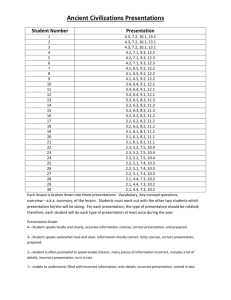
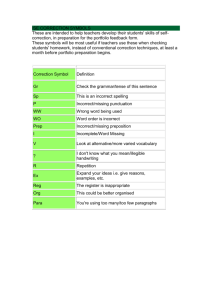
C UNIT Self-Quiz
advertisement

UNIT C Self-Quiz SUGGESTED ANSWERS 1. (c); Choice (c) is an example of a solution because solder is an alloy, which is an example of a solid solution. Choice (a) is incorrect because cereal is a mechanical mixture. Choice (b) is incorrect. Carbon is an element. Choice (d) is incorrect because water is a pure substance. 2. (b); When a liquid reaches its boiling point, it changes to a gas. Choice (a) is incorrect because it describes melting point, not boiling point. Choice (c) is incorrect because it describes freezing point, not boiling point. Choice (d) is incorrect because the change from a gas to a solid is deposition, not boiling. 3. (a); The particle theory states that particles are constantly moving. Choice (b) is incorrect because, according to the particle theory, different substances are made up of different kinds of particles. Choice (c) is incorrect because the particle theory states that particles attract each other. Choice (d) is incorrect because the particles of a substance move more slowly as its temperature decreases. 4. (b); Malleability is the ability of a substance to be hammered into a thin sheet. Choice (a) is incorrect because lustre is shininess or dullness. Choice (c) is incorrect because ductility is the ability of a substance to be drawn into a finer strand. Choice (d) is incorrect because viscosity is the ability of a substance to flow. 5. False. An alloy is a solution. A true version of the statement would be: An alloy is a solution composed of two or more metals. 6. True. The attraction between positive and negative ions forms the bonds in ionic compounds. 7. False. Water is a pure substance, which is a type of matter that consists of only one type of particle. A true version of the statement would be: Water is an example of a pure substance. 8. cations; A cation is an ion with a positive charge. It is formed by the loss of electrons from a neutral atom. 9. qualitative, quantitative; Colour and texture are not measured and do not have numerical values, so they are qualitative. Mass and temperature have numerical values, so they are quantitative. 10. (a)(iii) Copper is an element because it is a pure substance that cannot be broken down; (b)(ii) A compound is a substance that is made up of at least two different types of particles; (c)(i) Clear apple juice is a solution because it is a uniform mixture of two or more substances; (d)(iv) Trail mix is a mechanical mixture because you can distinguish different types of matter. 11. (a) (b) (c) (d) (e) physical; Colour can be observed without changing the identity of the substance. chemical; This property is observed during a chemical reaction. chemical; This property can only be determined by attempting a chemical reaction. physical; Freezing point can be observed by changing temperature, which is a physical change. physical; Odour can be determined without a chemical reaction. 12. They are similar because they all have the same chemical formula, H2O. They are different because they are in different states (liquid, gas, and solid). 13. Indicate whether J. J. Thompson would have agreed or disagreed with each of the following statements. [K/U] (a) agree; Thompson theorized that all atoms have electrons. (b) agree; Thompson theorized that certain subatomic particles, called electrons, are negatively charged. (c) disagree; Thompson theorized that electrons are evenly distributed throughout the atom. NEL 55219_04_ch07_p379-438_pp4.indd 437 Unit C Self-Quiz 437 12/3/09 3:37:44 PM 14. The bottom plates are made of metals because they need to be able to conduct heat. The handles are made from nonmetals because these materials generally do not conduct heat as well as metals. This allows the handles to stay cool and comfortable to touch. 15. (a) Element A: group 16; Element B: group 1; (b) Element A, 6; Element B: 1; (c) Element A 22; Element B 11; (d) AB2 16. (a) CaCl2; (b) N2O4; (c) Na2S2 17. (a) The arrangement of the elements is based on their atomic number. Groups of elements show similarities in their chemical and physical properties. The metallic character of the elements decreases from left to right across the periodic table. (b) Sample answer: Calcium is a metal, it has 20 protons, it tends to form ionic compounds when combined with nonmetals, and it has two outer electrons. 18. The atomic number of an element is unique to that element, just as a fingerprint is unique for each person. No two humans have the same fingerprint, and no two elements have the same atomic number. 19. Sample answer: To demonstrate a physical change, use the scissors to cut the paper into smaller pieces. Explain that cutting the paper did not produce any new substances. To demonstrate a chemical change, use the match to burn a small part of the paper. Explain that burning the paper produced ashes and gases, such as carbon dioxide, which have different chemical properties from the paper. 20. Sublimation is a physical change. The process of sublimation does not produce a new substance. 21. (a) 22. P: non-metal; Ca: metal; N: non-metal; K: metal; O: non-metal; Si: metalloid (b) Sample answer: Ca and O; Ionic compounds tend to form from metals and nonmetals. (c) Sample answer: N and O; Molecular compounds tend to form from nonmetals. The word “periodic” means “repeated at regular intervals.” The properties of the elements recur at regular intervals throughout the periodic table. 23. (a) (b) 24. (a) d 5 2.90 g/0.15 cm3 5 19g/cm3; gold d 5 55.7 g/11.1 cm3 5 5.02 g/cm3 ; pyrite As the substance inside the can is heated, the particles move faster and spread far apart in all directions. The volume of the substance increases and this could case the can to explode. (b) The increase in temperature causes the particles of the lid to move apart in all directions. This will cause the lid to expand so it comes off easily. (c) As the temperature changes, particles move closer together or farther apart, which could cause the structure to crack. Expansion joints allow the structure to expand in hot weather and shrink in cold weather without cracking. 25. (a) 7pⴙ 7n0 N 26. 438 15pⴙ 16n0 P (b) The diagrams are similar because they both show how many protons, neutrons, electrons, and electron orbits each atom has, and they both have five electrons in the outer orbit. The diagrams are different because the two atoms have different numbers of protons, neutrons, electrons, and electron orbits. (c) These two elements would both form ions with a charge of –3. Lead and cadmium cannot be burned for disposal because the gas given off would be toxic to humans. Unit C: Atoms, Elements, and Compounds 55219_04_ch07_p379-438_pp4.indd 438 NEL 12/3/09 3:37:45 PM