Information in Radio Waves Introduction:
advertisement

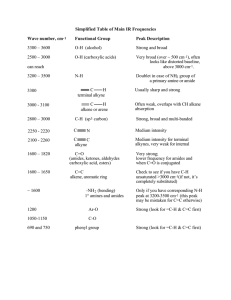
Information in Radio Waves Name: ________________________________________ Class: ________ Date: ________ Identifying Life and its Origins Through Spectroscopy Introduction: One area of study that has always intrigued scientists is the question of where did life begin, and not only where but how? Ancient civilizations have explored this question through studies in philosophy and religion and those studies continue today. Now though, the methods of science and observation allow us to study origins of life in a standardized and clear way. We now have an understanding of the basic organic molecules that make life possible. Through the work of organic chemists, these compounds have been broken down and analyzed to the scale of their molecular and even atomic structure. Because of this, scientists have been able to pinpoint likely compounds that served as intermediate steps in building organic molecules as well as likely conditions in which they formed. Proof of this is the Miller-Urey experiment in 1953. The Miller-Urey experiment tested an earlier hypothesis that stated early conditions on Earth were favorable for the production of organic compounds from pre-existing organic precursors. This theory that life was able to originate from non-living precursor compounds is called abiogenesis. Stanley Miller and Harold Urey were successfully able to, through an interesting lab set-up, produce amino acids artificially. Amino acids as you should remember are the building blocks of protein and are theorized to be the original organic molecules. The question is, how did the scientists know they produced those molecules? How did they detect them? In the first part of this activity, you will be working with spectral data to identify functional groups and eventually full amino acids. If you remember, spectroscopy is the study of detecting emission and absorption of specific electromagnetic wavelengths, aka light. Part 1: Identifying Functional Groups and Amino Acids One type of spectroscopy particularly useful in identifying organic compounds is infrared spectroscopy. In this type of spectroscopy, infrared light is passed through a compound and particular wavelengths are absorbed in this range based on what types of functional groups the compound contains. The resulting spectra can usually be broken down into two regions, the functional group region along with the “fingerprint” region. The functional group region is going to be similar to all compounds containing the same groups while the fingerprint region will be unique to each and every compound. Because of this, the functional group region is usually the first place you look when identifying a compound as each functional group contained will have a characteristic band and shape. Assignment: Refer to Handout A and use that information to identify the functional groups present in each spectra provided in numbers 1 - 4. In numbers 5-8, use Handouts A and B to identify which amino acid that spectra identifies overall. For numbers 5-8, there will likely be more than one answer. Information in Radio Waves 1.) Functional groups present: _____________________ ____________________ _______________________ 2.) Functional groups present: _____________________ ____________________ _______________________ Information in Radio Waves 3.) Functional groups present: _____________________ ____________________ _______________________ 4.) Functional groups present: _____________________ ____________________ _______________________ Information in Radio Waves 5.) Functional groups present: _____________________ ____________________ _______________________ Which amino acids could this represent? Explain. 6.) Information in Radio Waves Functional groups present: _____________________ ____________________ _______________________ Which amino acids could this represent? Explain. 7.) Functional groups present: _____________________ ____________________ _______________________ Which amino acids could this represent? Explain. Information in Radio Waves 8.) Functional groups present: _____________________ ____________________ _______________________ Which amino acids could this represent? Explain. Part 2: Take Home In 2007, scientists looked back at the vials that were sealed from the Miller-Urey experiment. They re-tested these vials and confirmed that well over twenty different amino acids were artificially produced by recreating what was thought to be early Earth atmospheric conditions. Since then, our view of what early Earth conditions were has changed but despite this, scientists argue that the updated version would be even more favorable to forming early organic compounds. There is only one problem with all of this, we can’t actually see early Earth. So how do you prove definitively that these early organic compounds can form? The answer is to look elsewhere: space! Information in Radio Waves There is one small issue here though, the IR portion of the EM spectrum that we use here on Earth to identify molecules cannot penetrate our atmosphere. So how do we see and prove that certain molecules exist elsewhere? The answer simply lies in another part of the spectrum. Just as the absorption and emission of specific frequencies in the IR spectrum can be used for identity, so can the same in other parts of the spectrum. In particular, radio waves. Detection via radio waves differs slightly from using IR. In infrared spectroscopy, molecules absorb and emit wavelengths based upon the stretching, bending, and rocking of atoms around their chemical bonds. Radio spectroscopy relies on the slight energy differences between two of more energy states in a compound. The best example of this is neutral hydrogen. Neutral hydrogen has hyperfine energy structure based upon the spin states of its one electron in the 1s orbital. When a hydrogen undergoes a ‘spin-flip’ between the two levels, it gives off a specific packet of energy with a wavelength of 21-cm (frequency of 1420 MHz). Therefore, if a radio astronomer points a radio telescope out in space and observes that wavelength, then a source containing neutral hydrogen has been found. Since hydrogen’s detection, many more compounds have been detected based on their unique radio signatures. A list of common ones can be found in the accompanying handout titled “List of Chemicals in Space.” Of extra interest though is a newly discovered molecule in space, cyanomethanimine. Information in Radio Waves Your assignment: Read the following excerpts and answer the accompanying discussion questions. Excerpt 1: https://public.nrao.edu/news/pressreleases/icy-cosmic-start-for-amino-acids-and-dna Discussion questions: 1.) What is a precursor molecule? 2.) What is significant about the finding of cyanomethanimine and ethanamine? 3.) What kinds of implications does this have for the origination of life? 4.) Does this finding support or go against there possibly being life outside of Earth? Why? Excerpt 2: http://helix.northwestern.edu/article/origin-life-panspermia-theory Discussion questions: 1.) Briefly, in your own words, explain what the panspermia theory states. 2.) Provide at least four examples of evidence used to support this theory. You may look at and use other sources for your information. 3.) What kinds of implications does this have for the origination of life? 4.) Does this finding support or go against there possibly being life outside of Earth? Why? Information in Radio Waves Handout A: Functional Groups and their Accompanying Spectral Bands First it needs to be stated that identifying molecules or even functional groups is no easy task. Hidden within the infrared absorption bands is a lot of information and it isn’t easily deciphered. Usually, this area of study is reserved for students in their second semester in Organic Chemistry or in Analytical Chemistry as an undergraduate student. Because of this, these spectra have been greatly simplified to show general shapes and regions where each functional group can be identified. Functional Groups: C-H, O-H, C=C Functional Groups: C-H, O-H (by C=O), C=O Information in Radio Waves Functional Groups: C-H, NH2, C=N, Benzene ring Functional Groups: C-H, S Information in Radio Waves Handout B: Amino Acids and their Functional Groups Alanine Functional Groups Contained: C-H C=O O-H (by C=O) NH2 Arginine Functional Groups Contained: C-H C=O O-H (by C=O) NH / NH2 C=N Asparagine Functional Groups Contained: C-H C=O O-H (by C=O) NH2 Aspartic Acid Functional Groups Contained: C-H C=O O-H (by C=O) NH2 Information in Radio Waves Cysteine Functional Groups Contained: C-H C=O O-H (by C=O) NH2 S Glutamic Acid Functional Groups Contained: C-H C=O O-H (by C=O) O-H NH2 Glutamine Functional Groups Contained: C-H C=O O-H (by C=O) NH2 Glycine Functional Groups Contained: C-H C=O O-H (by C=O) NH2 Information in Radio Waves Histidine Functional Groups Contained: C-H C=C C=O O-H (by C=O) NH / NH2 C=N Isoleucine Functional Groups Contained: C-H C=O O-H (by C=O) NH2 Leucine Functional Groups Contained: C-H C=O O-H (by C=O) NH2 Lysine Functional Groups Contained: C-H C=O O-H (by C=O) NH2 Information in Radio Waves Methionine Functional Groups Contained: C-H C=O O-H (by C=O) NH2 S Phenylalanine Functional Groups Contained: C-H C=C C=O O-H (by C=O) NH2 Benzene ring Proline Functional Groups Contained: C-H C=O O-H (by C=O) NH Serine Functional Groups Contained: C-H C=O O-H (by C=O) O-H NH2 Information in Radio Waves Threonine Functional Groups Contained: C-H C=O O-H (by C=O) O-H NH2 Tryptophan Functional Groups Contained: C-H C=C C=O O-H (by C=O) NH / NH2 Benzene ring Tyrosine Functional Groups Contained: C-H C=C C=O O-H (by C=O) O-H NH2 Benzene ring Valine Functional Groups Contained: C-H C=O O-H (by C=O) NH2