G P E : D
advertisement

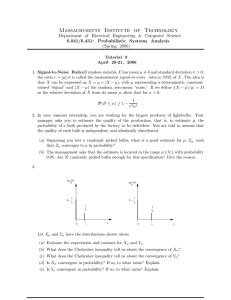
Physical Chemistry Laboratory Experiment I-4 GAS PHASE EQUILIBRIUM: DISSOCIATION OF N2O4 References: See “References to Experiments” Giauque,W. F.; Kemp J. D. J. Chem. Phys. 6 (1939) 40. Objectives: To measure the equilibrium constant for the dissociation of dinitrogen tetroxide at a series of temperatures To compute • the standard Gibbs free energy of the dissociation reaction at each temperature • the standard enthalpy and the entropy of the reaction at 25oC and at 127oC. To know the following and their interrelations: • Enthalpy of Reaction • Entropy of Reaction • Gibbs Free Energy of Reaction • Gibbs-Helmholtz Equation • Equilibrium Constant, Kp Materials and Apparatus: Cylinder of N2O4 gas. Gas handling manifold Gas measurement bulbs Tra p Platform balance Bul b Mettler analytical balance Variable temperature water bath Pump N 2O4 Figure 1. Gas-handling manifold Background: The equilibrium between nitrogen tetroxide and nitrogen dioxide is N2O4 2NO2 If a portion α of each 1 mole of N2 O4 dissociate into 2α moles of NO2, this leaves 1–α moles of N2O4. However, the total number of moles in the system is (1–α)+2α=1+α moles. Calculating the euilibrium constant, assuming a total pressure P Physical Chemistry Laboratory Kp = 2 pNO 2 p N2O4 = x 2NO2 P 2 xN 2 O 4 P = ( 2! (1 + ! )) Experiment I-4 2 (1 " ! ) (1 + ! ) P= 4! 2 1 " !2 where xA is the mole fraction of any compound A. Procedure: Week One 1) Determine the volume of three or four 250 mL glass bulbs, using freshly boiled distilled water that has been cooled to about room temperature. Accurately measure the water temperature so that you may look up the correct water density. Weigh the evacuated bulb on a platform balance, then fill it with water with a syringe, and reweigh. Be careful to eliminate any air bubbles inside the bulb or water drops external to the stopcock seal. Repeat this for each bulb you use in the experiment. Record and report the I.D.# for each bulb used. Carefully empty the bulbs and place them (without stopcocks) in an oven to dry. Week Two 1. Prepare the gas-handling manifold (see Figure 1) for use, under the direction of the instructor by replacing the cold trap, evacuating the line, then filling the Dewar flask around the trap with liquid nitrogen LN2 CAUTION: LN2 can cause severe frostbite. CAUTION: An LN2-cooled trap can condense oxygen and argon if left open to the atmosphere CAUTION: Evacuated glassware, including Dewar flasks are potential implosion hazards 2. Attach the glass bulbs to the manifold by ground joints with a small amount of silicone stopcock grease, evacuate the bulbs to less than 0.1 mm Hg and weigh on an analytical balance. Be sure to wipe the joint as clean as possible before weighing. 3. Reattach the bulbs to the manifold, checking each joint for leaks by isolating the pump and monitoring the Hg manometer. If a leak is found, remove the bulb, clean both parts of the joint, regrease lightly and reattach. 4. Fill each bulb with N2 O4. The vapor pressure of N2O4 is about one atmosphere at room temperature. Therefore the cylinder needle valve can be opened slowly with little danger of over-pressurizing the system. However, always use caution with such systems to avoid damage to glass manifolds. Once the system has been pressurized to the vapor pressure of the gas, cool the gas bulb with ice water until the first bit of liquid is formed. At this point, shut off the bulb and system, and separate the bulb from the system, carefully cleaning any grease from the joint. Condense any excess N2O4 from the manifold into the cold trap. CAUTION: N2O4 is very toxic and corrosive. All manipulations must be performed in a wellventilated fume hood. 5. Allow the bulbs to warm to room temperature. Vent the bulbs (in the hood) several times by opening and rapidly reclosing the stopcock until no liquid is left and no brown NO2 fumes escape. 6. Sequentially place the bulbs (to just below the stopcock) into a water bath to thermally equilibrate at approximately 30°C, venting occasionally to ensure that the bulb pressure is the same as the ambient pressure. While Venting: keep the body of the bulb immersed in the constant temperature bath within the hood. Dry the bulbs, allow them to cool to room temperature, and weigh on the analytical balance. Be sure to clean off any fingerprints and that no water has been trapped around the stopcock or bulb inlet. While cooling and weighing the bulbs, increase the temperature of the bath by ~5°C. Repeat this procedure up to 60°C. CAUTION: Pressure can build up rapidly when the bulb is subjected to a large, rapid temperature change. Physical Chemistry Laboratory 7. Experiment I-4 Shut down the gas-manifold (under direction of the instructor) by isolating the pump, venting the manifold, and rapidly removing the LN2 Dewar. The cold trap should be disconnected and clamped horizontally in the hood to allow frozen N2O4 to vaporize overnight. Treatment of Data: 1. Assume an ideal mixture of ideal gases. From your data, compute the average molecular weight of the gas mixture at each temperature. Using these values, determine the degree of dissociation and the equilibrium constant, Kp, ΔGo and ΔSo for the dissociation at each temperature. From an analysis of a graph of ln Kp vs. 1/T for each bulb, determine the enthalpy of the reaction (assumed constant over the temperature range used). From the parameters obtained from the graph, compute α, Kp, ΔGo, and ΔSo at 127oC and at 25oC. 2. In your report, tabulate the volume calibration data as well as the experimental values for mass, average molar mass, α, Kp, ΔGo , ΔHo, 1/T, ln Kp, and ΔSo at each of the temperatures of the experiment as well as at 25oC and at 127oC. Discuss the significance of the sign of ΔGo as well as of the change in magnitude of the value of ΔGo with temperature. Treat each bulb separately, do not average the values from the two bulbs. 3. From the following literature values (in kJ/mol) of free energies and enthalpies of formation, compute α, ΔHo, ΔGo, Kp and ΔSo for the reaction at 25oC and at 127oC. NO2 N2O4 ΔH ΔG ΔH ΔGfo 298.15 33.095 51.258 9.079 97.787 400.15 32.217 57.534 8.514 128.110 Temp /K o f o f o f Error Analysis: 1. From the standard deviation of slope (from linear least-squares analysis), calculate the standard deviation of ΔH0. 2. From the propagation of error computation of K calculate the uncertainty in ΔG0 at a temperature in the middle of the range, 3. From the standard deviation in (1) and the propagated error in (2), calculate the uncertainty in ΔS0 at the same temperature in (2). 4. Discuss sources of systematic error in this experiment, and suggest ways of eliminating as many of them as possible.