Homework I
advertisement

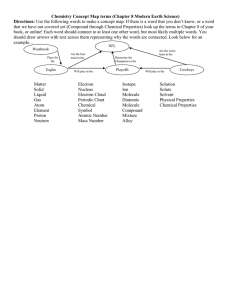
Chemistry 5.0 Name ______________________________ Date ____________________ Period ____ Homework I Bohr reasoned that the obits of electrons surrounding the nucleus must have a definite diameter. He determined that an electron could emit energy of one or two quanta, but not 1.5 or 3.2 quanta, as it fell to a lower energy level. A ball bouncing from one step to another represents the motion of an electron as it falls from one energy level to another. The bottom of the staircase represents the lowest energy level in an atom. The spectra produced by a compound can be used to determine the elements in the compound. Each line of the spectrum represents one frequency of light and therefore a certain energy. This energy is determined by the movement of electrons between energy levels which are specific for each element. The same set of energy levels will always produce the same spectrum. When an electron falls from a higher to lower energy level, a photon of light is emitted. The greater the difference in energy levels is the greater the energy of the emitted light. As an electron moves farther from the nucleus, it absorbs energy. _______________________a. According to the formula c=, as the frequency gets larger, the wavelength gets ____. _______________________b. Which light waves contain more energy, infrared or ultraviolet? _______________________c. Do microwave ovens produce waves with short or long wavelengths? _______________________d. Microwaves are high-energy waves that can be used for cooking. What equation can be used to show why a microwave has high energy? _______________________ e. The smallest orbit of an electron is called its ____ state. _______________________ f. _____________ said it is impossible to state the exact position and momentum of a moving object. _______________________ g. Light travels in waves, but it acts like matter when it interacts with small matter like an electron. This shows the ____ nature of light. _______________________ h. An electron must _______ energy to move to a higher energy level. _______________________ i. Visible radiations are less destructive because they have less ______ than UV radiation. _______________________ j. An electron is in a(n) ____ state when it goes from a lower energy level to a higher energy level.