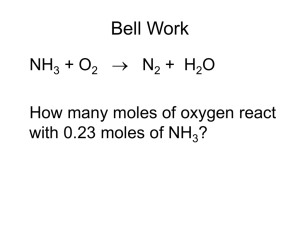Stoichiometric Calculations A Directed Learning Activity for Hartnell College
advertisement

Stoichiometric Calculations A Directed Learning Activity for Hartnell College Chemistry 1 Funded by the Title V – STEM Grant #P031S090007 through Hartnell College For information contact lyee@hartnell.edu Start Student Learning Objectives This tutorial will help you to: 1. 2. 3. Determine limiting and/or excess reagents for a balanced equation Use data from gravimetric analysis to predict mass of product and determine percent yield Use data from volumetric analysis to calculate titration results Next Getting Started This set of Power Point slides will lead you through a series of short lessons and quizzes on the topics covered by this Directed Learning Activity tutorial. Move through the slideshow at your own pace. There are several hyperlinks you can click on to take you to additional information, take quizzes, get answers to quizzes, and to go to other lessons. You can end this slide show at any time by hitting the “ESC” key on your computer keyboard. Next Table of Topics What You Need to Know What is “Stoichiometry”? Balanced Chemical Equations Limiting Reagents Percent Yield Titrations Next slide What You Need to Know How to write chemical formulas and balanced chemical equations to describe chemical reactions Systems of expressing concentration of solutes in solution (molarity, normality, percent) Conversion between mass and moles and different units of concentration The Ideal Gas Laws Please check with your textbook or other tutorials in this series for help on these subjects. Next slide What is “Stoichiometry”? “Stoichiometry” is a term that comes from two Greek words that mean “measuring elements”. Stoichiometric calculations make use of the quantitative relationships between the substances in a chemical reaction to convert from the amount of one substance in the chemical reaction to the amount of another substance in that same reaction. In order to do so, you need to know the balanced chemical equation. Next Slide Balanced Chemical Equations How to Use Balanced Chemical Equations to Convert Quantities Next Slide What a Balanced Chemical Equation Can Tell You If you have a balanced chemical equation, it can tell you many things: 1. The formulas and symbols of the reactants and products 2. The physical state of a substance (solid, liquid, gas, or dissolved in solution) 3. If special conditions (e.g., heat, light, catalyst) are needed, and 4. The relationships between the molecules or moles of the reactants and products. Next slide A balanced chemical equation must obey the Law of Conservation of Mass – i.e., the sum of the masses of all the reactants must equal the sum of the masses of all the products. Another way of looking at a balanced chemical equation is that the number and kind of atoms on one side of the reaction arrow must equal the number and kind of atoms on the other side of the arrow. We can use these facts to our advantage when we are trying to calculate how much of a product or reactant is present. Next Slide Mole-to-Mole Conversions The coefficients of the balanced equation give us the relationship between the moles of each chemical species. If we look at this reaction: 𝐶𝐶 𝑠 + 2 𝐴𝐴𝐴𝐴3 𝑎𝑎 → 𝐶𝐶 𝑁𝑁3 2 𝑎𝑎 + 2 𝐴𝐴(𝑠) We see these molar relationships: 1 mole Cu: 2 moles of AgNO3 : 1 mole Cu(NO3)2: 2 moles Ag, so if we know the quantity of one of the reactants or products, we can calculate the amounts of the other chemical species. Next Slide Mole-to-Mole cont’d You can set up a simple equation using a conversion factor like this: 𝑚𝑚𝑚𝑚𝑚 𝑜𝑜 𝐷𝐷𝐷𝐷𝐷𝐷𝐷 𝑠𝑠𝑠𝑠𝑠𝑠𝑠 = 𝑐𝑐𝑐𝑐𝑐𝑐𝑐𝑐𝑐𝑐𝑐 𝑜𝑜 𝐷𝐷𝐷𝐷𝐷𝐷𝐷 𝑠𝑠𝑠𝑠𝑠𝑠𝑠 𝑚𝑚𝑚𝑚𝑚 𝑜𝑜 𝐾𝐾𝐾𝐾𝐾 𝑠𝑠𝑠𝑠𝑠𝑠𝑠 𝑐𝑐𝑐𝑐𝑐𝑐𝑐𝑐𝑐𝑐𝑐 𝑜𝑜 𝐾𝐾𝐾𝐾𝐾 𝑠𝑠𝑠𝑠𝑠𝑠𝑠 So if we were given a problem to calculate how much Ag(s) would be formed if 0.295 mole of Cu(s) is reacted, Ag (s) would be the DESIRED species and Cu(s) would be the known species, which would lead to 2 𝑚𝑚𝑚𝑚 𝐴𝐴 𝑚𝑚𝑚𝑚𝑚 𝐴𝐴 = 0.295 𝑚𝑚𝑚𝑚 𝐶𝐶 = 0. 590 𝑚𝑚𝑚𝑚 𝐴𝐴 (1 𝑚𝑚𝑚𝑚 𝐶𝐶) Next Slide Mole-to-Mass and Mass-Mole Conversions So, continuing this process of examining balanced equations, we can convert from moles of one substance mass of another substance, or vice-versa, as follows: 𝑚𝑚𝑚𝑚 𝑜𝑜 𝐾𝐾𝐾𝐾𝐾 → 𝑚𝑚𝑚𝑚𝑚 𝑜𝑜 𝐾𝐾𝐾𝐾𝐾 → 𝑚𝑚𝑚𝑚 𝑜𝑜 𝐷𝐷𝐷𝐷𝐷𝐷𝐷 or 𝑚𝑚𝑚𝑚 𝑜𝑜 𝐾𝐾𝐾𝐾𝐾 → 𝑚𝑚𝑚𝑚 𝑜𝑜 𝐷𝐷𝐷𝐷𝐷𝐷𝐷 → 𝑚𝑚𝑚𝑚 𝑜𝑜 𝐷𝐷𝐷𝐷𝐷𝐷𝐷 Next Slide Mass-to-Mass Conversions We can also begin with a known mass of one substance, and using the balanced chemical equation, then calculate the mass of another substance. This calculation takes several steps: 1. Convert known mass of substance “A” to moles of substance “A” 2. Convert from moles of substance “A” to moles of some desired substance “B” 3. Convert from moles of “B” to mass of “B” Next Lesson Limiting Reagent Calculations How Much or How Little? Next slide The Limiting Reagent In a reaction with two or more reagents, the reagent which is completely consumed first is referred to the “limiting reagent”. In other words, this is the reagent that dictates how much product it is possible to produce. In order to determine the limiting reagent, we are typically given the mass of the two reagents. From this information and the balanced equation, you can calculate the amount of product the reagents will produce. Let’s look at an example. Next Slide Limiting Reagent Example If 1.0 mole of Cr is mixed with 1.0 mole O2 and the reaction is heated to produce Cr2O3(s), what would be the amount of product? The balanced equation is: 4 Cr(s) + 3 O2 (g) → 2 Cr2O3(s) First calculate the number of moles of O2 (g) needed to react with 1.0 mole Cr. 𝑚𝑚𝑚𝑚𝑚 𝑜𝑜 𝑂2 = 3 𝑚𝑚𝑚𝑚𝑚 𝑂2 1.0 𝑚𝑚𝑚𝑚 𝑜𝑜 𝐶𝐶 = 0.75 𝑚𝑚𝑚𝑚𝑚 𝑂2 4 𝑚𝑚𝑚𝑚𝑚 𝐶𝐶 Next Slide Example cont’d So, there is more than enough oxygen to react with all the Cr. Therefore, what limits the amount of product is Cr, which is the limiting reagent. Note that you could have started with the oxygen instead and found that there was not enough Cr to react with the available oxygen. You would reach the same conclusion. Now what? Well, you know that only 0.75 mole Cr is consumed, and also know the ratio between Cr and Cr2O3(s). Next Slide Example cont’d 2𝑚𝑚𝑚𝑚𝑚𝑚𝑚2𝑂3 𝑚𝑚𝑚𝑚𝑚 𝐶𝐶2𝑂3 = 1 𝑚𝑚𝑚𝑚 𝐶𝐶 4 𝑚𝑚𝑚𝑚𝑚 𝐶𝐶 = 0.500 𝑚𝑚𝑚𝑚𝑚 𝐶𝐶2𝑂3 Note that if you had a gas as a product, you could use the ideal gas laws to calculate the volume of gas produced. Quiz Questions Quiz Questions Ammonia reacts with oxygen to produce nitrogen monoxide and liquid water NH3(g) + O2(g) → NO(g) + H2O(l) 1. Balance the chemical equation 2. Determine the limiting reagent if 100g ammonia and 100g oxygen are present when the reaction begins. 3. Which is the excess reagent and how much of it will be left when the reaction is complete? Review Examples Check Answer Answer to Quiz The balanced equation is 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(l) Remember that you need to have the same kind and number of atoms on each side of the equation. 1. Oxygen is the limiting reagent. You can start the limiting reagent calculations with either of the starting reagents. 2. Next Slide Quiz Answer cont’d Starting with ammonia, this is how to calculate the amount of oxygen consumed: 1𝑚𝑚𝑚𝑚𝑚3 100𝑔𝑔𝑔3 17.0𝑔𝑔𝑔3 5𝑚𝑚𝑚𝑚2 4𝑚𝑚𝑚𝑚𝑚3 32.0𝑔𝑔2 1𝑚𝑚𝑚𝑚2 So oxygen is the limiting reagent. 3. = 235𝑔𝑔2 Ammonia is in excess and 57.5 g is left. 1𝑚𝑚𝑚𝑚2 4𝑚𝑚𝑚𝑚𝑚3 17.0𝑔𝑔𝑔3 100𝑔𝑔2 32.0𝑔𝑔2 5𝑚𝑚𝑚𝑚2 1𝑚𝑚𝑚𝑚𝑚3 = 42.5𝑔𝑔𝑔3 Next Slide Quiz Answer cont’d That tells us how much ammonia is consumed. The excess would be difference between the 100g you started with and the amount consumed, or 57.5g NH3. Return to Examples Next Lesson Percent Yield Reality Compared to Theory Next slide Using More of What You Have Learned In the previous lesson, you saw how to predict how much product would result from given quantities of starting material. That amount produced is referred to as the “theoretical yield”. In other words, all things being perfect, that amount would be the maximum quantity of product you could create from the given quantities of reactants. First, you had to determine the limiting reagent and then use that to calculate the (theoretical) yield. Next Slide Continued 𝑃𝑃𝑃𝑃𝑃𝑃𝑃 𝑌𝑌𝑌𝑌𝑌 = 𝐴𝐴𝐴𝐴𝐴𝐴 𝑌𝑌𝑌𝑌𝑌 𝑥 𝑇𝑇𝑇𝑇𝑇𝑇𝑇𝑇𝑇𝑇𝑇 𝑌𝑌𝑌𝑌𝑌 100% Let’s look at an example – Calculate the percent yield of sodium sulfate if 32.18 g sulfuric acid reacts with excess sodium hydroxide to give 37.91g sodium sulfate. Next Slide Continued 32.18𝑔 𝐻2𝑆𝑆4 1𝑚𝑚𝑚 𝐻2𝑆𝑆4 1𝑚𝑚𝑚𝑚𝑚2𝑆𝑆4 142.0𝑔 𝑁𝑁2𝑆𝑆4 𝑥 𝑥 𝑥 98.08𝑔 𝐻2𝑆𝑆4 1𝑚𝑚𝑚 𝐻2𝑆𝑆4 1𝑚𝑚𝑚 𝑁𝑁2𝑆𝑆4 = 46.59𝑔 𝑁𝑁2𝑆𝑆4 However, the experimental yield was 37.91g 𝑁𝑁2𝑆𝑆4, so the percent yield is: % yield = 100% 𝑥 Review Lesson 37.91𝑔 46.59𝑔 = 81.37% Go to Quiz Quiz Question SO2(g) + H2O(l) → H2SO3(aq) If this reaction yields 21.61g sulfurous acid when 19.07g of sulfur dioxide reacts with excess water, what is the percent yield? Check Answer Answer to Quiz Solution: 88.46% yield. The equation given is balanced. So, first you have to calculate the theoretical yield based on the limiting reagent, which is given as sulfur dioxide. The theoretical yield is: 1𝑚𝑚𝑚𝑚𝑚3 1𝑚𝑚𝑚𝑚2𝑆𝑆3 82.07𝑔𝑔2𝑆𝑆3 19.07𝑔𝑔𝑔3 𝑥 64.05𝑔𝑔𝑔3 1𝑚𝑚𝑚𝑚𝑚3 1𝑚𝑚𝑚𝑚2𝑆𝑆3 = 24.43𝑔𝑔2𝑆𝑆3 21.61𝑔 % 𝑦𝑦𝑦𝑦𝑦 = 𝑥𝑥𝑥𝑥𝑥 = 88.46% 24.43𝑔 Review Lesson Next Slide Titrations Using Liquid Concentrations Next slide Using Molarity, Normality and Percent Concentrations Sometimes we are asked to do quantitative calculations of reactions based on volumes and concentrations of solutions. Suppose you have two different solutions containing solutes that can react with each other to form a product. A simple example would be reaction of an acid solution and a base solution. If you know the concentration of one of the two solutions, by using some simple mathematics, you can calculate the concentration of the second solution. The type of experiment used to determine the volumes required is called “titration”. Next Slide Continued Remember, if you know the concentration of a solute, it may be expressed in different ways. Molarity is the concentration of the solute in moles of solute per liter of solution. Normality is the concentration of the solute in equivalents per liter of solution. Percent concentration can be the percentage based on grams of solute in grams of solution. Next Slide Continued So, if the concentration of a solution is known and the volume is known, you know how much of the solute you have. You simply multiply the concentration by the volume (making sure the units are consistent). One useful equation for this type of problem is NA x VA = NB x VB Where N refers to normality and V refers to volume in liters; A and B represent two reactants, like an acid and a base. With the knowledge you have from previous lessons, you now have the ability to do stoichiometric calculations for solutions. Calculations like these may be done for neutralization reactions, precipitation reactions, or reactions that produce gases. Next Slide Acid-Base Neutralization What volume of 1.5M H3PO4 is necessary to neutralize 20 ml of 2N KOH? Solution: 2N KOH = 2 equivalents of KOH/L solution. 1.5M H3PO4 = 4.5 equivalents H3PO4/L solution Since NA x VA = NB x VB Solving for 𝑉𝐴 = 2𝑁𝑁𝑁𝑁𝑁𝑁 4.5𝑁 = 8.9𝑚𝑚 Next Slide Precipitation Reaction What is the minimum volume of 0.250M AgNO3(aq)that will precipitate all the chromate ion in 0.500L of 0.800M K2CrO4(aq)? Solution: The balanced equation is K2CrO4(aq) + 2 AgNO3(aq) → Ag2CrO4(s) + 2 KNO3(aq) In 0.500L of 0.800M K2CrO4(aq), 𝑚𝑚𝑚𝑚 0.500𝐿 𝑥 0.800 = 0.400 𝑚𝑚𝑚𝑚𝑚 K2CrO4 𝐿 Next Slide Precipitation cont’d 2𝑚𝑚𝑚 𝐴𝐴𝐴𝐴3 0.400𝑚𝑚𝑚 𝐾2𝐶𝐶𝐶4 𝑥 1𝑚𝑚𝑚 𝐾2𝐶𝐶𝐶4 = 0.800𝑚𝑚𝑚 𝐴𝐴𝐴𝐴3 The volume of 0.250 M AgNO3 solution required is 1.00𝐿 𝑉 𝐴𝐴𝐴𝐴3 = 0.800𝑚𝑚𝑚 𝑥 = 3.20 𝐿 0.250𝑚𝑚𝑚 Next Slide Gas Producing Reactions How many moles of carbon dioxide gas will form if 300. mL of 0.500 M HCl are added to 500. mL of 0.500 M NaHCO3? Solution: Balanced equation is: 𝑁𝑁𝑁𝑁𝑁3 𝑎𝑎 + 𝐻𝐻𝐻 (𝑎𝑎) → 𝑁𝑁𝑁𝑁 𝑎𝑎 + 𝐻2𝐶𝐶3 (𝑎𝑎) → 𝑁𝑁𝑁𝑁 𝑎𝑎 + 𝐻2𝑂 𝑙 + 𝐶𝐶2 (𝑔) Looking at the data given and the 1:1 mole ratio of the reactants, HCl is the limiting reagent. Next Slide Gas Producing Reactions cont’d 0.500𝑚𝑚𝑚𝑚𝑚𝑚 1𝑚𝑚𝑚𝑚𝑚2 𝑚𝑚𝑚 𝐶𝐶2 = 0.300𝐿 𝑥 𝐿 1𝑚𝑚𝑚𝑚𝑚𝑚 = 0.150𝑚𝑚𝑚 𝐶𝐶2 Note that at STP, we could use Avogadro’s Law to determine the volume of CO2. Review Lesson Go to Quiz Quiz Questions 1. What volume of 0.250 M HCl is necessary to precipitate the solute in 0.150 L of 0.100M Pb(NO3)2? 2. How many mL of 0.500M sulfuric acid will just neutralize 12.0g sodium hydroxide in 500 mL of water? Review Lesson Check Answers Quiz Answers Solution: 120 mL 0.100M HCl. Must first write the balanced equation: Pb(NO3)2(aq) + 2HCl(aq) → PbCl2(s) + 2HNO3(aq) Then calculate how many moles of Pb(NO3)2 there are in 0.150L of solution. You also know there is a 1:2 ratio of Pb(NO3)2 to HCl so you multiply the moles of Pb(NO3)2 by 2 to obtain the final answer. 1. Next Slide Quiz Answers cont’d Solution: 300mL 0.500M H2SO4. Need balanced equation: 2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(l) There is a 2:1 ratio between the moles of NaOH and moles of H2SO4. Have grams of NaOH – convert this to moles by using molar mass of NaOH (40.0g). Convert to moles of 1𝑚𝑚𝑚𝑚2𝑆𝑆4 H2SO4 by using ratio of 2. 2𝑚𝑚𝑚𝑚𝑚𝑚𝑚 Next Slide Congratulations! You have successfully completed this Directed Learning Activity tutorial. We hope that this has helped you to better understand this topic. Click here to end. Click here to repeat this activity. Information This document has been prepared in compliance with US & International Copyright Laws © 2011 Hartnell College Funded by the Title V – STEM Grant #P031S090007 through Hartnell College Hit the ESC key to end this slide show