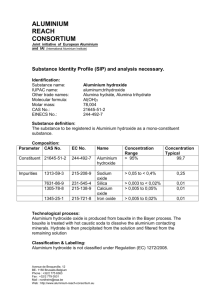
HIGH SPEED NICKEL PLATING ON DIFFICULT TO PLATE METAL (ALUMINIUM)
advertisement

PSZ 19:16 (Pind. 1/07) HIGH SPEED NICKEL PLATING ON DIFFICULT TO PLATE METAL (ALUMINIUM) NORZIANA BINTI LANI UNIVERSITI TEKNOLOGI MALAYSIA PSZ 19:16 (Pind. 1/07) HIGH SPEED NICKEL PLATING ON DIFFICULT TO PLATE METAL (ALUMINIUM) NORZIANA BINTI LANI A project report submitted in partial fulfilment of the requirements for the award of the degree of Master of Engineering (Materials) Faculty of Mechanical Engineering Universiti Teknologi Malaysia NOVEMBER 2010 iv For my beloved mother, father, family and all my friends v ACKNOWLEDGEMENT In the name of Allah, the Most Merciful and Most Beneficent It is with the deepest senses of gratitude I acknowledge that Almighty Allah gave me the strength and ability to complete this research work and write this thesis. I would like to express my sincere thanks to Dr. M. Sakhawat Hussain, my supervisor, for allowing me to use his award winning equipment for high speed plating and for all his guidance and encouragement that he provided in the course of this research work at Universiti Teknologi Malaysia, Skudai. His time, patience, consideration and also his creative ideas are greatly appreciated. I would also like to thanks all technicians for their help during my experiment. Their careful amendments and creative ideas are greatly appreciated. In addition, I would also like to thank to all my friends who sacrificed a lot of their time to help me with my research. Last but not least, I would like to thank my parents and my family for their love and encouragements. vi ABSTRACT Aluminium has been widely used in many field of application due to the low density, sensitive to corrosion, high mechanical strength and ease of fabrication. Plating of metal on aluminium is complex and difficult because aluminium always reacts with air to form oxide. It is difficult to obtain a good adhesive property on the aluminium surface. Thus, to plate metal on the aluminium, oxide layer must be eliminated by using an intermediate pre-treatment. However, this process involves several steps and even then the level of addition between the plated metal and the aluminium part is poor. The object of this project was to investigate the possibility of plating nickel directly on aluminium surface without any pre-treatment process, investigating the level adhesion between the deposited nickel and the aluminium base by high speed electroplating technique and also to investigate the effect of current density, temperature and different type of solution on weight of plated sample, thickness of plated sample and rate of deposition. The level adhesion of the nickel coating was determined qualitatively by using Adhesion Testing while morphology and thicknesses of Ni plated was studied using Scanning Electron Microscopy (SEM). It was found that the level of adhesion between nickel and aluminium became low at current density above 1.0 A/cm2 and Ni plated was found to peel off easily. Besides, by increasing the current density and temperature the weight and thickness of Ni plated increase and sulphate based Ni solution gave much higher rate of deposition compared to the traditional Watt’s based solution by increasing the current density and temperature. vii ABSTRAK Aluminium telah banyak digunakan dalam pelbagai bidang disebabkan sifat ketumpatannya yang rendah, kekuatan mekanik yang tinggi dan mudah untuk dibentuk. Penyaduran logam pada aluminium adalah sangat rumit dan sukar kerana aluminium .sentiasa bertindak balas dengan udara untuk membentuk oksida. Ini menyebabkan sukar untuk mendapatkan hasil lekatan yang baik pada permukaan aluminium. Oleh itu, untuk menyadur logam pada aluminium, lapisan oksida perlu dibuang dengan menggunakan pra-rawatan lanjutan. Namun begitu, proses ini melibatkan beberapa langkah dan tahap lekatan antara logam dan aluminium tidak begitu memuaskan. Objektif projek ini adalah untuk menyiasat kebolehan penyaduran nikel secara lansung pada permukaan aluminium tanpa melakukan proses pra-rawatan, menyiasat tahap lekatan antara nikel dan aluminium dengan menggunakan teknik “High Speed Electroplating” dan juga untuk menyiasat pengaruh ketumpatan arus, suhu dan jenis larutan pada berat, ketebalan, dan kadar penyaduran yang dihasilkan. Tahap lekatan saduran nikel ditentukan dengan menggunkan ujian perlekatan manakala morfologi dan ketebalan saduran nikel ditentukan dengan SEM. Didapati bahawa lekatan antara nikel dan aluminium menjadi rendah pada ketumpatan arus melebihi 1.0 A/cm2 dan saduran nikel yang dihasilkan mudah tertanggal. Selain itu, dengan pertambahan ketumpatan arus dan suhu, berat dan ketebalan saduran nickel juga bertambah. Larutan “Sulphate based Ni” memberikan kadar penyaduran yang tinggi berbanding dengan larutan “Watt’s” dengan pertambahan ketumpatan arus dan suhu. viii TABLE OF CONTENTS CHAPTER 1. 2. TITLE PAGE SUPERVISOR DECLARATION ii AUTHOR’S DECLARATION iii DEDICATION iv ACKNOWLEDGEMENT v ABSTRACT vi ABSTRAK vii TABLE OF CONTENTS viii LIST OF TABLES xii LIST OF FIGURES xiii LIST OF ABBREVIATIONS xvi LIST OF SYMBOLS xvii INTRODUCTION 1.1. Background of the project 1 1.2. Problem Statement 2 1.3. Objectives 3 1.4. Scope of the Project 3 1.5. Structure of Thesis 4 LITERATURE REVIEW 2.1. Overview of Electroplating 5 ix 2.2. Introduction of Aluminium 7 2.3. Problem of Plating on Aluminium 7 2.4. Nickel Electroplating 10 2.5. Type of Nickel Plating Solution 12 2.5.1. Watt’s Nickel Plating Solution 13 2.5.2. Nickel Sulphamate Solution 13 2.5.3. All Chloride Solution 14 2.5.4. Sulphate Chloride Solution 14 2.5.5. Fluoborate Solution 14 2.5.6. All-Sulphate Solution 15 2.5.7. Hard Nickel Solution 15 2.6. Direct Nickel Plating on Aluminium 15 2.7. High Speed Plating 18 2.8. Factor Affect the Rate of Electroplating 20 2.8.1. Surface Preparation 20 2.8.2. Temperature 20 2.8.3. Current Density 21 2.8.4. Electrolyte 21 2.8.5. Anodes 22 2.8.6. Current Efficiency 22 2.8.7. Filtration 22 2.8.8. Anti Pitting 23 2.8.9. Air Agitation 23 Introduction of Porous Anodic Aluminium 23 2.9. Oxide Film 2.10. Mechanism of Porous Anodic Aluminina 26 Formation 2.11. Disordered and Ordered of Pores of Anodic Alumina Oxide Film 29 x 3. 4. 5. METHODOLOGY 3.1. Experimental Design 32 3.2. Aluminium Sample Preparation 34 3.3. Solutions Preparation 34 3.4. Experiment Setup 35 3.5. Sample Preparation 37 3.6. Characterization 38 3.6.1. Morphology Analysis 38 3.6.2. Adhesion Testing 40 3.6.2.1. Adhesion Test by Knife 41 3.6.2.2. Adhesion Test by Tape 41 RESULTS AND DISCUSSION 4.1. Introduction 42 4.2. Nickel Plating Layer on Aluminium Sample 43 4.3. Adhesion Analysis 43 4.4. Morphology Analysis 44 4.5. Effect of Current Density 49 4.6. Effect of Temperature 51 4.7. Effect of Type of Solution 54 CONCLUSION 5.1. Conclusions 56 xi 6. RECOMMENDATION 6.1. REFERENCES Recommendations 57 58 xii LIST OF TABLES TABLE NO. TITLE PAGE 2.1 Electrode potential for pure elements 8 2.2 Electrode potential for second phase elements 9 2.3 Coefficient of thermal expansion for the range of 0 o to 10 100oc 2.4 Composition for various electroplating solutions 12 3.1 Composition of solutions preparation 35 3.2 Summary of sample preparation according current 37 density and temperature for Watt’s solution 3.3 Summary of sample preparation according current 38 density and temperature for sulphate based Ni solution 3.4 Preparation method (ASTM E3) 40 4.1 Weight of plated layer by using Watt’s solution 49 4.2 Weight of plated layer by using sulphate based Ni 49 solution 4.3 Coating thicknesses by using Watt’s solution 51 4.4 Coating thicknesses by using sulphate based Ni solution 52 4.5 Deposition rate at different type of solution. 55 xiii LIST OF FIGURES FIGURE NO. 2.1 Mechanism TITLE of electroplating PAGE and structure of 6 electroplated coatings 2.2 Schematic of the electrochemical plating of nickel 11 2.3 External potential versus limiting current density 19 2.4 The pore and hexagonal cell structure 24 2.5 The hexagonal arrays of porous oxide film 24 2.6 SEM micrographs of the bottom view of anodic alumina 26 layers. Anodization was conducted in (a) sulfuric acid, (b) oxalic acid and (c) phosphoric acid. The thicknessof the oxide film was approximately 120 µm 2.7 Porous anodic aluminium oxide film formation in term of 27 current and anodizing time 2.8 Schematic diagram of porous anodic alumina growth 29 2.9 The SEM images showing amorphous anodic aluminum 30 oxide layer produced in 10% phosphoric acid. (a) Plan view of the surface of the porous anodic aluminium oxide and (b) side profile of the aluminium with the porous aluminium oxide/ 2.10 SEM image of alumina pores array formed by two steps 31 anodic anodization. 3.1 Flowchart to conduct the electroplating process 33 3.2 Project specimen of aluminium rod 34 xiv 3.3 Schematic diagram of high speed plating equipment 36 3.4 High Speed Electroplating Equipment developed by 36 M.S.Hussain 3.5 Scanning Electron Microscope Equipment 39 3.6 Adhesion test by using knife 41 4.1 Nickel plating on aluminium sample 43 4.2 Nickel plated sample for (a) Good Ni adhesion and (b) 44 poor Ni adhesion on aluminium 4.3 Cross section nickel plated on aluminiun surface 45 morphology under 2000x Magnification. 4.4 The EDAX analysis for 55oC temperature and 0.3A/cm2 45 current density by Watt’s solution 4.5 Cross-section Ni plated on aluminium surface at 55oC for 46 (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. 4.6 Cross-section Ni plated on aluminium surface at 60oC for 46 (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. 4.7 Cross-section Ni plated on aluminium surface at 65oC for 47 (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. 4.8 Cross-section Ni plated on aluminium surface at 55oC for 47 (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. 4.9 Cross-section Ni plated on aluminium surface at 60oC for 48 (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. 4.10 Cross-section Ni plated on aluminium surface at 65oC for (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. 48 xv 4.11 Influence of current density on weight of Ni plated layer 50 by using Watt’s solution 4.12 Influence of current density on weight of Ni plated layer 50 by using sulphate based Ni solution 4.13 Influence of temperature on coating thickness by using 52 Watt’s solution 4.14 Influence of temperature on coating thickness by using 53 sulphate based Ni solution. 4.15 Influence of different type of solution on deposition rate 55 xvi LIST OF ABBREVIATIONS Al - Aluminium ASTM - American Society for Testing and Materials CuAl2 - Cuprum Alumina CuMgAl2 - Cuprum Magnesium Alumina EDAX - Energy Dispersive Spectroscopy FeAl3 - Ferum Alumina Mg - Magnesium Mg2Al3 - Magnesium II Alumina MgZn2 - Magnesium Zinc MnAl6 - Manganese Alumina Ni - Nickel NiAl3 - Nickel Alumina SEM - Scanning Electron Microscope Si - Silicon Zn - Zinc xvii LIST OF SYMBOLS aMZ+ - Bulk activity D - Diffusion of Coefficient of MZ+ e- - Electron iL - Limiting Current Density t - Transport number V - Electrode Potential δ - Thickness 1 CHAPTER 1 INTRODUCTION 1.1 Background of the Project The process for nickel plating on aluminum has advanced over the past decade from a rather complicated technology to a very consistent, easily reproducible procedure (George and Chadd, 2009). As a consequence of the continuous improvement in processing techniques, nickel plating is being relied upon more as a finish of choice by design engineers looking for lightweight, high strength materials in the manufacture of their products (George and Chadd, 2009; Mest, 2009). While aluminum has a good strength-to-weight ratio and excellent machinability, nickel plating can extend the ability of aluminum to function in applications where it could not be considered otherwise. Friction, wear and appearance are greatly enhanced by applying specific electroplating nickel coatings. For electronics applications, high phosphorous electroplating nickel can provide non-magnetic properties, corrosion resistance and extended wear. 2 The plating of metals on aluminium is a complex and highly developed (Satee and Schaumburg, 1980; Cooper et al., 1961; Bornhauser et al., 1932). For the most part, when it is desired to coated nickel on aluminium, the practice has been first to plate the aluminium with an intermediate layer of copper (Satee and Schaumburg, 1980). The nickel coating is then superimposed on the copper film, after which the object may be further processed by applying an outer chromium coating, if desired. In other processes the aluminium is first dipped in a cadmium plating bath, after which the article is electroplated or otherwise coated with such metal as copper, nickel, tin or any of other various metals as desired (Satee and Schaumburg, 1980; Cooper et al., 1961). In other processing techniques known as zincate process and the stannate process, a thin coating of zinc or tin is applied to the aluminium prior to the subsequent electrolytic deposition of other coating metals. But, these processes always require a copper or a bronze strike to prevent dissolution of the zinc or tin in the nickel bath (Kampert and Burell, 1976). This project relates to a process for depositing a film or coating of nickel directly on articles of aluminium without the need to preplate or otherwise to coat with an intermediate layer such as copper, zinc or cadmium. 1.2 Problem Statement Generally, electroplating method nickel on aluminium has been developed, but through this method involves several steps for plating nickel on aluminium by using an intermediate pre-treatment process and the deposition of a zincate layer (Miller, 1972). This is because oxide layer forms almost instantaneously on the aluminium surface. Because of the presence of oxide layer on aluminium surface, it is difficult to obtain a good adhesive property on the aluminium surface (Topelian and Newark, 1958; Kampert and Burell, 1976). Beside, the level addition between 3 the nickel plating and the aluminium part is poor (Frasch, 1941). Because of this problem the high speed plating method is chose to plate the nickel on aluminium. 1.3 Objectives The main objective of this research is to find out the possibility of nickel plating directly on aluminium surface without any pre-treatment process. The study also includes investigating the level adhesion between nickel and aluminium by high speed electroplating technique. The third objective is to investigate the effect of current density and temperature on weight and thickness of nickel plating layer. The fourth objective is to investigate the effect different type of solution on deposition rate of nickel plated. 1.4 Scope of the Project This project consists of three main tasks which include: 1. Deposition of nickel surface finish. The deposition of Ni will be conducted by high speed electroplating process on aluminium substrate. 2. The selection and determination of nickel plating parameters and also to design the experiment for high speed nickel electroplating process on aluminium. 3. Characterize the level of adhesion between nickel and aluminium and the thickness of Ni plated. 4 1.5 Structure of Thesis The thesis comprise of six chapters. The first chapter is the introduction, which clearly define the objective and scope of this project. Chapter two consists of literature review. In this chapter, it explains the problem of plating on aluminium, conventional plating process on aluminium, high speed electroplating process and lastly the porous anodic aluminium oxide film. Chapter three is the experimental methodology. In this chapter, a detailed account of materials, setup and procedures followed in the experimental work of this thesis are discussed thoroughly. Chapter four contains the results and discussion obtained from the experimental work conducted. Chapter five included the conclusion drawn based on the results and lastly, the final chapter, chapter six included the recommendations by the author for future work. 5 CHAPTER 2 LITERATURE REVIEW 2.1 Overview of Electroplating Electroplating is often also called "electrodeposition", and the two terms are used interchangeably. As a matter of fact, "electroplating" can be considered to occur by the process of electrodeposition. Electrodeposition is the process of producing a coating, usually metallic, on a surface by the action of electric current (Schlesingerl, 2002). If a metal object is placed in an aqueous solution of a salt of the same metal or a different metal and supplied with a strong cathodic electric potential, electroplating of the metal object may occur. Electroplating is caused by cathodic reactions leading to the reduction of metal from solution and evolution of hydrogen or oxygen. Typical examples of electroplating mostly involve aluminium or steel as the substrate and metals such as nickel chromium and cadmium as the plating metal. Electroplating is performed in large tanks of metal salt solution with an attached power supply and immersed metal (nickel) that functions as the anode. The range of metals that can be electroplated (Krishnam and Sanjeeb, 2007). 6 The formation of metal film on a substrate from metal ions in aqueous solution is a complex process that is still not fully understood. In most instances, this film formation process functions well without external intervention and is therefore usually overlooked. It is believed that electroplated coatings begin as small nodules of deposited metal, which then grow laterally to form a coating. The coating contains flaws at the boundary where the growth of one nodule joins the growth of another nodule. The principle of electroplating and structure of electroplated coatings are illustrated schematically in Figure 2.1 (Krishnam and Sanjeeb, 2007). (a) Electroplated coatings (b) Mechanism of coating formation Figure 2.1: Mechanism of electroplating and structure of electroplated coatings (Krishnam and Sanjeeb, 2007). 7 2.2 Introduction of Aluminium Aluminium has been widely used in many field of application, generally in electronic application and aerospace industries. There are three main properties which make aluminium an interesting base material which are low density of approximately 2.7g/cm3, sensitivities to the corrosion, high mechanical strength that is achieved by suitable alloying and heat treatments. Besides, it has other properties that are included in the aluminum such as high thermal and electrical conductance, high ductility, magnetic neutrality, high scrap-value and the non-poisonous and colourless nature of its corrosion products, which facilitates its use in the chemical and food processing industry (Luan et al., 2004). Aluminium in its pure state has a relatively high corrosion resistance and needs less protection than most metals. However, the commercial aluminium alloys, though mechanically more versatile are distinctly more sensitive to corrosion, and the development of high strength light alloys, containing quantities of heavy metal such nickel, copper or zinc has heightened the need for protective surface treatments. The nature of the heavy metal additions appreciably influences the alloy’s susceptibility to corrosion, and high mechanical strength and corrosion resistance have so far proved largely incompatible. The development of satisfactory protective finishes for these metals has been, therefore, of significant importance (Luan et al., 2004). 2.3 Problem of plating on Aluminium There are a number of difficulties to be considered when electroplating on aluminium. One of the major things to be considered when plating on aluminium is the condition of aluminium always react with the air to form oxide. It is difficult to 8 obtain a good adhesive property on the surface of aluminium due to the existence in the passive state of an oxide coating on the aluminium (Tsukamoto et al., 1978; Bornhauser et al., 1932; Kampert and Burell, 1976). It is important to note that aluminium is amphoteric i.e. aluminium can be dissolved in both acidic and alkaline solution. Due to the reactivity of aluminium unwanted ion-exchange processes are also likely to occur in the plating solution. All of these characteristics can be eliminated by using an intermediate pre-treatment layer deposited by zincate process (Tsukamoto et al., 1978; Moller, 1994; Satee and Schaumburg, 1980). According to Luan et al., (2004) two basic methods of pre-treatment have been developed which are the formation of a thin immersion coated layer of zinc, tin, iron, nickel and the treatment of a porous oxide film by chemical or anodic oxidation (Luan et al., 2004). Second problem to plate on aluminium is the electrode potential value of aluminium compared to some other metals. Its shows that difference between aluminium matrix and pure elements as well as second phase constituents can affect the deposition reaction. Table 2.1 and Table 2.2 show the electrode potential for pure elements and second phase elements. The position of aluminium in the electrochemical series can lead to the formation of immersion deposited in the plating solution. Because of this problem, some treatment should be taken when plating on aluminium to avoid alloying elements giving big electrode potential difference leading to unwanted electrochemical actions during the plating process (Moller, 1994a; Moller, 1994b). Table 2.1: Electrode Potential for pure elements (Moller, 1994a) Elements Electrode potential (V) Al (99.5%) -0.83 Al (99.5%) -0.85 Zn -1.10 Mg -1.73 9 Table 2.2: Electrode Potential for second phase elements (Moller, 1994a) Elements Electrode potential (V) Si -0.26 NiAl3 -0.52 FeAl3 -0.56 CuAl2 -0.73 MnAl6 -0.85 CuMgAl2 -1.00 MgZn2 -1.05 Mg2Al3 -1.24 The coefficient of thermal expansion of aluminium and its alloys differs from most of the metals commonly deposited. The coefficient of thermal expansion of aluminium gives the higher values compared to other metals. The consequence is that if the plated specimen is subjected to a temperature elevation the coating is mechanically stressed differently than the substrate and then can cause decohesion of the coating or form cracks through the coating. It frequently happens if the coating adhesion to the substrate or the pretreatment layer is poor decohesion of the coating can occur. Table 2.3 shows the coefficient of thermal expansion for the range of 0 o to 100oC (Moller, 1994a; Moller, 1994b). Plating on aluminium is a relatively expensive technique to apply in industry. However, plating on aluminium is an increasingly important technique in the industry because of the possibility to combining the low density of aluminium with the functional properties of the deposit. When plating on aluminium, the difference in atomic diameter and crystal lattice structure between the aluminium substrate and the metal deposited on it must be considered. The good results can be obtained with a suitable combination of metal and process. The achievable properties that can be added to aluminium are mechanical, magnetic, electrical, thermal, corrosive and decorative (Moller, 1994a; Moller, 1994b). 10 Table 2.3: Coefficient of thermal expansion for the range of 0 o to 100oC (Moller, 1994a) 2.4 Elements Coefficient of thermal expansion Chromium 7 x 10-5 / oC Steel 12 Nickel 13 Gold 14 Brass 18 Copper 18 Silver 19 Aluminium 24 Tin 27 Zinc 27 Cadmium 31 Nickel Electroplating Nickel coatings are most widely used for their high corrosion, coupled with an attractive visual appearance. Thus, taps and sanitary ware, automotive components and light fittings are but a few examples of this widely used coating. In electroforming, nickel coatings are widely used for their hardness, wear and corrosion-resistance (Nasser, 2004). Nickel electroplating is a process of nickel deposition on a part, immersed into an electrolyte solution and used as a cathode, when the nickel anode is dissolved into the electrolyte in the form of the nickel ions, travelling through the solution and depositing on the cathode surface (Dibari, 2000). Electrodeposited nickel can be strong adherent, ductile and resistant to corrosion, erosion and wear. Its mechanical properties can be varied at will between wide limits by changing plating conditions, by alloying with other elements, and by 11 incorporating particles and fibers within the electrodeposited nickel matrix (Liona, 2005). The process of nickel electroplating involves the dissolution of one electrode (anode) and the deposition of metallic nickel on the other electrode (cathode). The schematic of nickel electroplating cell is shown in Figure 2.2. Direct current is applied between the anode (positive) and the cathode (negative). When the power supply is turned on, the positive ions in the solution are attracted to the negatively biased cathode. The nickel ions that reach the cathode, gain electrons and are deposited on the surface of the cathode forming a layer. Simultaneously, another reaction that depends on the nickel solution used to plate, occurs at the anode, to produce ions and electrons for the power supply (Dibari, 2000). Figure 2.2: Schematic diagram showing the set-up of electrochemical plating of nickel (Liona, 2005) 12 2.5 Type of Nickel Plating Solution Various types of nickel plating solutions are used in depositing nickel these are Watts nickel plating solutions, nickel sulphamate solutions, all-chloride solutions, sulphate-chloride solutions and fluoborate solutions. Table 2.4 shows various electroplating solutions that are used in industry. Table 2.4: Composition for Various Electroplating Solutions Electrolyte Composition (oz/gal) Composition Nickel Sulphate, NiSO4.6H2O Watts 20 to 40 High All chloride Chloride Fluoborate Sulphamate 32 Nickel Fluoborate 45 to 60 Ni(SO3HN3)2 .4H2O Nickel Chloride NiCl2.6H2O Boric Acid H3BO3 6 to 12 12 32 4 to 6 4 to 5 4 0 to 3 4 4 to 6 Operating Conditions Temperature oF 90 to 160 100 to 160 100 to 145 90 to 160 90 to 140 10 to 60 10 to 60 50 to 100 50 to 100 5 to 260 Anodes Nickel Nickel Nickel Nickel Nickel pH 2 to 5.2 2 to 2.5 0.9 to 1.1 3.0 to 4.5 3.5 to 4.5 Cathode Current Density (asf) 13 2.5.1 Watts Nickel Plating Solutions The most common nickel plating bath is the sulphate bath known as the Watt’s bath. Watt’s solution was developed by Oliver P. Watts in 1916 (Kopeliovich, 2010; Graves, 2009). Plating operation in Watt’s solutions is low cost and simple (Kopeliovich, 2010). Watt’s bath contains three different solutions which are nickel sulphate, nickel chloride and boric acid. The large amount of nickel sulphate provides the necessary concentration of nickel ions. Nickel chloride improves anode corrosion and increases conductivity. Boric acid is used as a weak buffer to maintain pH. The Watt’s bath has four major advantages which are simple and easy to use, easily available in high purity grades and relatively inexpensive, less aggressive to plant equipment than nickel chloride solutions. The deposits plated from these solutions are less brittle and show lower internal stress than those plated from nickel chloride electrolytes (Graves, 2009). 2.5.2 Nickel Sulphamate Solutions Nickel sulphamate solution is used for electroforming and for producing functional nickel coating. Nickel coatings deposited nickel sulphamate baths possess lowest internal stress. High nickel concentrations of sulphamate electrolytes permit to conduct electroplating at high current densities (Kopeliovich, 2010). Nickel coatings from nickel sulphamate usually have very low stress values and high elongations. Another advantage is that it is possible to operate the sulphamate bath without difficulties related to anode dissolution at low chloride levels or even without chloride. The principle advantage of this bath is that it can be operated at nickel concentrations of 180-200 g/liter. This allows for the use of high current densities without losing the properties of the coating (Graves, 2009). 14 2.5.3 All-Chloride Solutions All-Chloride solutions operate at low voltage and permit deposition of thick coatings (Kopeliovich, 2010). The advantages of all-chloride solutions include the low voltage, good polishing characteristics, heavy coatings can be deposited, low pitting, improved cathode efficiency and no need to cool the plating solution (Graves, 2009). However, there are disadvantages to this bath. The main disadvantage of all-chloride baths is high internal stress of the coating (Kopeliovich, 2010). Another disadvantages include highly corrosive, nickel chloride is sometimes less pure than nickel sulphate and mechanical properties of the deposit are not as good as those from the Watts bath (Graves, 2009). 2.5.4 Sulphate-Chloride Solutions Sulphate-chloride solutions produce depositions with internal stress lower than the all-chloride solutions. Sulphate-chloride bath operate at voltages lower than Watts baths. This type of electrolyte permit deposition at high rates (high electric current) as compared to Watts bath (Kopeliovich, 2010). 2.5.5 Fluoborate solutions Fluoborate solutions permit high rate depositions due to higher (than in Watt’s solution) nickel concentration. Fluoborate solutions are mainly used for electroforming and for deposition of thick coatings (Kopeliovich, 2010). Anode dissolution in a nickel fluoborate bath not containing chloride is better than in a nickel sulphate solution with nickel chloride. The disadvantages of fluoborate baths include the high cost of chemicals and throwing power less than that of sulphate solutions (Graves, 2009). 15 2.5.6 All-Sulphate Solutions All-Sulphate solution are used mainly in applications where insoluble anodes are required (plating tubes and small fittings) (Kopeliovich, 2010). 2.5.7 Hard Nickel Solutions Hard nickel solutions are used in applications where high tensile strength and hardness are required (Kopeliovich, 2010). 2.6 Direct Nickel Plating on Aluminium Nickel plating on aluminium is one of the most versatile surface finish processes. Electroplated nickel is used extensively in many engineering applications, ranging from simple thin film for decorative purpose to corrosion and wear resistant coatings (Oliveira et al., 2006). The plating of nickel on aluminium is a complex and highly developed art (Satee and Schaumburg, 1980). Therefore, it has been considered to be extremely difficult on nickel plating to obtain a good adhesive property on the surface aluminium and aluminium alloys due to the existence in the passive state of an oxide coating on the aluminium (Tsukamoto et al., 1978). Therefore, many processes have been made prior to nickel plating on aluminium to overcome this problem. According to the Tsukamoto et al., (1978), the principal processes that currently used in industry can be generally classified into three types which are chemical plating process, anodized nickel coating process and molten metal plating 16 process (Tsukamoto et al., 1978). These three methods also known as indirect nickel plating. A chemical plating process is a process which first deposits a zinc layer upon an aluminium article by immersion, then deposit a copper on zinc layer by electrodeposition and firmly electroplated nickel on the copper layer (Asada, 1970). However, this process cannot withstand the severe condition of use due to the insufficient adhesive property between aluminium substrate and the plated metal (Tsukamoto et al., 1978). In addition, not all metal can be deposited onto zinc surface, thus special precaution are necessary because the zinc layer is very thin and any treatment which penetrates the zinc layer would attack the underlying aluminium and will result in a poor deposit (Topelian and Newark, 1958). Anodized nickel coating process is a process which first anodizes the aluminium article and then electroplates nickel on the coating produced by the anodization (Asada, 1970). This process provides usual adhesive property but have problem of quality limitation regarding the aluminium substrate as the chemical plating process. The third process is hot dipping process wherein a metal of low melting temperature is heated until molten and aluminium is immersed in the molten metal. This process give some advantages where the substrate aluminium would not be limited as to quality as in the chemical plating process and anodized process and also give a good adhesive property. However in this process, the large amount of heat source needed its inferior to the former two processes in producing an aluminium material in an annealed and soft condition and also presents a poor visual appearance (Tsukamoto et al., 1978). According to previous researchers all these three methods suffer from the disadvantages of involving several steps (Miller, 1972). According to Asada (1970) each method is complicated by necessity for repeated steps of etching by acids and alkalis of various kinds as well as rinsing in water. In addition, the selection of treating solution, including etching and washing and combination of treating process must be changed in accordance with the kind of base metal aluminium metal being plated (Asada, 1970). Another research found that, plating nickel on aluminium is a process reduction of the thin film of oxide on the metal, often include preliminary 17 coating such as copper, zinc or iron either by chemical transfer or electrolysis and the result in these method have not usually given satisfactory in the industrial application, since the alumina film is one more formed if the reduction is not instantaneously followed by the first chemical or electrolytic coating. Besides, the aluminium can be attacked by chlorides in which alumina will be dissolved and thus give unsatisfactory results owing to the absorption of chlorine compounds in the porosity of the metals, in which such chlorine will remain even after the subsequent electrolytical deposition. Although nickel plating is initially quite adherent, the absorbed chloride is liable to cause a separation of the nickel plating after a time, and especially when the temperature is raised (Frasch, 1941). All of these inconveniences in an indirect nickel plating processing were drawing the attention of researchers to make active investigation on direct nickel plating on aluminium. Direct nickel plating on aluminium is a process for the deposition of nickel directly on aluminium without the need to pre-plate or otherwise to precoat with an intermediate metal layer such as copper (Satee and Schaumburg, 1980). Based on previous research, direct nickel plating on aluminium offered some advantages compared to the indirect nickel plating such as the process is extremely simple wherein no equipment required other than what is ordinary used in carrying out conventional electrolytic process. The process is easy to prepare for plating by using conventional prewashing or pre-etching solution (Satee and Schaumburg, 1980). Plating of nickel directly onto the surface of aluminium metal would give a corrosion protection of the metal surface that provided by such a coating. A nickel coating also serves as an excellent substrate for receiving a coating of another metal which may be deposited by either electroless or electrolytic means (Miller, 1972). According to Satee and Schaumburg (1980) in direct nickel plating process, it is necessary only to rinse the pre-cleaned articles well with water prior to immersion in the treating composition which constitutes the preferred formulation. In addition, Satee and Schaumburg (1980) found that when nickel is only metal introduced into the plating tank, the plating bath is effectively protected from contamination. Other than that, a related advantages of a direct nickel plating process is that the need for a 18 strike bath process in indirect nickel plating can be eliminated, thus there being only nickel as the metal constituent in the plating system. By referring to the results obtained in the previous research, direct nickel plating give highly effective and produces products of excellent quality, with significant saving in material, time and manufacturing costs. This is because it is carried out in a series of simple steps (Satee and Schaumburg, 1980; Miller, 1972). 2.7 High Speed Plating The rate of electrodeposition, being governed as it is by Faraday’s law, depends directly upon the current density applied on the cathode, but this current density has a liminiting value above which acceptable plate is not obtained. The anion present in the nickel plating bath can affect this limiting current density, chloride and sulphamate ions being markedly beneficial in raising it. The use of nickel chloride solutions has been investigated by Wesley et al. amongst others, claims being made that, with a solution velocity of 0.38m/s, sound deposits could be obtained at 450 A/dm2. Sulphamate bath have been described in detail in many papers which Hammond had reviewed (Dennis and Such, 1993). The rate at which the electrolyte solution passes over the surface of the cathode has a considerable effect on the maximum current density at which satisfactory electrodeposits are obtained. This has been recognized for many years and was the reason for the introduction first of cathode movement, usually at the rate of about 0.1 m/s, and then air agitation, as means of providing solution movement over the cathode and thus reducing the thickness of the diffusion layer (Dennis and Such, 1993). 19 Current density increases as the applied external potential increases until the limiting current density is reached, as shown in Figure 2.3. Concentration polarization is largely responsible for this phenomenon. 𝒊𝑳 = 𝑫𝑴𝒁+ 𝒛 𝑭𝒂𝑴𝒁+ 𝒙 𝟏𝟎−𝟏 𝟏−𝒕 𝜹 where iL. the limiting current density, is in A/dm2, D is the diffusion coefficient of MZ+ in cm2/s and aMz+ its bulk activity in g ions/l, δ is the thickness of the diffusion layer in cm and t is transport number of MZ+. This equation shows the effect of D, a and δ on the limiting current density. Means of changing these terms in order to increase current density, however, it should be emphasized that the only way of obtaining a large increase in current density is to reduce δ, the thickness of the diffusion film. This is achieved by some means of agitation of the plating solution movement of the cathode. All methods of high speed plating are designed with this objective in mind (Dennis and Such, 1993). Figure 2.3: Electrode Potential versus Limiting Current Density (Dennis and Such, 1993). 20 2.8 Factor Affect the Rate of Electroplating There are many factors that affect the electroplating process. The surface area of the electrodes, the temperature, the kind of metal and the electrolyte, the magnitude of the applied current are some of these factors. 2.8.1 Surface Preparation For good quality electroplating and good adhesion of the deposit, the condition of the surface to be plated is important. Most plating defects arise from unclean surfaces prior to plating. The surface to be plated must be clean and free from grease, dirt, oxides and tarnish films, polishing compounds, etc. Greasy, dirty surfaces will not be wetted by the electrolyte and may not be plated. It also helps to have a smooth polished surface, free from defects and imperfections, if one wants a bright polished electroplated deposit. Plating should not be used to hide defects and to improve the surface polish (reduction in surface roughness). Defects to be avoided include casting porosity, inclusions and embedded polishing compounds, scratches and tool marks, and pitting from over-pickling (Cristopher, 2002). 2.8.2 Temperature The temperature of the electrolyte can also play a role in getting good plating, particularly in alloy plating. Nickel electroplating processes are conducted at increased temperature, which results in lower electrolyte resistance and therefore permits to decrease the voltage. Additionally higher temperatures aid dissolution and prevent precipitation of boric acid and other components (Gay et al., 1988). 21 2.8.3 Current Density The electrical conditions during plating are also important for plating quality. In particular, the current density (the current divided by surface area of the piece) plays an important role, particularly in alloy plating where deposit composition is controlled by current density. If the current is too high, the plating speed is increased but one may get a porous, dendritic deposit rather than a bright one and it may be accompanied by gas evolution which affects the surface finish. If it is very low, then the deposit may not have a good appearance and plating will be slow (Christopher, 2002). One way to avoid working at lower current densities is by employing a discontinuous direct current to the electroplating solution. By allowing between one and three seconds of break time between every eight to fifteen seconds of electrical current, high current densities can produce a higher level of quality. A discontinuous current is also beneficial for avoiding over-plating of specific sections on the base material (Hibbard et al., 2008) 2.8.4 Electrolyte A good electrolyte will contain the metal (or metals) to be deposited in solution in a sufficient concentration. In cyanide based gold baths, this will be in the form of gold potassium cyanide salt. It will also contain other additives to give good plating properties, These include, for example, additives to improve (Christopher, 2002): 1. The throwing power of the bath which means good uniformity of thickness over the piece being plated. 2. The brightness of the deposit. Special brighteners are added to assist. 3. The internal stress in the deposit. These additives control the build-up of stress to prevent cracking and spalling. 22 4. The chemical stability of the electrolyte and may include buffering agents to control pH which is a measure of the acidity or alkalinity of the electrolyte. 2.8.5 Anodes The anode area and position are important in order to obtain efficient electrodeposition and uniformity of deposit. There is a tendency for plating to be thicker on cathode areas closest to the anode and thinner in areas hidden (or out of line of sight) from the anode. Correct positioning of the anodes (more than one may be used) and a large anode area (compared to cathode area) is desirable for good plating (Christopher, 2002). 2.8.6 Current Efficiency Current efficiency is a ratio of the current producing nickel deposit to the total passing current. Anode current efficiency in nickel electroplating is about 100%. It may decrease at high pH when nickel dissolution is accompanied by discharging hydroxyl ions (OH -). Cathode efficiency of nickel electroplating is 9097%. 3-10% of the electric current is consumed by discharging hydrogen ions (H+), which form bubbles of gaseous Hydrogen (H2) on the cathode surface (Gay et al., 1988). 2.8.7 Filtration Continuous filtration of nickel plating baths with active carbon filters permits to control both presence of foreign particles and organic contaminations (products of 23 brightener decomposition etc). The filtration pumps should turn over the solution a minimum 1-2 times tank volume per hour (Gay et al., 1988). 2.8.8 Anti Pitting Hydrogen bubbles formed on the cathode surface and adhered to it may cause pitting of the deposit. In order to enhance removal of the bubbles wetting agents are added to the electrolyte. Wetting (anti-pitting) agents e.g. sodium lauryl sulphate (SDS) decrease the surface tension of the cathode and force the hydrogen bubbles out of the surface (Gay et al., 1988). 2.8.9 Air agitation Air agitation by low pressure blowers is used in nickel electroplating to enhance removal of the hydrogen bubbles discharged at the cathode (Gay et al., 1988). 2.9 Introduction of Porous Anodic Aluminium Oxide Film Since 1932, it has been known that the aluminium oxide layer consists of three distinct layers; aluminium metal, a thin compact oxide layer (barrier layer) and a porous oxide layer (Le et al., 1980; Juhl, 1999). The schematic diagram of oxide layer shows in Figures 2.4. Figure 2.5, shows a plane view of the porous oxide layer. The porous oxide layer is a relatively thick outer layer with regularly spaced pores extending from the outer surface towards the aluminium metal, while the barrier layer is located at the adjacent to the metal oxide interface (Miney et al., 2003). 24 Depending on the application of the anodic aluminium oxide film as template for nano-structure materials, the characteristics of porous anodic aluminium oxide film that play important roles in determining the properties of the nano-structure from which formed by electroplating process on the anodic aluminium oxide template are its pore’s diameter, pore array’s organization and also its porous layer thickness. Figure 2.4: The pore and hexagonal cell structure (Juhl, 1999) Figure 2.5: The hexagonal arrays of porous oxide film (Juhl, 1999) Anodic aluminium oxide thin film has attracted much attention in nanofabrication industry due to its self-organization properties that owing to the fact that 25 it enables mass production without the use of expensive lithographical tools, such as an electron beam exposure system (Miney et al., 2003). As mentioned by Metzger et al. (2000) ordered pores only occur in aluminium and no other metals. The highly self-ordered hexagonal pore array of anodic aluminium oxide film was first found by Masuda et al. (2004), by utilizing a prolonged anodization, followed by stripping of the thick oxide and re-anodizing process (Metzger et al., 2000). As explained by Metzger et al. (2000), and Ghorbani et al. (2006), the selforganized arrangements of neighboring pores in hexagonal arrays come from any repulsive force interaction between the pores. According to the Li et al., (1998), a possible origin of these forces between neighboring pores is the mechanical stress, which is associated with the expansion during oxide formation interface and leads to form curved shape metal/ porous oxide interface. It is claimed that the pores are formed during electropolishing and/ or anodizing on the aluminium surface can become hexagonally ordered at certain voltages and times of the initial electropolishing or by long term anodization and re-anodizing or also by a dynamic process depending on the mobility of ions within the barrier oxide and of aluminium atoms within the metal. It is well known that a porous anodized film is formed on the surface of aluminium by anodization in acid or alkaline solution (Wada et al., 1986). Selforganized hexagonal pore arrays with a 50 – 420 nm inter-pore distance in anodic alumina have been obtained by anodizing aluminium in oxalic, sulphuric and phosphoric acid solution. Figure 2.6 represent the pore arrangement anodized in sulphuric, oxalic and phosphoric acid solutions under optimum parameters, where SEM micrographs of the porous films are shown with same magnification. Hexagonal ordered pore arrays with distance as large as 420 nm were obtained under a constant anodic potential in phosphoric acid. By comparison of the ordered pore formation in the three types of electrolyte, it was found that the ordered pore arrays in phosphoric acid show a polycrystalline structure of a few micrometers in size (Li et al., 1998) 26 Figure 2.6: SEM micrographs of the bottom view of anodic alumina layers. Anodization was conducted in (a) sulphuric acid, (b) oxalic acid and (c) phosphoric acid. The thickness of the oxide film was approximately 120 µm (Li et al., 1998). 2.10 Mechanism of Porous Anodic Alumina Formation The initial stages of oxide growth correspond to a relatively uniform oxide thickness. But by the entrance of Al3+ ions into the electrolyte penetrations paths will develop in top of the barrier layer. The local field will be concentrated in these penetrations paths giving field strength of up to about 2.1 x 107 V/cm. Between these penetration paths there will be a decrease in field strength and hence a decrease in the 27 field-assisted dissolution rate. The variation in field strength results the interface aluminum/barrier layer to adopt the form of a fine scalloped structure. Concentration of electric field and ionic current are higher at the bottom-middle region of the scalloped structure and aid the formation of aluminum oxide layer. Based on this theory, the influence of current and voltage behavior during anodization process need to be taking in consideration (Juhl, 1999). Figure 2.7 represents the current-anodizing time curved during porous anodic alumina formation in anodizing process. At the beginning of period a, the current is high due to the fact that the current only passes through the metallic aluminum. Then, as time increase, the current is decreasing along with the formation of thin compact aluminum oxide layer. This is because; oxide layer has higher resistance than the metallic aluminum. The increase in thickness and therefore an increasing resistance result in a further decrease in the current in period b (Juhl, 1999). Compact oxide layer become thicker and roughened Porous oxide layer starts to form Porous oxide layer continue to form with constant current Oxide layer Metallic aluminium Figure 2.7: Porous Anodic Aluminium Oxide film formation in terms of current and anodizing time (Juhl, 1999). 28 The increasing thickness of barrier layer will subsequently increase the resistance and thus cause further decrease in current within period b. However, within the period b, at certain point, the current-time curve tends to turn upward as the current increases with time. This is due to roughness that forms on the top of compact oxide layer (barrier layer). The roughness of the barrier layer is built by the concentration of the current in areas with thinner oxide than on the rest of the surface. The localized thinner oxide areas are usually the subgrain boundaries found in aluminum. It was originally suggested by Metzger et al. (2000) that the pores initiated at cracks or other defects in oxide initially produced during anodizing. It appears, however, that any process that thins the oxide locally can initiate pore formation. The natural oxide film on either side of these subgrain boundaries is not compact or as uniform as on the rest of the surface, thus it posses less electrical resistance which will become the places or sites for initial pore cell formation to start (Metzger et al., 2000). Then, in period c, the current is concentrated in areas with thinner oxide, consequently increase electrolyte temperature at that area and results more dissolution of compact alumina (barrier layer) layer. Then, the barrier layer become even thinner and leads to increasing of current that favor the formation of porous oxide layer to starts. This means that all oxides have been a part of the barrier layer before becoming a porous oxide layer (Juhl, 1999). The current will increase up to certain point where it becomes constant as in period d. This is where the formation and dissolution of oxide layer process reach a steady level (occurs at same rate). The growth of porous anodic aluminum oxide film (Figure 2.8) can be concluded to involve below sequence (Wan , 2005): a) Formation of barrier layer. b) Pore nucleation due to local thickening of the oxide layer. 29 c) Field enhanced oxide dissolution at oxide/electrolyte interface. d) Oxidation at the aluminum/oxide interface due to field enhanced migration of O2- ions. e) Sustenance of pore growth due to the fact that electric field is highest in the region around the pore bottom, leading to field assisted oxide formation and growth of alumina side walls at the expense of field assisted oxide dissolution at the pore bottom. Figure 2.8: Schematic diagram of porous anodic alumina growth (Wan , 2005). 2.11 Disordered and Ordered Pores of Anodic Alumina Oxide Film There are actually two types of aluminium oxide layer were formed during anodizing which are disordered or amorphous pores formation and ordered (crystalline) pores formation. The disordered pores arrangement can be formed in a very wide processing using variety of a single acid solution such as sulphuric acid (H2SO4), oxalic acid (H2C2O4), phosphoric acid (H3PO4), chromic acid (H2CrO4) or by using the combination of two acids or with other organic solution such as ethanol. 30 Metzger et al. (2000) mentions that the pore growth rate is 0.1 µm per minute. During the formation, amorphous and anhydrous alumina is formed but the pore bottom of alumina may be partially crystalline (Metzger et al., 2000). The image of disordered alumina pores is shown in Figure 2.9 below. Figure 2.7(a) shows a typical SEM image of the sample surface obtained after anodization and Figure 2.7 (b) shows a cross section of the original aluminium and the porous aluminium oxide layer. The surface image shows the pores of varying size and it is difficult to know whether the cross-sectional image shows the widest wall-to-wall distance or not because, it is not sure if the sample were cleavage down the center of the pore. Miney et al. (2003) estimated the maximum pore width from those images is approximately 100nm. Figure 2.9: The SEM images showing amorphous anodic aluminum oxide layer produced in 10% phosphoric acid. (a) Plan view of the surface of the porous anodic aluminium oxide and (b) side profile of the aluminium with the porous aluminium (a) (b) oxide (Miney et al., 2003). As for the highly ordered pore formation as shown in Figure 2.10 (Singubara , 2003), it has only narrow processing window and several methods has been found such as long term-anodizing and re-anodizing steps and also initial electro polishing to aid the highly ordered anodic aluminium oxide pores formation (Metzger et al., 2000). Besides, highly ordered pores also have been produced by using imprint periodic (pre-pattern) concave on aluminium metal to control pore initial position (Singubara, 2003; Masuda et al., 2004). The methods of synthesizing highly ordered 31 hexagonal pores arrays in aluminium oxide are still intensively being studied due to its application as templates in nano-structure fabrication industry. Figure 2.10: SEM image of alumina pores array formed by two step anodic anodization. (Singubara , 2003). 32 CHAPTER 3 METHODOLOGY 3.1 Experimental Design The methodology of this research is to directly electrodeposit nickel on aluminium by using high speed electroplating technique. High speed electroplating is a technique where the high speed movement of the plating solutions is 2.7 m/s and the high rate of plating is more than 600 μm/h (Hussain, 2009). In this research, three parameters are involved during the electroplating processes which are different values of current density, different values of temperature and different type of solutions used. The type of nickel plating solution which will be used to plate nickel on the aluminium is Watt’s bath solution and sulphate based Ni solution. Finally the nickel deposits will be analyzed by using Scanning Electron Microscope (SEM) and adhesion testing. Figure 3.1 shows the flowchart used to conduct the electroplating process in order to achieve the study objectives. 33 Sample preparations Anode: Nickel Cathode: Aluminium Current Density Effect Solution Effect Temperature Effect High Speed Plating Process Sample Testing Microstructure Testing Scanning Electron Microscope (SEM) Adhesion Testing Knife test Tape test Documentation Test Result Data Analysis Discussion of Analyze Data Conclusion and Recommendations Figure 3.1: Flowchart to conduct the electroplating process. 34 3.2 Aluminium Sample Preparation Specimen coupon size for the project study will be prepared where, aluminium rod will be of diameter 10 mm and length 40 mm. Figure 3.2 illustrates the dimension of the specimen coupon. 40 mm 10 mm Figure 3.2: Project specimen dimensions of the aluminium rod 3.3 Solutions Preparation In this experiment, two different solutions were prepared for plating nickel on aluminium which are Watt’s solution and sulphate based Ni solution. Table 3.1 shows the summary of solution preparation according to their composition. 35 Type Table 3.1: Composition of solution preparation Typical Cathode current concentration Constituents density (A/dm2) g/l pH Watt’s NiSO4.7H2O 300 3.5 – 5.5 solution NiCl2.6H2O 45 (Actual5) H3BO3 38 Sulphate NiCl2.6H2O 300 based Ni H3BO3 38 2.5 Temperature (oC) 2.5 – 10 45 – 100 (was varied) (was varied) 2.5 – 10 50 – 70 Solution 3.4 Experiment Setup Two specimens which are aluminium and nickel are placed at electrodes of high speed plating equipment. Aluminium specimen was put at the cathode and nickel specimen was put at anode. Nickel solution was put inside the solution bath and the temperature of the nickel solutions is control by using temperature control unit. Pump was turn on and the solution flows rapidly from the tank into the pipe through the anode and cathode and returned back to the tank. Power generator was turn on and the current density is adjusted. The electroplating process is performed at fixed speed of 2.7 m/s and fixed times of 4 minutes. Figure 3.3 shows the schematic diagram of the high speed plating equipment while Figure 3.4 show the high speed plating equipment developed by M S Hussain (Patent pending). 36 Direct current Anode Solution in Solution out Cathode Pump Tank Figure 3.3: Schematic diagram of the high speed plating equipment developed by M S Hussain (Patent pending) Solution in Solution out Anode Solution comes from tank Gap between anode and cathode ≤ 1mm Pump Cathode Figure 3.4: High Speed Eletroplating equipment developed by M S Hussain (Patent pending) 37 3.5 Sample preparation Sample preparation for test on solution effect, current density effect and temperature effect are discussed in this chapter. Each sample is coded as S/N which means sample number of the specimen, for example S/N 1 means sample number one. In solution or electrolyte preparation, Watt’s solution and sulphate based Ni solutions are prepared as solutions in current density and temperature effect test. Effect of current density studied by performing at 0.1 A/cm2, 0.3 A/cm2 and 0.6 A/cm2, while effect of temperature studied by performing temperature 550C, 600C and 65 0C. Table 3.2 and Table 3.3 show the summary of sample preparation according to the solution, current density and temperature. Table 3.2: Summary of sample preparation according current density and temperature for Watt’s solution Current Density Temperature Sample Number Solution (A/cm2) (0C) S/ N 1 0.1 S/N 2 0.3 S/N 3 0.6 S/N 4 0.1 S/N 5 Watt’s solution 0.3 S/N 6 0.6 S/N 7 0.1 S/N 8 0.3 S/N 9 0.6 55 60 65 38 Table 3.3: Summary of sample preparation according current density and temperature for sulphate based Ni solution Current Density Temperature ample Number Solution (A/cm2) (0C) S/N 1 0.1 S/N 2 0.3 S/N 3 0.6 S/N 4 S/N 5 3.6 55 0.1 Sulphate based Ni solution 0.3 S/N 6 0.6 S/N 7 0.1 S/N 8 0.3 S/N 9 0.6 60 65 Characterization For sample testing analysis, the sample produced through high speed plating method were tested and characterized by using scanning electron microscope (SEM) and Adhesion Testing. 3.6.1 Morphology Analysis. The morphology adhesion between the aluminium and nickel layer was observed using Scanning Electron Microscope (SEM). Figure 3.5 show the Scanning Electron Microscope equipment. SEM facilitates the observation of very fine details (high resolution) of materials and good focus over a wide range of specimen surface 39 (large depth of field). It also produces clear image of specimen ranging from object visible to the naked eye to a structure spanning few nanometers. Beside, the thickness of nickel layer will be determined to see the effect of different current density, temperature and solution used during the experiment. Figure 3.5: Scanning Electron Microscope (SEM) The specimens for metallographic examinations were prepared according to ASTM E3, Standard. Cleaning, grinding and polishing process in the specimen preparation is required as to promote clean and good surface finish. Water is used to remove the contaminants such as residue from grinder. Table 3.4 shows preparation sequence consist of a series of grinding and polishing steps. 40 Process Table 3.4: Preparation Method (ASTM E3) Abrasive Type Lubricant Time (s) ANSI Planar Grinding Stone 120 – 320 Water 15-45 Paper 240 grit SiC Water 15-45 Paper 320 grit SiC Water 15-45 Paper 600 grit SiC Water 15-45 grit SiC/Al2O3 Fine Grinding Rough Polishing 6μm diamond Final Polishing 1μm diamond Compatible Lubricant Compatible Lubricant 120-300 60-120 Rotation Complimentary Rotation Complimentary Rotation Complimentary Rotation Complimentary Rotation Complimentary Rotation Complimentary Rotation 3.6.2 Adhesion testing After the electroplating process, adhesion testing was required to quantify the strength of the bond between the nickel layer and the aluminium substrate. Adhesion testing in the paint and coating industries is necessary to ensure the paint or coating will adhere properly to the substrates to which they are applied. The adhesion between a coating and its substrate has been measured in various ways. For example, coatings are often evaluated by a scratch test. A calibrated scratching tool is applied to a test sample. A high quality adhesion between the coating and the substrate prevents the penetration of the scratching tool and the workpiece passes. If the adhesion is low, however, the tool can penetrate and scratch the coating, in which case the sample fails. 41 In adhesion testing where the material of the coating is relatively hard, pull tests are employed. A pulling apparatus having calibrated force and deflection gauges is attached to the coating. The substrate is then held statically and force is applied in a gradually increasing manner until the coating separates from the substrate, whereupon the degree of force required to cause the separation is recorded. However, in this study, two types of adhesion test are used which are adhesion by knife and adhesion by tape test. 3.6.2.1 Adhesion Test by Knife According ASTM D6677 – 07, this method is used to establish whether the adhesion of a coating to a substrate or to another coating is at generally adequate level by using a knife. Figure 3.6 show the adhesion testing using knife. Figure 3.6: Adhesion test by using knife 3.6.2.2 Adhesion Test by Tape test According ASTM D3359 – 09, this test method cover procedures for assessing the adhesion of coating films to metallic substrates by applying and removing pressures-sensitive tape over cuts made in the film. 42 CHAPTER 4 RESULT AND DISCUSSION 4.1 Introduction This chapter will discuss the results of plating nickel on aluminium samples that are produced via High Speed Electroplating Method. This result is divided into two different analyses: which are adhesion analysis, and morphology analysis for adhesion analysis, the samples were analyzed using the knife and tape test. For morphology analysis, the samples were characterized using a Scanning Electron Microscope (SEM) to measure the thickness of the layer. In this chapter, the effect of current density, temperature and type of solution on weight of Ni plated, thickness of Ni plated and rate of deposition are discussed. 43 4.2 Nickel Plating Layer on Aluminium Sample In this research work, nickel plating layer on aluminium substrate has been deposited using two different nickel solutions. Figure 4.1 show the sample of nickel plating on aluminium. Figure also shows the original diameter for the sample. The original diameter is 10 mm and ~ 10 mm thicknesses. From the observation of the sample, it shows that no defect occur on the sample after plating process. Nickel Layer Ø 10mm Aluminium Substrate Figure 4.1: Nickel plating on aluminium sample. 4.3 Adhesion Analysis After plating, all samples were subjected to an adhesion test using the knife test and tape test. Adhesion testing was necessary to ensure the Ni coating is adhered properly to the aluminium substrates. Based on this simple test, the results showed that Ni plating produced at current density below 1.0A/cm2 give a better level of adhesion, compared to the sample produced at current densities above 1.0A/cm2 for both Watt’s and sulphate based Ni solution. Figure 4.2(a) and Figure 4.2(b) show the adhesion test results for good and poor adhesion of Ni plating layer on aluminium substrate respectively. 44 (a) Current density: 0.3A/cm2 (b) Current density; 1.0A/cm2 Figure 4.2: Nickel plated sample for (a) Good Ni adhesion and (b) poor Ni adhesion on aluminium. 4.4 Morphology Analysis The morphology of the plated substrates was characterized by Scanning Electron Microscope (SEM) and Energy Dispersive X-Ray (EDAX). Before morphology testing was necessary to perform sample preparation on the sample to be tested in order to remove all the scratches and defects on the sample surface which can affect the observable process and also lead to confusion while interpreting morphology of the sample later.. After the morphology of the samples is obtained, elemental analysis was carried out on a selected number of samples were examined using Energy Dispersive X-Ray (EDAX). This observation was carried out in order to determine which elements were present on the samples being observed. Figure 4.3 shows the morphology of cross-section of nickel plating on aluminiun for 55oC at 0.3A/cm2 current density by using Watt’s solution. From the cross-section view taken by SEM, the result clearly shows that adhesion of Ni plated layer is properly deposited on aluminium substrate. The EDAX analysis for substrate plated at 55oC temperature and 0.3A/cm2 current density by Watt’s solution is shown in Figure 4.4. From EDAX analysis, it is indicated that Ni element is present on the sample. The graph also show that the Al element are exists on the sample but in a small percentage around 2.08%. 45 Nickel plating layer Aluminium Substrate Figure 4.3: Cross section nickel plated on aluminiun surface morphology under 2000x Magnification. Ni ~ 97.92% Al ~ 2.08% Figure 4.4: The EDAX analysis for 55oC temperature and 0.3A/cm2 current density by Watt’s solution In this study, the original deposited Ni plating thickness was determined. Cross-section samples were prepared after plating process and the thickness of Ni plated was measured for each sample using SEM. Figure 4.5, 4.6 and 4.7 show morphology for Watt’s solution while Figure 4.8, 4.9 and 4.10 show morphology for sulphate based Ni solution at temperature 55oC, 60oC and 65oC respectively, with different current density. 46 (a) (b) (c) Figure 4.5: Cross-section Ni plated on aluminium surface at 55oC for (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. (a) (b) (c) Figure 4.6: Cross-section Ni plated on aluminium surface at 60oC for (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. 47 (a) (b) (c) Figure 4.7: Cross-section Ni plated on aluminium surface at 65oC for (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. (a) (b) (c) Figure 4.8: Cross-section Ni plated on aluminium surface at 55oC for (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. 48 (a) (b) (c) Figure 4.9: Cross-section Ni plated on aluminium surface at 60oC for (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. (b) (a) (c) Figure 4.10: Cross-section Ni plated on aluminium surface at 65oC for (a) 0.1A/cm2, (b) 0.3A/cm2 and (c) 0.6A/cm2 current density. 49 4.5 Effect of Current Density Nine of aluminium samples were Ni plated sample by using Watt’s solution and sulphate based Ni solution. After rising with water and drying, the specimen was weighed using a digital microbalance (four decimal places). The differences in the weight before and after electrodeposition would be the weight of the plated Ni. The measured of weight Ni plating layer for Watt’s solution and sulphate based Ni solution are shown in Table 4.1 and Table 4.2. (A/cm2) Density Current Table 4.1: Weight of Plated Layer by using Watt’s solution Weight of Plating Layer (g) Temperature 55⁰C 60⁰C 65⁰C 0.1 0.0036 0.0048 0.0075 0.3 0.0085 0.0098 0.0132 0.6 0.0146 0.0153 0.0202 (A/cm2) Current Table 4.2: Weight of Plated Layer by using sulphate based Ni solution Weight of Layer (g) Temperature 55⁰C 60⁰C 65⁰C 0.1 0.0061 0.0073 0.0108 0.3 0.0120 0.0150 0.0185 0.6 0.0189 0.0224 0.0291 By using the data in Table 4.1 and Table 4.2, two graphs has been plotted to show the weight changes of Ni plated sample with increasing the current density. The results for weight of Ni plated sample are shown in Figure 4.11 and Figure 4.12. From both figure, it can be observed that by increasing the current density, the weight of Ni plated sample had increased. 50 0.025 Weight of Ni plated (g) 0.02 0.015 55⁰C 60⁰C 0.01 65⁰C 0.005 0 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 Current Density (A/cm2) Figure 4.11: Influence of current density on weight of Ni plated layer by using Watt’s solution 0.035 Weight of Ni plated (g) 0.03 0.025 0.02 55⁰C 60⁰C 0.015 65⁰C 0.01 0.005 0 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 Current Density (A/cm2) Figure 4.12: Influence of current density on weight of Ni plated layer by using sulphate based Ni solution. 51 According to Faraday’s first law of electrolysis, the amount of a substance produced or consumed in an electrochemical process depends quantitatively on the quantity of electricity that flows as the reaction takes place. The amount of electricity is the product of time and the magnitude of current (Bookrags Staff, 2005). By using the Faraday’s law equation it can be stated that, for a certain time interval, when the current applied to the electroplating cell is increased, the amount of metal that is coated should also increase. However, from the observation it can be seen that the current density has an affect on the plating adherence and the quality of plating. When the current density was increased to 1.0 A/cm2 or above the results were poor level of adhesion. At higher current densities the plated nickel layer were peeling off very easily from aluminium substrate surface. 4.6 Effect of Temperature The thickness of the plating nickel for Watt’s solution and sulphate based Ni solution were calculated from morphology analysis. Table 4.3 and Table 4.4 were show the measured thickness of nickel plated layers by using Watt’s solution and sulphate based Ni solution. Table 4.3: Coating thicknesses by using Watt’s solution Rate of solution movement (m/s) Time (min) 2.7 m/s 4 Influence of temperature on coating thicknesses Current Density (A/cm2) 0.1 0.3 0.6 55 Thickness layer (µm) 3.34 Thickness layer (µm) 12.00 Thickness layer (µm) 23.95 60 5.89 14.85 26.00 65 10.65 22.05 34.00 Temperature (oC) 52 Table 4.4: Coating thicknesses by using sulphate based Ni solution Rate of solution 2.7 m/s movement (m/s) 4 Time (min) Influence of temperature on coating thicknesses Current Density (A/cm2) 0.1 0.3 0.6 55 Thickness layer (µm) 6.27 Thickness layer (µm) 20.65 Thickness layer (µm) 31.15 60 10.55 25.15 36.20 65 16.35 30.45 43.40 Temperature (oC) From the data shown in Table 4.3 and Table 4.4, it is observed that for all the samples, as the temperature was increased from 55oC to 65oC the thickness of nickel plated layer increased linearly. The relationship between coating thicknesses and temperature are shown in Figures 4.13 and 4.14. Both figures show that by increasing the temperature, the thicknesses of Ni plated sample have shown a marked increase. 40 Coating thivknesses (µm) 35 30 25 55⁰C 20 60⁰C 15 65⁰C 10 5 0 0.1 0.3 0.6 Current Density (A/cm2) Figure 4.13: Influence of temperature on coating thickness by using Watt’s solution 53 50 45 Coating thivknesses (µm) 40 35 30 55⁰C 25 60⁰C 20 65⁰C 15 10 5 0 0.1 0.3 0.6 Current Density (A/cm2) Figure 4.14: Influence of temperature on coating thickness by using sulphate based Ni solution According to the Misbahul et al., (2002), it is stated that temperature is not a factor in influencing the weight gain of cathode according to the Faraday’s Law but it does influence the mobility of ions. Warm electroplating bath increases mobility and thus lets more metal ions diffuse in and out from electrodes into the electroplating bath and lowers the overall potential. However, temperature can affect the amount of plated metal because it affects the rate of the reactions at the electrodes. In electroplating processes, there is a very small amount of hydrogen gas production at the cathode, if the electrolyte is an aqueous solution. When the temperature is increased, the speed of formation of H2 increases more rapidly than the electrodeposition of metal and at the same time the cathodic efficiency decreases. Also the dissolution rate of the cathode increases and the decrease of over-potential hydrogen with the increase of the temperature produce an increase the H2 release (Barbato et al., 2008). Relying on this factor, it can be stated that the thickness of deposited metal increases as the temperature is increased. 54 Mibahul et al., (2002) also state that the ideal temperature of electroplating bath is ranged from 45 oC to 65oC. The electroplated samples, which were produced at low temperatures i.e. 30oC and 40oC, gave dull appearances whilst samples plated at higher temperatures 50oC to 70oC gave bright appearances. Finally, gradually dull and brownish colour appeared when the temperature was increased to 100oC. Electroplating in an over high temperature that was 100 oC, produced a sticky and brownish deposit but still possesed acceptable corrosion resistance ability. 4.7 Effect of Type of Solution In this research, the rate of deposition was calculated. The rate of deposition of the sample could be calculated using the following equation, 𝐷𝑅 = 𝑇 (𝜇𝑚 ) 𝑡 (min ) , where T and t stand for, thickness and plating time respectively. As discussed in Chapter 3, two bath solutions were tested in the present works which are Watt’s solution and sulphate based Ni solution. Table 4.5 shows the rate of deposition for both solutions with a different temperature and current density. From data in Table 4.5, a graph has been plotted to show the changes of Ni plated deposition rate for both solution at different temperature and different current density. From these graphs, it is obvious that the sulphate based Ni solution give the higher rate of deposition compared to the Watt’s solution as the current density was increased. From the observations it is concluded from deposition rate, higher the current densities the higher is the rate of deposition. The rates of deposition increased because the rate of transferring of nickel atoms was increased during the plating process and more nickel atoms were deposited on aluminium surface. 55 Table 4.5: Deposition rate at different type of solution Temperature 55 Watt’s Solutions Solution 0.6 0.3 0.1 Deposition rate (µm/min) Type of 60 Nickel Nickel Watt’s Sulphate Sulphate Solution Solution 65 Solution Watt’s Solution Sulphate Solution 1.5675 1.4725 2.5450 2.6625 4.0875 3.0000 5.1625 3.7125 6.2875 5.5125 7.6125 5.9250 7.7875 6.5000 9.0500 8.5000 10.8500 55oC 60oC 65oC 10 8 6 4 2 0 Nickel 0.8350 12 Deposition Rate (µm/min) Current Density (A/cm2) (oC) Watt’s solution Sulphate based Ni solution Watt’s solution Type of Solution 0.1 A/cm2 Sulphate based Ni solution 0.3 A/cm2 Watt’s solution Sulphate based Ni solution 0.6 A/cm2 Figure 4.15: Influence of different type of solution on deposition rate 56 CHAPTER 5 CONCLUSION 5.1 Conclusion 1. It has been possible to plate nickel directly on aluminium without any pretreatment process by using high speed electroplating technique. 2. Very good level of adhesion has been obtained at relatively lower current densities. 3. The adhesion test results showed that nickel plating produced at current density below 1.0A/cm2 gave better level of adhesion, compared to the sample produced at current density above 1.0A/cm2 for both Watt’s and sulphate based Ni solution. Nickel plating peeled off easily at current densities above 1.0A/cm2. 4. By increasing the current density and temperature, the weight and thicknesses of Ni plated layer also increased for both solutions. 5. Sulphate based Ni solution gives higher rate of deposition compare to the Watt’s solution as the current density and temperature are increased. 57 CHAPTER 6 RECOMMENDATION 6.1 Recommendations Direct Ni plating on aluminium surface has just been accomplished; however this has opened up avenues for many improvements that can be accomplished. Thus based on the experience from this research project, the following recommendation or suggestions are made for further work; a) Study the effect of increasing and decreasing the speed of the electrolyte on the rate of deposition and microstructure and properties of the Ni deposits. b) Further study by comparing effect of current density, temperature and solution by using the different of material such as titanium or stainless steel. c) More parameter needs to be added into the study such as different plating time, different value of temperature and different type of solution so that more information can be analyzed and investigated on the sample. 58 REFERENCES Anynomous (2010). “The Electro Nickel Plating Process [Online]. Thomas publishing Company. Available at: http;//www.thomasnet.com/articles/custom-manufacturing fabricating/ electroplating-process.(Access: 6 November 2010). Asada, T. (1970). Method of Electroplating Nickel on an Aluminium Article. (U.S Patent 3, 515, 650). Barbato, S., Ponce, J., Marcelo, J.L., Jacqueline. C. and Rodrigo, E. (2008). Study of the Effect of Temperature on the Hardness, Grain Size, and Yield in Electrodeposition of Chromium on 1045 Steel. J.Chill, Chemistry Soc., vol 53, pp 1440-1443 [Online]. Available at: http://www.bookrags.com/research/faradays-laws-woc/. (Access: 6 Bookrags Staff (2005). “Faraday’s Law”. November 2010) Bornhauser, O., Strasbourg and France. (1932). Electroplating of Aluminium. (U.S Patent 2, 028, 312). Christopher, W. C (2002). Back to Basics: Electroplating and Electropolishing of Jewellery. International Technology, World Gold Council London. Cooper, C.F., Lake, G. and Mich. (1961). Electroplating Process. (U.S Patent 2, 977, 295). Dennis, J. K and Such, T. E. (1993). Nickel and Chromium Plating.(3rd ed.) Abington Hall; Woodhead Publishing Ltd. 59 Dibari, G. A. (2000). “Nickel Plating”. International Nickel Inc., Saddle Brook. pp 224 – 241. Frasch, J. (1941). Process for Direct Nickel Plating of Aluminium and its Alloys. (U.S Patent 2, 233, 410). Gay, R. N. and Wayne, K.R., (1988). Process for Electroplating Nickel over Stainless Steel. (US Patent 4,764,260). George, T. and Chadd, K. (2009). “Aluminum/Electroless nickel... a choice finish”. Tectmetals, INC. [Online]. Available at: http://www.techmetals.com/Aluminum-Electroless-Nickel-A-ChoiceFinish.aspx. (Access: 29 March 2010) Ghorbani, M., Nasirpouri, F., Zad, A. I, and Saedi, A. (2006). “On the Growth Sequence of Highly Ordered Nanoporous Anodic Aluminium Oxide.” Material and Design. vol. 27, Issues 10, pp. 983 – 988. Graves, B. A. (2009). “Nickel Plating Primer” Products Finishing Magazine [Online]. Available at: http://www.pfonline.com/articles/040102.html. (Access: 29 March 2010) Hibbard, G.D., Radmilovic, V., Aust, K.T. and Erb, U. (2008). “Grain Boundary Migration during Abnormal Grain Growth in Nanocrystalline Ni.” Materials Science and Engineering. Vol 494, Issues 1-2, pp 232-238. Hussain, M.S. Patent Pending (2010). “Nanocrystalline Ni Plating Directly on Aluminium by High Speed Electroforming.” Hussain, M.S. Direct Electrolytic Ni deposition on non-conductive Al Surfaces without Any Pre-treatment (To be published). 60 Juhl, A. D. (1999). Theoretical Introduction to Pulse Anodizing. Doctor Philosophy. AluConsult, Denmark. Kampert, W. P. and Burell, L. (1976). Metal Plating on Aluminium. (U.S Patent 3, 989, 606). Kopeliovich, D. (2010). “Nickel (Substances&Technologies) Electroplating” [Online]. Available http://www.substech.com/dokuwiki/doku.php?id= SubsTech at: nickel _electroplating&DokuWiki=5dcdca7f7e263a7de3372d0f2880af50#watts _nickel_plating_solutions. (Access: 29 March 2010). Krishnam, S. C. and Sanjeeb, K. L (2007). Effect of Substrate Texture on Electroplating. Bachelor of Technology in Metallurgical and Materials Engineering. National Institute of Technology, Rourkela. Le, S. H., Camerlingo, A., Pham, H. T. M., Rejaei, B. and Sarro, P. M. Anodic Aluminum Oxide Templates for Nanocapacitor Array Fabrication. Delft University of Technology, Delft Institute of Microelectronics and Submicrontechnology (DIMES); pp. 96-100. Li, A. P., Muller, F., Birner, A., Nielsch, K. and Gosele, U. (1998). “Hexagonal Pore Arrays with a 50 – 420 nm Interpore Distance Formed by Selforganization in Anodic Alumina.” Journal of Applied Physics. vol. 84. Issues. 11, pp. 6023 – 6026. Liona, L. V. (2005). “Nickel Electroplating”. Transducers Science and Technology [Online]. Available at: http://tst.ewi.utwente.nl/research/microfabrication/mmflowcontrollers/ind ex.html. (Access: 29 March 2010). Luan, B., Le, T., and Nagata, J. (2004). “An Investigation on the Coating of 3003 aluminium alloys.” Surface and Coating Technology. vol 186, Issues 3, pp. 431 – 443. 61 Masuda, H., Matsui, Y., Yotsuya, M., Matsumoto, F., and Nishio, K. (2004). “Fabrication of Highly Ordered Anodic Porous Alumina Using Selforganized Polystyrene Particle Array.” Chemistry Letter. vol. 33. Issues 5. pp. 584 – 585. Mest, G. T. (2009). “Nickel Electroplating”. Products Finishing Magazine [Online]. Available at: http://www.pfonline.com/articles/pfdmest01.html. (Access: 29 March 2010) Metzger, R. M., Konovalov, V. V., Ming, Sun., Tao, Xu., Zangari, G., Bin, Xu., Benakli, M. and Doyle, W. D. (2000). “Magnetic Nanowires in Hexagonally Ordered Pores of Alumina.” IEEE Transitions on Magnetics. vol. 36. Issues 1, pp. 30 – 35. Miller, G. A. (1972). Processes for Nickel Plating Metals. (U.S Patent 3, 667, 991). Miney, P. G., Colavita, P. E., Schiza, M. V., Priore, R. J., Haibach, F. G. and Myrick, M. L. (2003). “Growth and Characterization of a Porous Aluminium Oxide Film Formed on an Electrically Insulating Support.” Electrochemical and Solid State. vol 6. Issues 10, pp. B42 – B45. Moller, P. (1994a). Plating on Aluminium. European Aluminium Association (EAA). Dansk Technisca Hoogschool, Lyngby. Moller, P. (1994b). Surface Treatment of Aluminium. European Aluminium Association (EAA). Dansk Technisca Hoogschool, Lyngby Oliveira, E. M., Finazzi, G. A. and Carlos, I. A. (2006). “ Influence of Glycerol, Mannitol and Sorbitol on Electrodeposition of Nickel from a Watts Bath and on the Nickel Film Morphology. Surface and Coating Technology. vol 200, Issues 20 – 21, pp. 5978 – 5985. 62 Satee, R. and Schaumburg. (1980). Direct Nickel Plating of Aluminium. (U.S Patent 4, 196, 061). Schlesinger, M. (2002). “Electroplating.” Electrochemistry Encyclopedia [Online]. Department of Physics University of Windsor. Available at: http://electrochem.cwru.edu/encycl/art-e01-electroplat.htm. (Access: 29 March 2010). Singubara, S. (2003). “Fabrication of Nanomaterials using Porous Alumina Templates.” Journal of Nanoparticle Research. vol. 5. pp. 17 – 30. Topelian, P. J. and Newark, N.J. (1958). Electroplating. (U.S. Patent 2, 856, 333). Tsukamoto, T., Okamura, M. and Kutami, T. (1978). Process for Metal Plating on Aluminium and Aluminium Alloys. (U.S Patent 4, 115, 211). Wada, K., Shimohira, T., Yamada, M., and Baba, N. (1986). “Microstructure of Porous Anodic Oxide Films on Aluminium.” Journal of Material Science. vol. 21, pp. 3810 – 3816. Wan, Young Jang. (2005). “Electrical Transport Measurements of Individual Bismuth Nanowires and Carbon Nanotubes.” The University of Texas at Austin.