Work and Energy Synthesis Exchange Reactions Reactions
advertisement
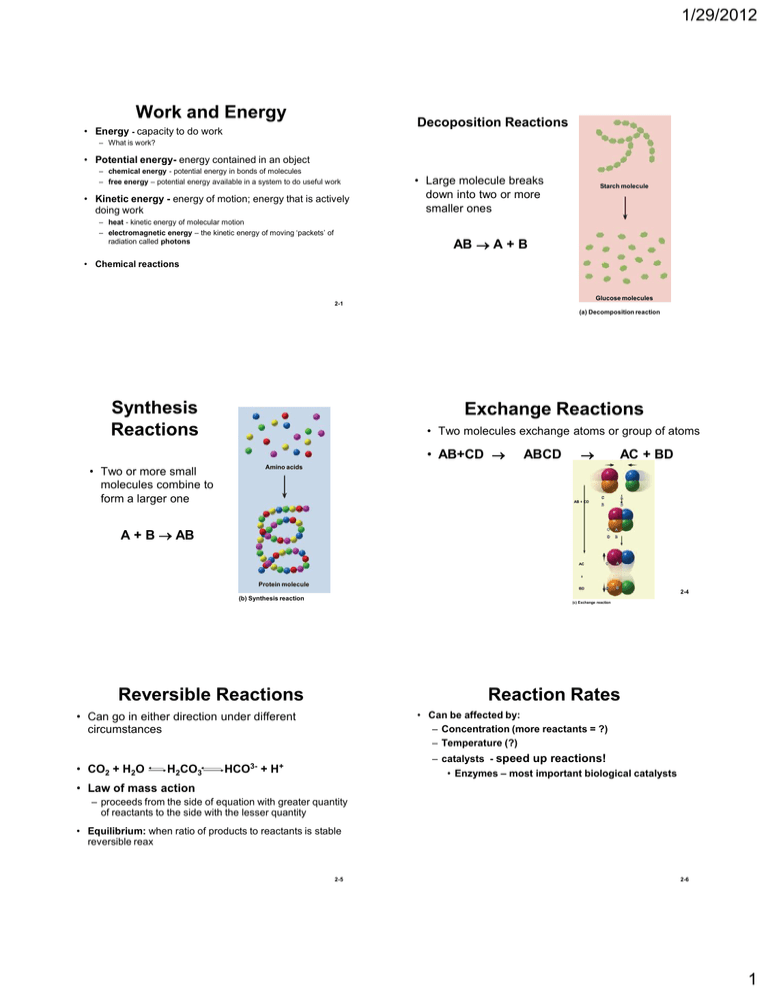
1/29/2012 Work and Energy Decoposition Reactions • Energy - capacity to do work – What is work? • Potential energy- energy contained in an object – chemical energy - potential energy in bonds of molecules – free energy – potential energy available in a system to do useful work • Kinetic energy - energy of motion; energy that is actively doing work – heat - kinetic energy of molecular motion – electromagnetic energy – the kinetic energy of moving ‘packets’ of radiation called photons • Large molecule breaks down into two or more smaller ones Starch molecule AB A + B • Chemical reactions Glucose molecules 2-1 (a) Decomposition reaction Synthesis Reactions Exchange Reactions • Two molecules exchange atoms or group of atoms • AB+CD • Two or more small molecules combine to form a larger one ABCD AC + BD Amino acids AB + CD A + B AB AC C A D B C A D B C A D B + Protein molecule BD (b) Synthesis reaction Reversible Reactions Reaction Rates • Can be affected by: – Concentration (more reactants = ?) – Temperature (?) • Can go in either direction under different circumstances • CO2 + H2O H2CO3 2-4 (c) Exchange reaction – catalysts - speed up reactions! • Enzymes – most important biological catalysts HCO3- + H+ • Law of mass action – proceeds from the side of equation with greater quantity of reactants to the side with the lesser quantity • Equilibrium: when ratio of products to reactants is stable reversible reax 2-5 2-6 1 1/29/2012 Oxidation-Reduction Reactions Metabolism • Oxidation • All the chemical reactions of the body – A molecule gives up electrons (or H+) – The molecule is oxidized in this process • Catabolism – energy releasing (exergonic) decomposition reax • produces smaller molecules • Anabolism • Reduction – a molecule gains electrons (or H+) – molecule is reduced when it accepts electrons • oxidation-reduction (redox) reactions – energy storing (endergonic) synthesis reax – oxidation of one molecule is always accompanied by the reduction of another – Electrons are often transferred as H • requires energy input • production of protein or fat • driven by energy that catabolism releases 2-7 2-8 Organic Molecules and Carbon Organic Compounds • 4 valence electrons – binds with other atoms that can provide it with four more electrons to fill its valence shell • 4 categories of carbon compounds – carbohydrates – lipids – proteins – Nucleotides & nucleic acids • carbon atoms bind readily with each other – carbon backbones – forms long chains, branched molecules and rings – forms covalent bonds with hydrogen, oxygen, nitrogen, sulfur, and other elements • carbon backbone carries a variety of functional groups 2-9 2-10 Functional Groups • small clusters of atoms attached to carbon backbone Name and Symbol Hydroxyl (—OH) Structure O H Sugars, alcohols H Fats, oils, steroids, amino acids AKA ―condensation reaction‖ – A hydroxyl group is removed from one monomer and a hydrogen from the next H Methyl (—CH3) C H • determines many of the properties of organic molecules Dehydration Synthesis Occurs in O Carboxyl (—COOH) Amino acids, sugars, proteins C Dimer O H Monomer 1 Monomer 2 H Amino (—NH2) Amino acids, proteins N H OH O HO H H+ + OH– O Phosphate (—H2PO4) O O P Nucleic acids, ATP H2O (a) Dehydration synthesis O H Figure 2.15a 2-12 2 1/29/2012 Organic Molecules: Carbohydrates Hydrolysis • Splitting a polymer (lysis) by addition of a water molecule – a covalent bond is broken • All digestion reax consists of hydrolysis reactions • hydrophilic organic molecule • general formula – (CH2O)n n = number of carbon atoms – for glucose, n = 6, so formula is C6H12O6 Dimer Monomer 1 Monomer 2 OH O H2O HO H+ + OH– (b) Hydrolysis Figure 2.15b 2-13 Monosaccharides • Simplest carbohydrates – simple sugars Glucose • composed of 2 monosaccharides O HO • 3 important monosaccharides – glucose, galactose and fructose – same molecular formula - C6H12O6 • Isomers! – produced by digestion of complex carbohydrates Disaccharides CH2OH H Galactose H H OH H H OH • 3 important disaccharides – sucrose - table sugar – lactose - sugar in milk • glucose + galactose – maltose - grain products • glucose + glucose CH2OH H H H OH H H OH OH Fructose O HOCH2 H OH H HO OH Figure 2.16 Sucrose CH2OH O H H OH H H H HO CH2OH OH OH CH2OH H H OH O HO H OH OH O OH H H H H H H OH H O H CH2OH Maltose CH2OH CH2OH O H H OH H H OH O H O HO 2-15 Polysaccharides H O Lactose H CH2OH CH2OH O H H HO OH O HO 2-14 H OH H H OH OH H Figure 2.17 2-16 Carbohydrate Functions • long chains of glucose • quickly mobilized source of energy • 3 polysaccharides of interest in humans – Glycogen: energy storage in animals • made in liver, muscles, brain, uterus, and vagina – Starch: energy storage polysaccharide in plants • only significant digestible polysaccharide in the human diet – all digested carbohydrates converted to glucose – oxidized to make ATP • Conjugated carbohydrate: bound to lipid or protein – Glycolipids: cell membrane – Glycoproteins: cell membrane – proteoglycans (Carb. dominant & peptide/or protein) • gels that hold cells and tissues together – Cellulose: structural molecule of plant cell walls (fiber) 2-17 2-18 3 1/29/2012 Fatty Acids Organic Molecules: Lipids • Chain of 4 to 24 carbon atoms • hydrophobic organic molecule – composed of carbon, hydrogen and oxygen – high ratio of hydrogen to oxygen • More energy than carbs! – carboxyl (acid) group on one end, methyl group on the other and hydrogen bonded along the sides • Classified – saturated - carbon atoms saturated with hydrogen – unsaturated - contains C=C bonds without hydrogen – polyunsaturated – contains many C=C bonds • Five primary types in humans – fatty acids – triglycerides – phospholipids – Eicosanoids (prostaglandins) – steroids H H H H H H H H H H H H H H H C C C C C C C C C C C C C C C H H H H H H H H H H H H H H H O C H HO Palmitic acid (saturated) CH3(CH2)14COOH 2-19 Lipids - Triglycerides Figure 2.19 2-20 Phospholipids • Formed by condensation of 1 glycerol and 3 fatty acids Dehydration Synthesis • structural foundation of cell membrane – fatty acid ―tails‖ are hydrophobic – phosphate ―head‖ is hydrophilic CH3 N+ CH3 CH3 Nitrogencontaining group (choline) CH2 CH2 O –O P Phosphate group O Hydrophilic region (head) O CH2 O O C (CH2)5 CH CH Glycerol CH2 O C O (CH2)12 Fatty acid tails CH3 Hydrophobic region (tails) CH (CH2)5 CH3 (a) (b) 2-36 Eicosanoids (prostaglandins) • Chemical regulators • produced in all tissues – role in inflammation, blood clotting, hormone action, labor contractions, blood vessel diameter O Steroids • 3 6-C rings bonded to a 5-C ring • Cholesterol – • precurser from which other steroids are synthesized – synthesized only by animals • especially liver cells – important component of cell membranes – required for proper nervous system function COOH Figure 2.21 2-23 OH 2-24 OH 4 1/29/2012 Organic Molecules: Proteins Representative Amino Acids Some nonpolar amino acids Some polar amino acids Methionine • protein - a polymer of amino acids H Cysteine H H N • amino acid – central carbon with 3 attachments C H – amino group (NH2), carboxyl group (COOH) and radical group (R group) CH2 CH2 S C H CH3 OH O Tyrosine H SH C H H N CH2 OH H C O OH Arginine H H N • Essential A.A.s CH2 C C O • 20 amino acids differ only in functional (R) group H N C NH2+ (CH2)3 NH O OH C NH2 C OH (a) • Note: they differ only in the R group 2-25 2-26 Structure of Proteins Naming of Peptides • peptide – molecule composed of two or more amino acids joined by peptide bonds Amino acids – amino group of one A.A. bonds to carboxyl group of other A.A. • dehydration synthesis/condensation H H • Peptides named number of amino acids H – dipeptides have 2 – tripeptides have 3 – oligopeptides < 15 – polypeptides > 15 – proteins have > 50 N H O C + C N OH R1 H OH C Amino acid 2 Beta sheet C C C N C C Secondary structure C C H Folding and coiling due to interactions among R groups and between R groups and surrounding water C Alpha helix A dipeptide O Tertiary structure C C R2 H Sequence of amino acids joined by peptide bonds O H Amino acid 1 Primary structure Peptide bonds H N C H R2 C O + C H2O Alpha helix or beta sheet formed by hydrogen bonding C Beta chain Alpha chain C C C Chain 1 H R1 OH Chain 2 Alpha chain Denature Beta chain 2-28 Peptide bond Protein Functions Quaternary structure Association of two or more polypeptide chains with each other Heme groups Enzymes • Structure • communication (hormones/receptors) • Membrane Transport – channels in cell membranes – carrier proteins on cell membranes • Muscle activity • Catalysts (enzymes) • Enzymes - function as biological catalysts – permit reactions to occur rapidly • Substrate: substance an enzyme acts upon • Products: result of chemical reax • Naming Convention – ends in ―ase‖ • Lipase = enzyme digests Lipids • Protease = digests proteins • Deoxyribonuclease = digests ?? • Immunity/Protection (recognition/antibodies/clotting proteins) • Movement (motor proteins) • Cell adhesion 2-29 2-30 5 1/29/2012 Enzymes and Activation Energy Enzyme Structure and Action • Substrate approaches active site on enzyme molecule Free energy content Activation energy Activation energy Net energy released by reaction Energy level of reactants Net energy released by reaction • Substrate binds to active site forming enzyme-substrate complex – highly specific fit – enzyme-substrate specificity • Reaction products released • Enzyme remains unchanged and is ready to repeat the process Energy level of products Time (a) Reaction occurring without a catalyst Time (b) Reaction occurring with a catalyst 2-31 2-32 Enzymatic Reaction Steps Sucrose (substrate) 1 Enzyme and substrate O Active site Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the ―Normal‖ or ―Slide Sorter‖ views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer. Sucrase (enzyme) 2 Enzyme–substrate complex O Glucose 3 Enzyme and reaction products Fructose Figure 2.27 2-33 Enzymatic Action: Important Points!! Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the ―Normal‖ or ―Slide Sorter‖ views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer. • Reusability of enzymes • Astonishing speed – one enzyme molecule can consume millions of substrate molecules per minute • Factors that change enzyme shape – pH and temperature 2-36 6 1/29/2012 Cofactors and Coenzymes Coenzyme NAD+ • Cofactors: inorganic non-protein helper Glycolysis – (iron, copper, zinc, magnesium and calcium ions) – binds to enzyme induces a change in shape, which activates active site Glucose Aerobic respiration Pyruvic acid ADP + Pi • Coenzymes – organic cofactors derived from water-soluble vitamins (niacin, riboflavin) – accept electrons from an enzyme in one pathway and transfer them to an enzyme in another ATP Pyruvic acid • 2-37 CO2 + H2O Figure 2.28 NAD+ transports electrons from one metabolic pathway to another 2-38 Metabolic Pathways Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the ―Normal‖ or ―Slide Sorter‖ views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer. • Chain of reactions, with each step usually catalyzed by a different enzyme • ABCD • A is initial reactant, B+C are intermediates and D is the end product • Regulation of metabolic pathways – activation or deactivation of enzymes – cells can turn on or off pathways 2-40 Organic Molecules: Nucleotides Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the ―Normal‖ or ―Slide Sorter‖ views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer. • 3 components of nucleotides 1. nitrogenous base 2. sugar (monosaccharide) 3. one or more phosphate groups • ATP – best known nucleotide – adenine (nitrogenous base) – ribose (sugar) – phosphate groups (3) 2-42 7 1/29/2012 ATP (Adenosine Triphosphate) Adenine NH2 C – Phosphate group, sugar, nitrogenous base C N N C N N C CH HC CH C HC C N N Nucleic Acids • polymers of nucleotides Adenine NH2 • DNA (deoxyribonucleic acid) N N • body’s most important Ribose energy-transfer molecule Adenosine Ribose Triphosphate O –O O P O –O P O –O O P Monophosphate O CH2 O O –O HO H H OH (a) Adenosine triphosphate (ATP) P CH2 OH O O H H H H O H H OH (b) Cyclic adenosine monophosphate (cAMP) ATP contains adenine, ribose and 3 phosphate groups – constitutes genes • instructions for synthesizing all of the body’s proteins • transfers hereditary information from cell to cell and generation to generation 2-43 • RNA (ribonucleic acid) – 3 types – messenger RNA, ribosomal RNA, transfer RNA 2-44 8





