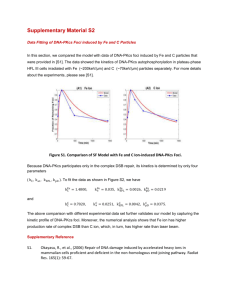
Tankyrase 1-Dependent pADPr Modification of DNA-PKcs is Essential
advertisement

22nd Annual NASA Space Radiation Investigators' Workshop (2011) 7037.pdf Tankyrase 1-Dependent pADPr Modification of DNA-PKcs is Essential for Holoenzyme Formation and Function R. C. Dregalla, D. G. Maranon, H. L. Liber and S. M. Bailey Department of Environmental & Radiological Health Sciences Colorado State University, Fort Collins, CO 80523-1618 Intrigued by the dynamics of the seemingly contradictory yet integrated cellular responses to the requisites of preserving telomere integrity while also efficiently repairing damaged DNA, we investigated roles of the telomere associated poly(adenosine diphosphate [ADP]‐ribose) polymerase (PARP) tankyrase 1 in both telomere function and the DNA damage response following exposure to ionizing radiation [1]. Tankyrase 1 siRNA knockdown in human cells significantly elevated recombination specifically within telomeres, hotspots for sister chromatid exchange (T‐SCE), a phenotype that quantitative modeling suggests has the potential of accelerating cellular senescence [2, 3]. We have also observed high T‐SCE rates in cells with deficiencies in WRN and BLM, the genes defective in Werner’s and Bloom’s syndromes, supporting a connection to premature aging [4]. Much more unexpected was the observation that depletion of tankyrase 1 resulted in concomitant and rapid reduction of the nonhomologous end‐joining (NHEJ) protein DNA dependent protein kinase catalytic subunit (DNA‐PKcs), while Ku86 and ATM protein levels remained unchanged; DNA‐PKcs mRNA levels were also unaffected. We demonstrated that depletion of tankyrase 1 resulted in proteasome‐mediated DNA‐PKcs degradation, thereby explaining the associated defective damage response observed; i.e., increased sensitivity to ionizing radiation‐induced cell killing, mutagenesis, chromosome aberration and telomere fusion, regardless of radiation quality. These studies were consistent with identification of DNA-PKcs amongst poly(ADP-ribose) protein complexes [5], and provided the first evidence for regulation of DNA-PKcs by tankyrase 1 PARP activity [1, 6]. However, any potential role of PARsylated DNA-PKcs in processes such as NHEJ remained to be elucidated. Here, we investigated the role of PARsylated DNA-PKcs in NHEJ-mediated double-strand break (DSB) repair in human cells. We found that tankyrase 1 PARP catalytic activity is required for recruitment to and function of DNA-PKcs at ionizing radiation-induced DSB sites, and so provide the first insights into the relevance of regulation of DNA-PKcs protein stability by pADPr modification. Our results demonstrate that inhibition of telomere-associated tankyrase 1 catalytic activity compromises DSB-repair kinetics as a consequence of abrogated DNA-PK holoenzyme assembly, and so provide new understanding into basic mechanisms of DNA repair following radiation injury. We propose a model in which the stepwise pADPr modification of DNA-PK subunits provides plausible explanation for the ‘DNA-dependent’ nature of the functional holoenzyme. The authors gratefully acknowledge support for this research from NASA (NNJ04HD83G and NNX08AB65G). References 1. Dregalla, R.C., et al., Regulatory roles of tankyrase 1 at telomeres and in DNA repair: suppression of T-SCE and stabilization of DNA-PKcs. Aging, 2010. 2(10): p. 691-708. 2. Blagoev, K.B., E.H. Goodwin, and S.M. Bailey, Telomere sister chromatid exchange and the process of aging. Aging, 2010. 2(10): p. 727-30. 3. Blagoev, K.B. and E.H. Goodwin, Telomere exchange and asymmetric segregation of chromosomes can account for the unlimited proliferative potential of ALT cell populations. DNA Repair (Amst), 2008. 7(2): p. 199-204. 4. Hagelstrom, R.T., et al., Hyper telomere recombination accelerates replicative senescence and may promote premature aging. Proc Natl Acad Sci U S A, 2010. 107(36): p. 15768-73. 5. Gagne, J.P., et al., Proteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexes. Nucleic Acids Res, 2008. 36(22): p. 6959-76. 6. Chang, S., The telomere protein tankyrase 1 regulates DNA damage responses at telomeres. Aging, 2010. 2(10): p. 639-42.