Acid-Base Titration and pH
advertisement

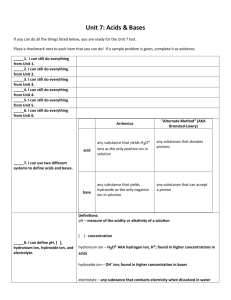
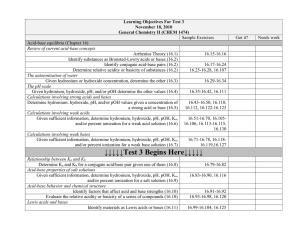
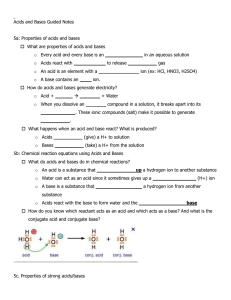
Acid-Base Titration and pH Aqueous Solutions and the Concept of pH • In the self-ionization of water, two water molecules produce a hydronium ion and a hydroxide ion by the transfer of a proton • At 25 degrees C, the concentration of [OH] and [H3O] equals 1.0 x 10-7 • The ionization constant for water is written as Kw and is equal to [H3O]x[OH] • Kw = 1.0 x 10-14 • Since the concentrations of hydroxide and hydronium are equal in water, water is neutral – If the hydronium concentration is greater, it is acidic, if the hydroxide concentration is greater it is basic • Since the ionization constant is known, the hydronium or hydroxide concentration can be calculated if the other is known • The pH of a solution is defined as the negative of the common logarithm of the hydronium ion concentration – pH – French for pouvoir hydrogene “hydrogen power” – pH = -log [H3O] – pOH is the negative log of the hydroxide ion concentration • To calculate the pH simply take the –log of the concentration – If only the OH concentration is known, divide this from 1.0 x 10-14 and take the –log of this number • To calculate the hydronium ion concentration from pH, take the antilog of the pH – (2nd/log/(-)/pH/enter) • The molarity and ion concentration are only equal in strong acids and bases (weak ones must be calculated) Determining pH and Titrations • Acid base indicators are compounds whose colors are sensitive to pH • They are weak acids or bases • A pH meter determines the pH of a solutions by measuring the voltage between the two electrodes that are placed in the solution • A titration is the controlled addition and measurement of the amount of a solution of known concentration required to react completely with a measured amount of a solution of unknown concentration • The point at which the two solutions used in a titration are present in chemically equivalent amounts is the equivalence point • The point in a titration at which an indicator changes color is called the end point of the indicator • In titrations of strong acids and strong bases, the equivalence point will be 7 • In titrations of strong acids and weak bases, the equivalence point will be less than 7, since the salt formed is a weak acid • In titrations of strong bases and weak acids, the equivalence point will be greater than 7, since the salt formed is a weak base