16.1 a) RATE EXPRESSION
advertisement

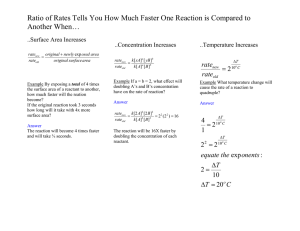
16.1 a) RATE EXPRESSION UNDERSTANDINGS - Reactions may occur by more than one step and the slowest step determines the rate of reaction (rate-determining step / RDS). - The molecularity of an elementary step is the number of reactant particles taking part in that step. - The order of reaction can be either integer or fractional in nature. The order of a reaction can describe, with respect to a reactant, the number of particles taking part in the rate-determining step. - Rate equations can only be determined experimentally. - The value of the rate constant (k) is affected by temperature and its units are determined from the overall order of the reaction. - Catalysts alter a reaction mechanism, introducing a step with lower activation energy. APPLICATION AND SKILLS - Deduction of the rate equation from experimental data and solving problems involving the rate equation. - Sketching, identifying, and analyzing graphical representations for zero, first, and second order reactions. - Evaluation of proposed reaction mechanisms to be consistent with kinetic and stoichiometric data. NATURE OF SCIENCE - Principle of Occam’s Razor – newer theories need to remain as simple as possible while maximizing explanatory power. The low probability of three-molecule collisions means stepwise reaction mechanisms are more likely. EXAMPLE: DETERMINING A RATE LAW EXPRESSION FROM EXPERIMENTAL DATA 1. When aqueous bromate and bisulfite ions react to produce bromine, the overall equation is: 2BrO3-(aq) + 5HSO3-(aq) Br2(g) + 5SO42-(aq) + H2O(l) + 3H+(aq) a) Consider the series of experiments recorded in the following table, in which initial reactant concentrations are varied and rates are compared. From the data provided, determine the rate law expression. b) Determine the value of the rate constant, K, by substituting values from _________ trial into the rate law equation. c) Determine the units of K. (You will almost always be asked to do this.) Trial Initial [BrO3-(aq)] / mmol dm-3 Initial [HSO3-(aq)] / mmol dm-3 1 2 3 4.0 2.0 2.0 6.0 6.0 3.0 Initial rate of Br2(g) production / mmol dm-3 s-1 16.0 8.0 2.0 *THE RATE LAW EXPRESSION: The rate will always be proportional to the product of the ____________ concentrations of the reactants, where these concentrations are raised to some exponential value. The Rate Expression is always _____________________determined. *This can be expressed Rate *The rate law equation Rate = Where: k = __________ constant, [X] and [Y] = _____________ concentrations of reactants, m is the order with respect to reactant _____, n is the order with respect to reactant _____, m + n is the _____________ order of reaction. Example: rate = k[X]1[Y]2 [Z]0 *actually written *Why? *The Overall Order of Reaction = (i.e. This equation represents a _______ order reaction) Individual Orders: *order of reaction with respect to X = ____ *order of reaction with respect to Y = ____ *order of reaction with respect to Z = ____ *What do the exponents (i.e. individual orders) mean? a) Exponent 0 (e.g. r α [Z]0): Doubling the initial concentration of Z, has ____ __________ on the rate. Tripling the initial concentration of Z, has ___ _________ on the rate, and so on. (*i.e., changing the initial concentration of the reactant has ____ _______ on the rate when the order is equal to ________.) *This is why “[Z]0” does not appear in the rate equation. b) Exponent 1 (e.g. r α [X]1): Doubling the initial concentration of X, __________ the rate. Tripling the initial concentration of X, ___________ the rate, and so on. (*i.e., whatever happens to the initial concentration of X ______ happens to the rate when the order is equal to ______.) c) Exponent 2 (e.g. r α [Y]2): Doubling the initial concentration of Y, _______________(22) the rate. Tripling the initial concentration of Y, increases the rate by a factor of _____ (32), and so on. (*i.e., the rate change is equal to whatever happens to the initial concentration of Y, __________… when the order is equal to _______.) Summary: ORDER OF REACTION Concentration 0 1 2 3 Change x1 x 2 (doubling) x 3(tripling)