What Happens When You Breathe?
advertisement

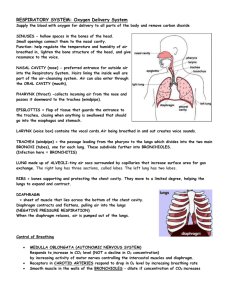
What Happens When You Breathe? Ventilation – the mechanical aspect of breathing To move air into the lungs, the diaphragm must contract. The diaphragm is a large, dome-shaped muscle at the bottom of the thoracic (chest) cavity. Inhalation: the diaphragm contracts, flattens and moves down. The chest muscles (intercostals muscles) also thrust the chest up and out. These two muscle actions create a partial vacuum in the thoracic cavity. Because the mouth or nose is open, air will rush inwards to equalize the pressure between the thoracic cavity and outside the body. Exhalation: the diaphragm relaxes, pushes back up, intercostal muscles pull the rib cage back and down. These muscles relaxing create a higher pressure inside the thoracic cavity, pushing air out of the lungs. Air entering the nose: - contains foreign particles (dust) - may be cold due to low air temperature Turbinate bones: - three bones in the nose that are covered by mucous membranes. - Incoming air is moistened rapidly - Ciliated cells help trap dust and move it to the throat to be swallowed - Cilia move with a whip-like action. Incoming air passes the tonsils and the adenoids. These are small pieces of soft tissue that help fight infectious agents like viruses and bacteria. Air passes through the larynx (voice box). Membranes (vocal folds) stretched across the larynx vibrate with the incoming air to produce sounds when you exhale. When you learn to speak, you learn to control these vocal folds. Trachea - is also lined with mucous membrane to trap particles that get past the turbinate bones - ciliated cells also move trapped particles up toward pharynx so they can be swallowed or discharged Bronchi - two large passageways that branch off of trachea, one for each lung. Bronchioles - number approximately 250 000, each about 0.5 mm in diameter - each is surrounded by smooth muscle and lined with a thin epithelium (tissue layer) Alveoli - each bronchiole is capped with several bubble like sacs called alveoli (singular: alveolus) that resemble a bunch of grapes - many blood capillaries surround each alveolus - the epithelium of the alveoli are coated in a surfactant which increases the likelihood that air will contact the epithelium - this increases the chance of oxygen diffusing into the lungs - O2 as well as CO2 diffusion occurs here 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Hazards to Breathing CO – carbon monoxide – colourless, odourless gas byproduct of incomplete combustion – your hemoglobin in the red blood cells has an affinity for CO over O2 – a concentration of 0.04 – 0.06% of CO in the air will cause headaches in one hour – higher concentrations and longer exposure increases the risk – many effects are reversible: headache, nausea, cherry-red discoloration of the skin – to aid someone with CO poisoning, immediately get them into fresh air Asbestos – fibrous material that occurs naturally in rock formations - properties are well suited for use as an insulating material: i) heat resistant ii) can be woven into fabric - was commonly used in school ceilings, curtains, car brake linings, hair dryers, protective gloves/suits. - small particles break off and are inhaled into the lungs - lungs cannot expel material, so normal lung tissue grows scar tissue around particles – this can block alveoli and reduces effective lung volume - causes difficulty in breathing and a dry cough - asbestos is also a carcinogen and so can cause cancer - preventative measures: o reduce dust particles in the air through the use of fans, air filters o good industrial housekeeping o covering the nose and mouth with a protective filter mask o use alternative fire retardant materials - major changes in industry have reduced the amount of asbestos used since the 1980s. Air Quality Index - an indicator of air quality, based on hourly pollutant measurements of some or all of the six most common air pollutants: sulphur dioxide, ozone, nitrogen dioxide, total reduced sulphur compounds, carbon monoxide and fine particulate matter - Several state-of-the-art air monitoring stations, operated by the Ministry across the province, form the Air Quality Index (AQI) network - At the end of each hour, the concentration of each pollutant that the AQI station monitors is converted into a number ranging from zero upwards, using a common scale, or index. The pollutant with the highest number at a given hour becomes the AQI reading. As the air quality changes, the AQI reading increases or decreases. The lower the AQI reading, the cleaner the air. What do the readings mean? Below 16, the air quality is in the very good category. From 16 to 31, the air quality is in the good category. From 32 to 49, the air quality is in the moderate category, and there may be some adverse effects for very sensitive people. From 50 to 99, the air quality is in the poor category, and may have adverse effects for sensitive members of human and animal populations, and may cause significant damage to vegetation and property. Above 99, the air quality is in the very poor category, and may have adverse effects for a large proportion of those exposed. www.airqualityontario.com Smoking - cigarette smoke contains about 4,000 chemical agents - these include over 60 carcinogens such as formaldehyde, as well as four others which affect normal cell development such as nicotine and CO. - which chemicals are present depend on factors such as the type of tobacco, the chemicals added to the tobacco, how the product is smoked, and the paper in which the tobacco is wrapped - in addition, many of these substances, such as carbon monoxide, tar, arsenic, and lead, are poisonous and toxic to the human body. - see www.smoke-free.ca/Health/Healtheffectssmoke.htm for a list of several chemicals in cigarette smoke and the effects each one can have on human health - nicotine is a drug that is naturally present in the tobacco plant and is primarily responsible for a person’s addiction to tobacco products - when smoke is inhaled, tar is deposited on mucous membrane of the entire respiratory system (mouth, pharynx, trachea, bronchi, bronchioles, alveoli) - nicotine paralyzes the ciliated cells – mucous cannot be moved towards throat - tar accumulates until the cells become altered - altered cells being to produce abnormal cells – cancerous cells - besides the risk to the smoker, there is an increased risk to others through second-hand smoke environmental tobacco smoke (ETS) - Smoking is responsible for one in five deaths in Canada. This is roughly five times the number of deaths caused by car accidents, suicides, drug abuse, murder and AIDS combined – this is about 45 000 deaths per year in Canada due to smoking related illnesses How is Gas Exchanged? - in order to understand gas exchange, you must first understand a little about the physical nature of gases - Dalton’s Law of Partial Pressure states that each gas in a mixture exerts its own pressure, or partial pressure. - In general, the partial pressure of a gas can be calculated using the percentage of volume that it makes up out of the atmosphere - Standard atmospheric pressure is approximately 101 kPa (at sea level) – all the gases in the atmosphere make up a part of this pressure. o e.g.: oxygen makes up approximately 21% of the gas in the atmosphere, so if we take 21% of 101 kPa, the partial pressure of O2 is 21.21 kPa. o e.g.: carbon dioxide makes up approx. 0.03% of the atmosphere, so it’s partial pressure is (101 kPa) x 0.0003 = 0.0303 kPa. - Gases always diffuse from an area of high partial pressure to an area of lower partial pressure. - This works well for gas exchange, because the partial pressure of oxygen is high inside the alveoli and low in the blood in the alveolar capillaries. - Likewise (but opposite) the CO2 partial pressure is high in the alveolar capillaries, and low in the air in the alveoli. Despite diffusion working in our favour, this is not enough to provide adequate O2 to the tissues. Oxygen transport - oxygen is not very soluble – only about 0.3 mL of O2 can dissolve in 100 mL of plasma - hemoglobin in the erythrocytes carries most of the O2 (about 20mL per 100 mL of plasma) - the amount of O2 that hemoglobin can carry depends on the partial pressure of O2 - this characteristic of hemoglobin is ideal for gas exchange - when partial pressure of O2 is high (at lungs) the hemoglobin has a very high affinity for O2 – this means it is easy for O2 to attach to the hemoglobin (see figure 2 on page 292 or below) - when partial pressure of O2 is low (at tissues) hemoglobin’s affinity for O2 drops significantly, which releases causes hemoglobin to release it’s oxygen. - only when the partial pressure of O2 drops to about 5.3 kPa does it become easy to release O2 – fortunately, this only occurs after the blood has traveled through the arteries, arterioles and then into the capillaries – precisely where it is supposed to be dropped off. - Not all of the oxygen is dropped off at the capillaries - the blood still contains about 70% of its oxygen when it returns to the heart – but exchange only occurs at the capillaries - Each hemoglobin molecule can carry four O2 molecules – when the first one attaches to hemoglobin, it becomes easier for the hemoglobin to attach a second O2 molecule, then a third, and so forth – in other words, the affinity increases with each O2 molecule added. Carbon dioxide transport - carbon dioxide is transported in three ways in the blood o about 9% is dissolved in the plasma o about 27% combines with hemoglobin to form carbaminohemoglobin o about 64% is converted into carbonic acid - that last method involves a series of reactions: 1) 2) 3) carbon dioxide + water in plasma → carbonic acid CO2 + H2O → H2CO3 carbonic acid → hydrogen ion + bicarbonate ion H2CO3 → H+ + HCO3hydrogen ion + bicarbonate ion → water + carbon dioxide + H + HCO3 → H2O + CO2 - in step one, an enzyme called carbon anhydrase speeds up this reaction 250 times - this reduces the concentration of CO2 in the plasma, so that more can diffuse and dissolve into the plasma - this raises the acidity (lowers the pH) of the blood – this can be bad, so hemoglobin also acts like a buffer - when the carbonic acid forms in reaction one, reaction two occurs quickly on its own. + - these free H ions attach to hemoglobin (and in the process they displace O2) – this is good because it occurs at the tissues – just where we want O2 to be dropped off. + - By picking up H ions, this brings the pH back down to normal + - When the blood returns to the lungs, it is still carrying the H ions, but these are displaced by the high partial pressure of O2 + - the H ions are now free to combine with the bicarbonate ions and the entire set of reactions go in reverse. - Even carbonic anhydrase can make the reverse reaction for reaction #1. Maintaining gas levels: - there are several chemoreceptors (special sensors) in the body which constantly measure CO2 and O2 levels in the blood - in the aorta and the carotid artery, there are oxygen sensors - in the medulla oblongata (part of the brain) there is a CO2 - the medulla will send a nerve impulse to the diaphragm to contract at a greater rate or lower rate depending on the conditions