6 (first order reaction), we substitute Eq. 8 into Eq. 10 ra = ⎧ G − (1 − β ) nFφ ⎫ ia = ka' cR exp ⎨− a ⎬ nF RT ⎩ ⎭ We have assumed a reference potential, therefore the subscripts 1 and 2 have been dropped Eq. 11 37 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman • We can redefine the reaction constant including the activation energy at our reference potential: – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • ra = Reference Electrodes ⎧ (1 − β ) nFφ ⎫ ia = ka cR exp ⎨ ⎬ nF RT ⎩ ⎭ Eq. 12 38 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Similarly for the cathodic reaction: rc = ic ⎧ − β nFφ ⎫ = kc cO exp ⎨ ⎬ nF ⎩ RT ⎭ Eq. 13 • The net current density (i=ia-ic) is the difference between the anodic and cathodic current densities (Eq. 12-Eq. 13) r = ra − rc = ⎧ (1 − β ) nFφ ⎫ i ⎧ − β nFφ ⎫ = ka cR exp ⎨ ⎬ − kc cO exp ⎨ ⎬ nF RT ⎩ RT ⎭ ⎩ ⎭ Eq. 14 39 13 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • • At equilibrium the net current density is zero, but the rates of the anodic and cathodic reaction are not zero. The magnitude of both (ia and ic) are the same and this is called exchange current density (i0) Reference Electrodes 40 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • If we designate the equilibrium potential as f0 ⎧⎪ (1 − β ) nFφ 0 ⎪⎫ ⎧ −β nFφ 0 ⎫ i0 = ka cR exp ⎨ ⎬ = kc cO exp ⎨ ⎬ Eq. 15 nF RT ⎩ RT ⎭ ⎩⎪ ⎭⎪ • Taking the logarithm of Eq. 15 and rearranging: φ0 = RT ⎛ kc ⎞ RT ⎛ CR ⎞ ln ⎜ ⎟ − ln ⎜ ⎟ nF ⎝ ka ⎠ nF ⎝ CO ⎠ Eq. 16 41 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Substituting Eq. 16 into Eq. 14 and using the definition of overpotential: ⎧⎪ (1 − β ) nF ⎛ i RT kc RT CO ⎞ ⎪⎫ = ka cR exp ⎨ ln + ln ⎜η s + ⎟⎬ nF nF ka nF CR ⎠ ⎭⎪ ⎝ ⎩⎪ RT ⎧⎪ − β nF ⎛ RT kc RT CO − kc cO exp ⎨ ln + ln ⎜η s + nF ka nF CR ⎪⎩ RT ⎝ ⎞ ⎫⎪ ⎟⎬ ⎠ ⎪⎭ Eq. 17 42 14 Electrode Kinetics • Rearranging Eq. 17: • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes ⎡ ⎧ (1 − β ) nF ⎫ ⎧ −β nF ⎫⎤ i = nFkc1− β k a− β c1O− β c Rβ ⎢exp ⎨ ηs ⎬ − exp ⎨ ηs ⎬⎥ Eq. 18 ⎩ RT ⎭⎦⎥ ⎩ RT ⎭ ⎣⎢ • Eq. 18 is a general kinetics expression for the first order elementary step given in Eq. 6 • The concentration of the reactants are at the surface of the electrode • The cathodic and anodic kinetic constants can be evaluated at equilibrium from the exchange current density: kc = i0 i Where the superscript 0 represents and ka = 0 nFCO0ChE 455/555 nFCR0 equilibrium conditions 43 Electrode Kinetics • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes • Substituting the kinetic constants into Eq. 18 we obtain: 1− β ⎛c ⎞ i = i0 ⎜ O0 ⎟ ⎝ cO ⎠ ⎛ cR ⎞ ⎜ 0⎟ ⎝ cR ⎠ β ⎡ ⎧ (1 − β ) nF ⎫ ⎧ − β nF ⎫⎤ ηs ⎬ − exp ⎨ η s ⎬⎥ ⎢exp ⎨ ⎩ RT ⎭⎥⎦ ⎢⎣ ⎩ RT ⎭ – Butler-Volmer – Tafel Eq. 19 44 Butler-Volmer Equation • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • Redefining the transfer coefficients for the anodic and cathodic components as: α a = (1 − β ) n αc = β n • And assuming the concentration at the surface is equal to the concentration at the bulk which will be the case of equilibrium condition, then Eq. 19 becomes: ⎡ ⎧α F ⎫ ⎧ −α F ⎫⎤ i = i0 ⎢exp ⎨ a ηs ⎬ − exp ⎨ c η s ⎬⎥ ⎩ RT ⎭ ⎩ RT ⎭⎦ ⎣ Eq. 20 Eq. 20 is known as the Butler-Volmer Equation 45 15 Butler-Volmer Equation • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Three variables aa, ac, and i0 need to be determined to use Butler-Volmer Equation • Butler-Volmer equations gives a good representation of experimental data for many systems • The exchange current density is a strong function of temperature • When the exchange current density is very large, the reactions is said to be reversible 46 Butler-Volmer Equation • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • When two reactions take place simultaneously, on the same electrode surface, we can use the Butler-Volmer equation for each of them • We will have to determine the individual parameters for both reactions 47 Linear form of Butler-Volmer Equations • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • One of the disadvantages of the Butler Volmer equation is that the overpotential can’t be expressed implicitly • To confront this several approximations have been made – Small surface overpotential – Large surface overpotential 48 16 Linear form of Butler-Volmer Equations • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • When the overpotential is very small, the exponential term in Eq. 20 can be expanded using Maclaurin series, neglecting some of the terms in the series: i= i0 (α a + α c ) F ηs RT Eq. 21 49 Linear form of Butler-Volmer Equations • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • Eq. 21 is the linear form of the Butler-Volmer Equation • The current density is a function of only one parameter (i0 and the transfer coefficients can be defined as one constant) • It is used to model systems operating at low current densities • It’s often used when the overpotential is 10mV or less • If the current density does not vary widely (±30%), the linear expression can be used even in the high current density 50 Tafel Equation • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • If the overpotential is large and positive, the second term in Eq. 20 can be neglected: ⎛α F ⎞ i = i0 exp ⎜ a η s ⎟ ⎝ RT ⎠ Eq. 22 • If the overpotential is large and negative, the first term in Eq. 20 can be neglected: ⎛ α F ⎞ i = −i0 exp ⎜ − c η s ⎟ ⎝ RT ⎠ Eq. 23 51 17 Tafel Equation • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Eqs. 22 and 23 are known as Tafel equations • Taking the logarithm of Eq.22 and rearranging: Eq. 24 η s = B log i − A B= 2.303RT αa F A= 2.303RT log i0 αa F • The constant B is called the Tafel Slope • Use of the Tafel approximation depends on the error that can be tolerated • It is general used when the overpotential is at least 50 to 100 mV • The Tafel slope varies between 30 to 300 mV/decade 52 Tafel Equation • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • Values of the exchange current density and the transfer coefficient are obtained experimentally • Plot overpotential vs. log(i). The slope of the line will give the transfer coefficient, and the intercept will give the exchange current density 53 Example 1 • Solve problem 3 of chapter 5 in your text book 54 18 Example 2 • Solve problem 2 of chapter 5 in your text book 55 Reference Electrodes • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • So far we have learned how to estimate kinetic expressions as a function of the overpotential • We have also learned that the overpotential is given by: ηs = φ − φ 0 56 Reference Electrodes • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • When using the overpotential equation, we need to make sure that the potential that we measure is only due to the electrochemical reaction • One of the ways to accomplish that is by using reference electrodes • Before discussing more details about reference electrodes, we will present a discussion in the factors that affect the potential 57 19 Contributions to the Potential in Galvanic Cells • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • The maximum potential that we can measure in a galvanic cell is the equilibrium potential. – The equilibrium potential is only obtained when no current (or very small current) flows through the circuit • When a current flows through the circuit the potential measure will always be smaller than the equilibrium potential 58 Contributions to the Potential in Galvanic Cells • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes • The decrease in the potential of the cell is due to several limitations, and this is often called “Potential loss, Eloss” • Therefore, the potential of a cell is defined as: φ = E − Eloss – Butler-Volmer – Tafel Eq. 24 Where E: equilibrium potential F: potential of the cell 59 Contributions to the Potential in Galvanic Cells • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • The potential limitations include: – Surface overpotential limitations, due to kinetics limitations – Concentration overpotential, due to diffusion and convection limitations in the electrolyte (also known as liquid-junction potential) – Ohmic drop, due to the mobility limitations (ion interactions) – Solid diffusion limitations, due to diffusion limitations in porous electrodes, e.g., electrodes that the ones used in lithium ion batteries 60 20 Contributions to the Potential in Galvanic Cells • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Accounting for all the limitations the potential loss is given by: Eloss = Δφohm + ηs ,a + ηs ,c + ηcn + ηsd Eq. 25 Where: Δφohm : Ohmic drop ηs,a : Anodic surface overpotential ηs,c : Cathodic surface overpotential ηcn : Concentration overpotential ChE 455/555 ηsd : Solid diffusion overpotential 61 Contributions to the Potential in Electrolytic Cells • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • In an electrolytic cell the minimum potential that we need to apply for the reaction to take place is the equilibrium potential • Therefore the potential in an electrolytic cell is given by: φ = E + Eloss Eq. 26 • The potential loss is calculated using Eq. 25. 62 Reasons for Using Reference Electrodes • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • Measurement of the potential at equilibrium conditions is relatively easy. All we need to make sure is that the current that flows through the circuit is very small • Such determination depends on the use of a counter-electrode having a known reversible potential 63 21 Reasons for Using Reference Electrodes • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Under load the measurement of the potential respect to a reference electrode becomes more complicated (due to the Eloss). In addition we will have significant reactions at both electrodes • Then a simple two electrode approach is not longer satisfactory for making accurate measurements • A technique to overcome this problem is to use a third electrode into the electrolyte 64 Reasons for Using Reference Electrodes • • Interface Role Electric Double Layer V – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel Counter Reference Working electrode electrode electrode • The reference electrode should be place very close to the working electrode • In theory it should be placed just outside the electrical double layer • However, the electrical field of the electrode can affect the measurement of the overpotential • A rule of thumbs suggest to place the reference electrode at least 4 diameters (of the reference electrode) away from the working electrode 65 Reasons for Using Reference Electrodes • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • An approach to avoid the effect of the reference electrode field on the working electrode is to use a luggin capillary • Ohmic drop in the capillary tube is small because the current that flows through it is very small 66 22 Reasons for Using Reference Electrodes • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Sometimes a second reference electrode is added to measure the ohmic drop between two points in the solution. This approach is known as the four electrode arrangement • Another use of reference electrodes is to measure current distributions in a cell having a non uniform current distribution 67 Reasons for Using Reference Electrodes • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • Summarizing the reasons for using reference electrodes are: – Accurate measurement of surface overpotentials – Measurement of ohmic drops in solution – Measurement of current distribution 68 Types of Reference Electrodes • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • When choosing a reference electrode we have the following criteria – Reproducibility – Stability – Small temperature sensitivity • Sometimes a reference electrode of the same type as the working electrode is chosen, this is known as a pseudo reference electrode: – Avoids: • Contamination problems • Liquid junction potential problems – The problem with doing this is that sometimes the results are not reproducible and they are more difficult to generalize 69 23 Types of Reference Electrodes • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • Generally the reference electrode should be chosen that is reversible to one of the ions in solution. However, this is difficult to accomplish all the time • The practical approach is to choose a reference electrode that is standard built for some electrolyte conditions 70 Types of Reference Electrodes • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • Typical reference electrodes – Hydrogen electrode – Calomel electrode (SCE) – Mercury-Mercuric oxide electrode – Mercury-mercurous sulfate electrode – Silver-Silver Chloride electrode 71 Calomel Electrode • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • It is constructed by covering a pool of mercury with mercurous chloride (calomel) • Potassium chloride is the electrolyte for the following reaction: Hg2Cl2 + 2e− ⇔ 2Hg + 2Cl − 72 24 Calomel Electrode • The equilibrium potential is given by: • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes 2.303RT log aCl2 − 2F E 0 = 0.268 E = E0 − • Commercial electrodes are commonly prepared with three concentrations of KCl: 0.1 N, 1 N and saturated • The calomel electrode is best used in acid solutions • Reproducibility is about 2 mV 73 Ag/AgCl Electrode • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • Reference Electrodes • It is used when mercury contamination is not allowed • KCl is used as the electrolyte. The most common concentration is 4M • It is also used in acidic solutions • The reaction involved is: AgCl + e− ⇔ Ag + Cl − • The equilibrium potential is given by: E = E 0 − 0.059log aCl − 0 EChE =455/555 0.222 74 Mercury/Mercurous electrode • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • It is used in acid solution • When chloride contamination is not allowed • The reactions if given by: Hg2 SO4 + 2e− ⇔ 2Hg + SO4= 2.303RT log aSO= 4 2F 0 E = 0.615 ChE 455/555 E = E0 − 75 25 Mercury/Mercuric oxide electrode • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models – Butler-Volmer – Tafel • • It is used in basic solutions • It uses KOH as the electrolyte • The reaction is given by: HgO + H 2O + 2e− ⇔ Hg + 2OH − Reference Electrodes E = E 0 − 0.059log aOH − E 0 = 0.0986 76 Calculation of overpotentials • • Interface Role Electric Double Layer – Helmholtz – Gouy-Chapman – Stern • Electrode Kinetics Models • Reference Electrodes – Butler-Volmer – Tafel • For the calculation of overpotential we need to use Eq. ηs = φ − φ 0 • Because the applied potential is measured respect to a reference electrode, the Equilibrium potential for the reaction needs to be expressed respect to the reference electrode: φ 0 = Ew − Eref Eq. 27 Where: Ew: equilibrium potential of the working electrode Eref: equilibrium potential of the reference electrode 77 Example 3 • The deposition of nickel takes place in a typical sulfate bath that contains: NiSO4, H2O, NiCl2 and H3BO4 • Typical operating conditions are T=25oC, pH=5 and nickel concentration of 1 M • A SCE reports a measurement of -0.67 V. At this conditions the ESCE=0.22V vs. SHE • We know that hydrogen will be evolved as a parasitic reaction and we would like to estimate the current efficiency • We know that hydrogen generation and nickel deposition can be characterized by Tafel equation according to: ⎛ i ⎞ −5 ⎟ ⎝ 10 ⎠ i ⎛ ⎞ = −ChE 0.1log 455/555 ⎜ −4 ⎟ ⎝ 10 ⎠ η Ni = −0.06 log ⎜ ηH 2 with i in A/cm2 78 26 Summary • At the end of this chapter you must be able to: – Use and understand the different expression to express electrode kinetic rates – Calculate overpotentials – Correct surface overpotentials from other loss effects – Know the uses and applications of reference electrodes – Understand the effect of the double layer on the electrode kinetics (e.g., what do you do experimentally do reduce this effect?) 79 27