5.1 Properties and changes
advertisement

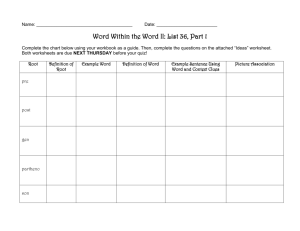
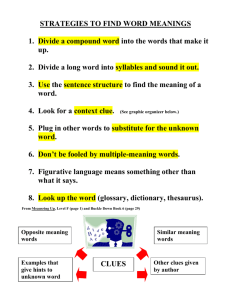
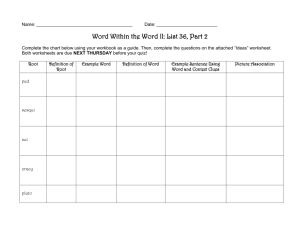
5.1 Properties and changes Properties of matter Physical Property: • A characteristic or description of a substance – Examples: colour, texture, density, smell, solubility, taste, melting point, physical state Two types: Qualitative - described using your senses Quantitative - A property of a substance that is measured and HAS a numerical value. Properties of matter Chemical Property: • A characteristic behaviour that occurs when the substance changes into something new – Examples: flammability, corrosion, bleaching ability Changes Physical Change: • A change that does not produce a new substance • Most physical changes can be reversed Examples: – Change of State – Dissolving sugar in water Changes Chemical Change: • A change that produces a new substance • Many chemical changes cannot be reversed Examples: – Forest fire – Boiling an egg Five Clues that a Chemical Change occurred 1. A new colour appears. Five Clues that a Chemical Change occurred 2. Heat or light is produced or absorbed. Five Clues that a Chemical Change occurred 3. Bubbles of gas are formed. Five Clues that a Chemical Change occurred 4. A solid material (precipitate) forms in a liquid. Five Clues that a Chemical Change occurred 5. The change is generally difficult to reverse.