Molarity and Dilutions Lab
advertisement

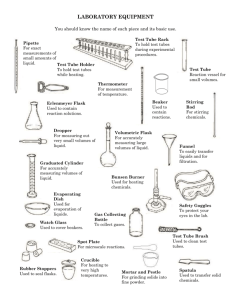
Molarity and Dilutions Lab Name_________________________________ 15 Points Period______Date_______________________ Background: The following lab is meant to give you hands-on experience with making solutions using both the molarity formula (M=n/V), as well as the formula for dilutions (M1 x V1 = M2 x V2). In particular you will make a 0.050 M stock solution of C6H12O6. From that stock solution you will make four dilutions resulting in solution concentrations of 0.040 M, 0.030 M, 0.020 M, and 0.010 M respectively. Materials: 25.0 mL volumetric flask 4 test tubes food coloring (green or blue) dropper test tube rack sugar 10.0 mL graduated cylinder small weigh boat Procedure and Calculations: 1. Clean and rinse 4 test tubes, graduated cylinder and volumetric flask well. 2. Determine the mass of sugar needed for 25.0 mL of a 0.0500 M C6H12O6 stock solution. Show all your Work (3 pts). Add the sugar to the volumetric flask first using a small weigh boat into a funnel, then add the water to the line. DO NOT OVERFILL! Finally add 1 drop of food coloring to the flask. Shake well. 3. In the 4 test tubes make the following dilutions from your stock solution. You will need to calculate how much stock solution you need to make each dilution. Do your calculations in milliliters first using the dilution formula: M1 x V1 = M2 x V2 (2 pts each) a. From the stock solution make 10.0 mL of 0.040 M C6H12O6 in the 1st test tube. b. From the stock solution make 10.0 mL of 0.030 M C6H12O6 in the 2nd test tube. c. From the stock solution make 10.0 mL of 0.020 M C6H12O6 in the 3rd test tube. d. From the stock solution make 10.0 mL of 0.010 M C6H12O6 in the 4th test tube. 4. Before you discard the solutions, show your teacher the test tubes and stock solution and have him/her sign your lab. Rinse everything well and return the volumetric flask. Answer the questions on the back. Questions: 1. Observe the solutions in each test tube. Which molarity has the deepest color? ______ The lightest? _____ 2. What does the depth of color indicate about the number of C6H12O6 molecules (or concentration) in each solution you made?