The Relationship Between Atomic Size, Charge, and Polarizability Introduction
advertisement
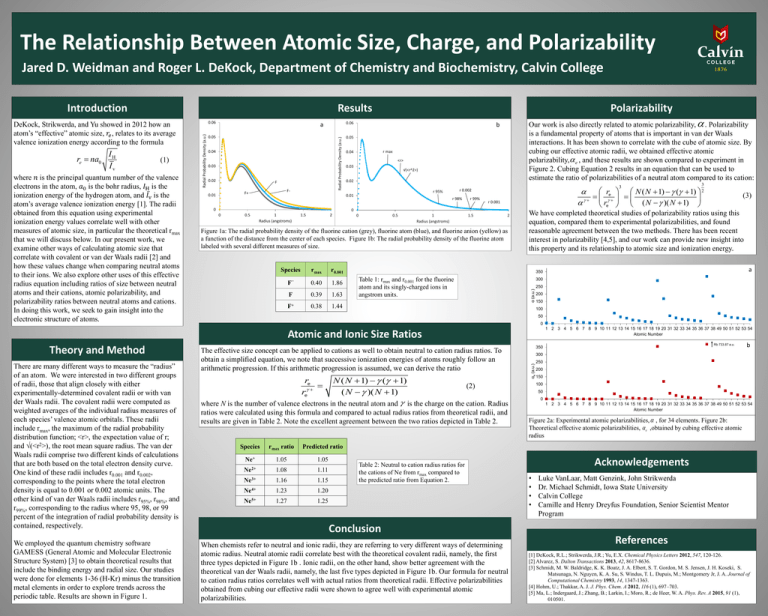
The Relationship Between Atomic Size, Charge, and Polarizability Jared D. Weidman and Roger L. DeKock, Department of Chemistry and Biochemistry, Calvin College Results Introduction where 𝑛 is the principal quantum number of the valence electrons in the atom, 𝑎0 is the bohr radius, 𝐼H is the ionization energy of the hydrogen atom, and 𝐼v is the atom’s average valence ionization energy [1]. The radii obtained from this equation using experimental ionization energy values correlate well with other measures of atomic size, in particular the theoretical rmax that we will discuss below. In our present work, we examine other ways of calculating atomic size that correlate with covalent or van der Waals radii [2] and how these values change when comparing neutral atoms to their ions. We also explore other uses of this effective radius equation including ratios of size between neutral atoms and their cations, atomic polarizability, and polarizability ratios between neutral atoms and cations. In doing this work, we seek to gain insight into the electronic structure of atoms. 0.05 0.04 0.03 0.02 F F- F+ 0.01 0.05 r max 0.04 <r> 0.03 √(<r^2>) 0.02 3 re N ( N 1) ( 1) re ( N )( N 1) r 0.002 r 95% 0.01 0 Our work is also directly related to atomic polarizability, . Polarizability is a fundamental property of atoms that is important in van der Waals interactions. It has been shown to correlate with the cube of atomic size. By cubing our effective atomic radii, we obtained effective atomic polarizability, e , and these results are shown compared to experiment in Figure 2. Cubing Equation 2 results in an equation that can be used to estimate the ratio of polarizabilities of a neutral atom compared to its cation: b r 98% r 99% r 0.001 0 0 0.5 1 Radius (angstroms) 1.5 2 0 0.5 1 Radius (angstroms) 1.5 2 Figure 1a: The radial probability density of the fluorine cation (grey), fluorine atom (blue), and fluorine anion (yellow) as a function of the distance from the center of each species. Figure 1b: The radial probability density of the fluorine atom labeled with several different measures of size. Species – rmax r0.001 F 0.40 1.86 F 0.39 1.63 F+ 0.38 Table 1: rmax and r0.001 for the fluorine atom and its singly-charged ions in angstrom units. We employed the quantum chemistry software GAMESS (General Atomic and Molecular Electronic Structure System) [3] to obtain theoretical results that include the binding energy and radial size. Our studies were done for elements 1-36 (H-Kr) minus the transition metal elements in order to explore trends across the periodic table. Results are shown in Figure 1. a 300 250 1.44 200 150 100 50 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 31 32 33 34 35 36 37 38 49 50 51 52 53 54 Atomic Number The effective size concept can be applied to cations as well to obtain neutral to cation radius ratios. To obtain a simplified equation, we note that successive ionization energies of atoms roughly follow an arithmetic progression. If this arithmetic progression is assumed, we can derive the ratio N ( N 1) ( 1) (2) ( N )( N 1) where N is the number of valence electrons in the neutral atom and is the charge on the cation. Radius re re ratios were calculated using this formula and compared to actual radius ratios from theoretical radii, and results are given in Table 2. Note the excellent agreement between the two ratios depicted in Table 2. Species rmax ratio Predicted ratio Ne+ 1.05 1.05 Ne2+ 1.08 1.11 Ne3+ 1.16 1.15 Ne4+ 1.23 1.20 Ne5+ 1.27 1.25 Table 2: Neutral to cation radius ratios for the cations of Ne from rmax compared to the predicted ratio from Equation 2. Conclusion When chemists refer to neutral and ionic radii, they are referring to very different ways of determining atomic radius. Neutral atomic radii correlate best with the theoretical covalent radii, namely, the first three types depicted in Figure 1b . Ionic radii, on the other hand, show better agreement with the theoretical van der Waals radii, namely, the last five types depicted in Figure 1b. Our formula for neutral to cation radius ratios correlates well with actual ratios from theoretical radii. Effective polarizabilities obtained from cubing our effective radii were shown to agree well with experimental atomic polarizabilities. Rb 733.87 a.u. 350 b 300 αe (a.u.) There are many different ways to measure the “radius” of an atom. We were interested in two different groups of radii, those that align closely with either experimentally-determined covalent radii or with van der Waals radii. The covalent radii were computed as weighted averages of the individual radius measures of each species’ valence atomic orbitals. These radii include rmax, the maximum of the radial probability distribution function; <r>, the expectation value of r; and √(<r2>), the root mean square radius. The van der Waals radii comprise two different kinds of calculations that are both based on the total electron density curve. One kind of these radii includes r0.001 and r0.002, corresponding to the points where the total electron density is equal to 0.001 or 0.002 atomic units. The other kind of van der Waals radii includes r95%, r98%, and r99%, corresponding to the radius where 95, 98, or 99 percent of the integration of radial probability density is contained, respectively. (3) 350 Atomic and Ionic Size Ratios Theory and Method 3 2 We have completed theoretical studies of polarizability ratios using this equation, compared them to experimental polarizabilities, and found reasonable agreement between the two methods. There has been recent interest in polarizability [4,5], and our work can provide new insight into this property and its relationship to atomic size and ionization energy. α (a.u.) (1) 0.06 a Radial Probability Density (a.u.) re na0 IH Iv 0.06 Radial Probability Density (a.u.) DeKock, Strikwerda, and Yu showed in 2012 how an atom’s “effective” atomic size, 𝑟𝑒 , relates to its average valence ionization energy according to the formula Polarizability 250 200 150 100 50 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 31 32 33 34 35 36 37 38 49 50 51 52 53 54 Atomic Number Figure 2a: Experimental atomic polarizabilities, , for 34 elements. Figure 2b: Theoretical effective atomic polarizabilities, e ,obtained by cubing effective atomic radius Acknowledgements • • • • Luke VanLaar, Matt Genzink, John Strikwerda Dr. Michael Schmidt, Iowa State University Calvin College Camille and Henry Dreyfus Foundation, Senior Scientist Mentor Program References [1] DeKock, R.L.; Strikwerda, J.R.; Yu, E.X. Chemical Physics Letters 2012, 547, 120-126. [2] Alvarez, S. Dalton Transactions 2013, 42, 8617-8636. [3] Schmidt, M. W. Baldridge, K. K. Boatz, J. A. Elbert, S. T. Gordon, M. S. Jensen, J. H. Koseki, S. Matsunaga, N. Nguyen, K. A. Su, S. Windus, T. L. Dupuis, M.; Montgomery Jr, J. A. Journal of Computational Chemistry 1993, 14, 1347-1363. [4] Hohm, U.; Thakkar, A. J. J. Phys. Chem. A 2012, 116 (1), 697–703. [5] Ma, L.; Indergaard, J.; Zhang, B.; Larkin, I.; Moro, R.; de Heer, W. A. Phys. Rev. A 2015, 91 (1), 010501.