Document 14260766
advertisement

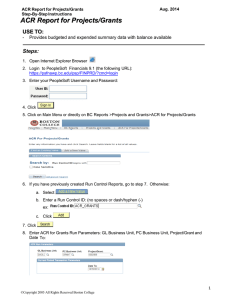
3/14/12 ACR MAP QC in the Digital Era Douglas Pfeiffer, MS, DABR Boulder Community Hospital Some New Additions To The Fold ✦ Agfa CR Mammography ✦ Carestream CR Mammography ✦ Giotto FFDM ✦ And t c o ntin he conf Lorad Dimensions u ues to g sion r Philips/Secta ow… Fuji FDR Mammography ✦ Planmed monitor ✦ ✦ ACR Subcommittee on Quality Assurance ✦ Clinical Representatives ✦ MITA Representatives ✦ ACR Representatives Clinical Representatives ✦ Chris Adent-Delaney, RT - Northwestern Memorial Hospital ✦ Jay Baker, MD – Duke University Medical Center ✦ ✦ Shelli Dixon, RT – The Women’s Imaging Center of Denver ✦ R. Edward Hendrick, PhD ✦ ✦ Lawrence Bassett, MD – UCLA Medical Center ✦ Chair, Joint Committee on Breast Imaging for Appropriateness Criteria and Guidelines Chaired by Eric Berns, PhD (Denver Health) Debra Monticciolo, MD – Texas A&M Health Sciences Center ✦ Chair of ACR Accreditation Program Chairs ✦ Chair of ACR Mammography Accreditation ✦ Douglas Pfeiffer, MS – Boulder Community Hospital ✦ Margarita Zuley, MD – University of Pittsburgh Medical Center 1 3/14/12 MITA Representatives ✦ ACR Representatives Medical Imaging & Technology Alliance ✦ [John Sandrik, PhD (Ret.) – GE Medical Systems] ✦ Robert Uzenoff – FUJIFILM Medical Systems ✦ [Stephen Vastagh – MITA (now in new position)] ✦ ✦ ✦ Moustafa Zerhouni – Computerized Imaging References Systems Subcommittee Charge ✦ ✦ ✦ Update the 1999 ACR Screen-Film QC Manual Priscilla Butler, MS – Senior Director, ACR Breast Imaging Accreditation Programs Subcommittee Goals Design ACR Accreditation Phantom for FFDM Write QC Manual for ACR FFDM Mammography Accreditation Program Marion Boston, RT – Manager, ACR Breast Imaging Accreditation ✦ Standardize all QC tests for all digital manufacturers ✦ Standardize test frequencies ✦ Standardize performance criteria ✦ ACR FFDM QC Manual to become basis of new regulations 2 3/14/12 QC Tests ✦ Process Based on a variety of sources ✦ MQSA ✦ SFM ✦ ACRIN DMIST ✦ Manufacturer’s QC programs ✦ MITA ✦ Subcommittee clinical experience ✦ Apply to all manufacturers ✦ Be clinically relevant ✦ Be user friendly Phantom Design Goals ✦ More challenging targets ✦ More sensitive to changes ✦ Fewer digital processing artifacts ✦ Full field ✦ Same attenuation as current phantom ✦ Same Pass/Fail targets as current phantom ✦ Design & build new phantom ✦ Write QC Manual ✦ ✦ When complete, draft will be sent to manufacturers for their input When final, ACR will apply for the alternative standard under current regulations Current Phantom 3 3/14/12 Everything I Say From Now On Might Be A Lie Cousins Prototype Phantom Prototype in Clinical Environment 4 3/14/12 ACR Phantom Prototype Wax Insert Specifications with Virtual “Placement Grid” ID Tag Specs Depth of CNR Cavity = 0.1 + 0.005 cm Wax = 0.70 cm + 0.02 cm Total Insert Depth = 0.75 cm 1.5 cm Tolerances (Insert Well & CNR Cavity) Compensator = 0.023 cm Total Thickness Under Insert = 3.05 cm • Wax insert well depth : ±0.005 cm (+ 2 mils). • Wax insert well width and length : +0.04 / -0.00 cm • CNR cavity depth : ±0.005 cm (+ 2 mils). • CNR diameter : ± 0.05 cm. Location of Virtual Box 0.49 cm ID Tag 2.0 cm 6.98 cm Test object distance from base of wax = 0.35 + 0.10 cm Total Thickness = 4.10 + 0.03 cm Virtual Box: height = 0.5 cm, length = 1.8 cm 12.98 cm (+ 0.00, - 0.04 cm) Cover =Nominal 0.3 cm Air Gap = 0.027 cm Nominal (+ 0.00, - 0.04 cm) 2.0 cm 31.0 + 0.1 cm 2.0 cm 0.49 cm 2.0 + 0.05 cm CNR Cavity (0.1 + 0.005 cm Deep) Centered Left to Right 19.0 + 0.1 cm 1.0 + 0.05 cm 9.5 cm 0.49 cm Milled out wax insert area 13.0 cm (+0.04, -0.00 cm) 2.0 cm each 0.49 cm Notes: Test objects to be centered on their respective “placement grid” locations. 0.49 cm perimeter around test object “placement grid”. ID Tag 0.635 cm (1/4 inch) radius on corners of wax insert. Milled out wax insert area 7.0 cm (+0.04, -0.00 cm) Fiber Placement specs Fiber specifications Speck Placement & Specs 1. Specks to be placed at points on star and middle of star 2. Speck Size (spherical) = See Table 3. Center speck placement to be within + 0.1 cm of center of virtual grid 4. Distance from center speck to center of speck on perimeter = 0.5 cm + 0.1 cm Fiber Length = 1.0 cm + 0.1 cm Screws Fiber Fiber Fiber Diameter = See Table 2.5 + 0.05 cm 1.0 + 0.05 cm o o 45 + 5 45o + 5o Mass Placement & Specs 1. Mass pre-cut sphere diameter = 5/8 inch 2. Mass placement to be within + 0.1 cm of center of virtual grid 1.0 + 0.05 cm Attenuation Compensator Compensator is 9 mil polyvinylidene (C2H2Cl2) Clinical Unit Image Lorad/Hologic Digital Mo/Mo Auto-Filter 52 mm Mo/Mo 29 kVp 66 mAs 1.64 mGy 5 3/14/12 The New Insert Attenuation Equalization Specks are lime glass spheres Expanded View of Specks Insert Design 0.89 0.75 0.61 0.54 0.40 0.30 (mm) Fiber Diameter 0.33 0.28 0.23 0.20 0.17 0.14 (mm) Speck Diameter 1.00 0.75 0.50 0.38 0.25 0.20 (mm) Mass Sliver Thickness 6 3/14/12 Test Visual Object Comparison ^! Fibers (mm) Specks (mm) Fibers (mm) Masses (mm) FFDM ACR 156 FFDM (Glass Spheres) ACR 156 Specks (mm) Masses (mm) Test Object Test Object ACR 156 Pass/Fail Criteria ACR 156 FFDM FFDM ACR 156 FFDM ACR 156 FFDM 1.56 1.56 2.00 0.89 0.40 1.00 1.00 0.75 0.75 0.32 0.33 0.75 0.75 0.28 0.50 0.50 0.61 0.54 0.54 0.40 0.40 0.30 0.24 0.23 0.20 0.16 0.25 0.17 0.14 Phantom Scoring 0.54 1.12 0.54 0.89 1.12 Fail Pass 4 0.89 0.89 0.40 0.75 0.75 0.32 2 1.00 0.33 0.28 0.61 0.38 0.54 0.54 0.25 0.40 0.40 0.20 3 2.00 0.30 0.24 3 0.50 0.23 0.20 0.16 3 0.75 1.00 2 0.75 0.50 0.38 0.25 0.17 0.25 0.20 0.14 CR Imaging 7 3/14/12 CR Imaging Screen-Film Imaging Screen-Film Imaging AEC Technique Comparison Heel effect Screen defects Mode Dark center to light edge Roller marks Lorad – Mo Lorad - W Fuji CR 18 x 24 cm Fischer Auto-Filter Auto-Filter AA Auto-Technique Phantom FFDM SFM FFDM SFM FFDM SFM FFDM SFM Compression Thickness (cm) 5.2 5.2 5.2 5.2 4.0 4.0 5.74 4.05 Target/Filter Mo/Mo Mo/Mo W/Rh W/Rh Mo/Mo Mo/Mo W/Al W/Al kVp 29 29 28 28 27 27 31 27 mAs 66.4 65.4 92.5 97.6 90 89 177 mA 158 mA Machine Reported Dose (mGy) 1.64 1.61 1.03 1.08 ** ** 0.954 1.211 Dust 8 3/14/12 Manual Technique Signal Comparison Measurements Using Phantom Lorad/Hologic – Mo/Mo ✦ ACR Phantom Image Quality Manual ✦ Compression Thickness Consistency & Verification Mode Phantom FFDM SFM ✦ Phantom AGD Check Target/Filter Mo/Mo Mo/Mo ✦ AEC Consistency kVp 29 29 ✦ Phantom Scoring on Monitors mAs 65 65 ✦ SNR/CNR Consistency Signal in Wax 542.0 546.5 ✦ Artifact Evaluation ✦ Exposure Duration St. Dev. In Wax 9.7 9.7 Measurements Using Phantom ✦ Laser Printer QC Measurements Using Phantom ✦ Monitors Phantom Scoring ✦ ✦ ACR Phantom Scoring Artifact Evaluation ✦ ✦ Artifact Check ✦ Background OD Check ✦ Dmax OD Check ✦ Contrast OD ✦ ACR Phantom Printed Size Check 50% 41% RT tes ts Phy si use t cs tests he new phan tom ✦ Ghost Image Evaluation ✦ Spatial Resolution ✦ Average Glandular Dose ✦ CR Artifact Evaluation (if applicable) ✦ CR SNR Inter-Plate Consistency (if applicable) 9 3/14/12 QC Manual ACR Digital QC Draft Manual • Structure of Manual: – Radiologist’s Section ✦ Focussing on FFDM ✦ Selective, high-yield tests ✦ Applicable to all units ✦ Will eventually unify with screen-film manual – Clinical Image Quality Section – Radiologic Technologist’s Section – Medical Physicist’s Section – Educational, Guidance, and Troubleshooting Section – Glossary – References – Index What Will Be New? • Radiologist Section – Image ID regulations – Hanging protocols (left vs. right) – Monitor and viewing conditions guidance – Section on diagnostic tools for analyzing poor images What Will Be New? • Tech Section – Enhanced positioning and image quality section – New Test: Monitor QC for the Radiologist – New Test: Facility QC Review – New Format: Corrective Action Log – New Documentation: Facility Equipment Inventory – How to score the ACR FFDM Phantom – Improved QC Forms – Guides for understanding their role and responsibility for – Instructions for Mobile Units overseeing the QC program – Eliminate calculations (TBD) 10 3/14/12 What Will Be New? • Medical Physicist Section – Theme: provide better documentation and communication – Single MP Summary Form What Will Be New? • Medical Physicist Section – Provide QC forms in both PDF and Excel Worksheets – For Facility, ACR, State and MQSA Inspectors – Include an Action Item Summary – MP form for Tech for Operating Levels (if app.) and QC instructions – Will include guidance on how to test – Multiple units (FFDMs, AWs, RWs, Printers, etc) – Procedures for evaluating and documenting Tech QC – MP letter to the Radiologist – Multiple facilities – MP to use same Corrective Action Log form as Techs What Will Be New? • Facility – Guidance on how to handle multiple units at multiple locations – Guidance on who/what/when tests need to be performed when “major” and “minor” repairs are performed on unit Technologist QC Tests – Facility QC Review (Tech Test) – Quarterly 11 3/14/12 ACR Digital QC Draft Manual Tech Tests Frequency: Quarterly Objective Name (# of Test Elements) Frequency: Tech Quarterly Facility MAP ID-Unit# (00000-00) Procedure Technologist QC Tests Test Number 8. Monitor QC For The Radiologist 1. This test is to be performed or supervised by the Lead Interpreting Radiologist. 2. The Technologist should deliver this form to the Radiologist, ensure correct completion, follow-up on any failures, and place the form into QC notebook. 3. This test should be performed for each FFDM or CR unit within a facility on a quarterly basis. 4. This test can be performed on the same workstation for multiple FFDM or CR units. 5. Example: A Radiologist can sit at one workstation and view the images for all the FFDM and/or CR units within a facility. The Radiologist does not need to evaluate every monitor at every workstation. This test is for the Radiologist to perform an evalution of the entire mammographic imaging chain with the focus being primarily on the detector and secondarily on the monitors. This test is not intended to evaluate technologist issues such as positioning, compression, etc. Minimum Frequency Required Corrective Action 1 ACR Phantom Image Quality (5) Weekly Before Clinical Use Procedure for Radiologist 2 Acquisition Workstation (AW) Monitor QC (3) Weekly Before Clinical Use Step 1: 3 Radiologist Workstation (RW) Monitor QC (5) Weekly Before Clinical Use 4 Laser Printer QC (5) Weekly Before Clinical Use 5 Viewbox Cleanliness (1) Weekly Before Clinical Use 6 Visual Checklist (1) Monthly Before Clinical Use 7 Repeat Analysis (1) Quarterly Within 30 Days 8 Monitor QC for the Radiologist (1) Quarterly Before Clinical Use 9 Facility QC Review (1) Quarterly Not Applicable 10 Compression Force (1) Semiannual Before Clinical Use Room ID FFDM Unit Mfr & Model Monitor ID Complete the demographics: Radiologist Name Date of Evaluation Step 2. Pull up a any recent mammographic study from the above listed FFDM unit and record ID & Study Date. Step 3. Place the same LMLO image on each monitor. Step 4. Evaluate the images for the following artifacts and check the appropriate boxes. Image ID: Study Date: Left Monitor Right Monitor Yes No Comparing the monitors, do the background area (outside of breast tissue) appear different (darker or lighter, etc…) Is there a difference in contrast within the breast between monitors? Does the image contain excessive noise (not patient motion)? Manufacturer Detector Calibration (If Applicable) Per Mfr Recommendation For examples and more detailed descriptions, please see the Guide on Identifying Artifacts. Before Clinical Use Do you see ghosting? Do you see "bad pixels" (singular or clusters) (white or black)? Do you see white dots that could be from excessive dust? Do you see any form of image distortion (not architectural distortion)? Supplemental Forms Do you see gridlines? Step 5. If necessary, document any Do you see artifacts that could be due to image processing? failures on the "Corrective Corrective Action Log Do you see "line artifacts" (single or multiple pixels that form lines extending across image - horizontally or vertically)? Action Log" form and ensure Are there any other artifacts that are present and clinically significant (impeding interpretation)? items are resolved. Facility Equipment Inventory Form If any box is checked "Yes", then seek service. Action Limits Timeframe: If an image quality problem or artifact impedes clinical interpretation, then seek service immediately or before further imaging or interpretations are performed. If the artifact does not impede clinical interpretation, then seek service within 30 days. Tech Tests 9. Facility QC Review Frequency: Tech Quarterly Facility: Date of QC Mtg: Reviewed 1. Review and update "Facility Equipment Form" Tech Tests 9. Facility QC Review - Cont'd Facility: Frequency: Tech Quarterly Date of QC Mtg: QC Meeting Notes 2. Review Medical Physics Surveys and Results Room 1 Room 2 Room 3 Room 4 Room 5 Room ID Date of last Medical Physicist (MP) survey Written survey results communicated to Radiologist by MP? 6. Significant or notable findings during QC meeting "Executive Summarey for Facility" reviewed by Radiologist? Follow-up Confirmed (If App.) 11 All MP corrective action completed? ACR Phantom Dose (mGy) Fibers Specks Masses 3. Review Tech QC Test Frequency 1 ACR Phantom Image Quality Summary Comments from Last Quarter Weekly Room 1 Scores of most recent phantom image: Room 2 Room 3 Room 4 Room 5 Room ID 7. Items for quality improvement from QC Meeting Fibers Specks Masses Artifacts? (Yes/No) 2 AW Monitor QC 3 RW Monitor QC 4 Laser Printer QC Weekly Weekly Weekly 5 Viewbox Cleanliness Check Weekly 6 Visual Checklist Monthly 7 Repeat Analysis Quarterly 8 Monitor QC for the Radiologist % Repeats 8. Other QC Notes: Quarterly 9 Facility QC Review Quarterly 10 Compression Force Semiannual 11 Manufacturer Detector Calibration (If App.) 4. Review and verify completion of all "Corrective Action". 5. Technique Chart review for each room (see MP report for recommended chart) - (Annually). 6. Offsite RW(s) & Laser Printer(s) QC Reviewed. 7. Recent past and future service or service upgrades reviewed and discussed (if app). 8. Recent past and future State and/or MQSA inspections reviewed and discussed (if app). 9. ACR Accreditation issues discussed (if app.) Supervising radiologist and facility manager must review QC quarterly. Technologist should update the Facility Summary Form at least annually. Action Limit: Technologist and Lead Intepreting Radiologist should review technique charts at least annually for each FFDM system. Timeframe: NA Lead Interpreting Radiologist Facility Manager (If App) Lead QC Tech 12 3/14/12 Tech Tests 11. Manufacturer Detector Calibration (If Applicable) Facility MAP ID-Unit# (00000-00) Corrective Action Log Facility Name MAP ID# (00000-00) Date Room or Equipment ID QC Test Name and # (if app.): Frequency: _________________________ Description: Frequency: Per Manufacturer Recommendations (If Applicable). Manufacturer Procedure Tech Tests Note: See Medical Physicist for instructions (if any). Relevant Personnel Notified: Year Personnel Name: Date/Time of Call/Notification: (Rad, MP, Tech, Manager, Service Engineer) Date (Month & Day) Describe Actions Taken: Tech Initials Unit Description Confirmation of Resolution: Yes To Be Monitored NA Event resolved? Tech Signature Documentation from Service Engineer Obtained?: Date Date Room or Equipment ID QC Test Name and # (if app.): Description: Relevant Personnel Notified: Personnel Name: Date/Time of Call/Notification: (Rad, MP, Tech, Manager, Service Engineer) Describe Actions Taken: Pass = P Action Limits Fail = F Unit must pass manufacturer's prescribed periodic calibration test. Confirmation of Resolution: Yes Event resolved? Timeframe: Immediately Documentation from Service Engineer Obtained?: To Be Monitored NA Tech Signature Date Facility Equipment Inventory Form Tech Tests Facility Address Address Address QC Technologist: Lead Interpreting Radiologist Medical Physicist: Facility Manager Date Last Updated: List facility and equipment data for entire breast imaging network. Facility Location Example: Main Breast Center MAP ID Medical Physicist QC Tests 12345 Location Designation Room 3 Device (See Key Below) FFDM Manufacturer GE Model 2000D Serial Number 1234-56-7890 Customer Service ID 111-222-333 Service Telephone # 800-123-4567 Date of Manufacture December-09 MP Survey Date 9/17/2011 MP Survey Date 9/23/2012 MP Survey Date 9/5/2013 MP Survey Date 9/13/2014 MP Survey Date etc… MP Survey Date MP Survey Date MP Survey Date MP Survey Date MP Survey Date MP Survey Date Device Key FFDM = Full Field Digital Unit SFM = Screen-Film Unit CR = Computed Radiography Unit LP = Laser Printer FP = Film Processor for Screen-Film Unit AW = Acquisition Workstation RW = Radiologist Workstation. 13 3/14/12 ACR Digital QC Draft Manual ACR Digital QC Draft Manual Medical Physicists QC Tests Medical Physicists QC Tests Test Number Name (# of Test Elements) Test Number Minimum Frequency Required Corrective Action 1 ACR Phantom Image Quality (6) Annual Before Clinical Use 2 Ghost Image Evaluation (1) Annual Before Clinical Use 1 Medical Physicist Summary Report 3 Spatial Resolution (1) Annual Before Clinical Use 2 Technologist Operating Level Information and QC Instruction Form 4 Automatic Exposure Control System Performance (2) Annual Before Clinical Use 3 Medical Physicist Summary Letter for the Radiologist 5 Collimation Assessment (3) Annual Within 30 Days 4 Mammography Corrective Action Log 6 kVp Accuracy and Reproducibility (1) MEE Only Before Clinical Use 5 Technologist Pre-Inspection Interview Form 7 Beam Quality (Half-Value Layer) Assessment (1) Annual Within 30 Days 6 Technique Chart 8 Average Glandular Dose (2) Annual Before Clinical Use Before Clinical Use 9 Unit Checklist (1) Annual 10 Evaluation of Site’s Technologist QC Program (1) Annual Within 30 Days 11 MQSA Equipment Requirements (1) MEE Only Before Clinical Use 12 Computed Radiography (If Applicable) (3) Annual Before Clinical Use 13 Acquisition Workstation (AW) Monitor QC (6) Annual Before Clinical Use 14 Radiologist Workstation (RW) Monitor QC (11) Annual Before Clinical Use 15 Laser Printer QC (7) Annual Before Clinical Use 16 Viewbox Luminance and Room Illuminance (2) Annual Before Clinical Use 17 Evaluation of Off-Site Technologist QC Program (If Applicable) Annual Before Clinical Use Supplemental Forms ACR Digital QC Draft Manual Challenges Tech & MP Test Number ✦ Name Educational and Example Forms 1 Complete set of forms with example data, scores, and calculations 2 ACR Phantom Scoring Guide 3 SNR & CNR Calculation Guide 4 Monitor Test Pattern Evaluation Guide 5 Printed Film Evaluation Guide 6 FFDM Artifact Guide Name ✦ ✦ ✦ Accounting for, and incorporating, all the different FFDM technologies Handling offsite equipment Predicting and accounting for future FFDM systems Ensuring all necessary tests are included, meaningful, and relevant 14 3/14/12 What’s Next 3 Steps ✦ Preemptive Questions Cost of phantom? ✦ When ready, draft will be sent to manufacturers, FDA, and Implementation and roll-out? ✦ ✦ Subcommittee to review comments and edit manual ✦ Final draft to be sent to FDA from ACR to apply for alternative Don’t know. Reason to believe it will be affordable. ✦ select reviewers for preliminary feedback ACR to develop a plan to include some sort of training. ✦ standard under current regulations ✦ When? ✦ Goes to FDA Summer 2012 for review as alternative standard ✦ Alternative standard will allow facilities to use this instead of the manufacturer’s manuals ✦ Potential for ACR QC Manual to be basis for new MQSA Regulations Future Efforts Summary ✦ ✦ Finish manual ✦ Submit to MITA for comments ✦ ✦ ✦ Submit to FDA for approval as an alternative standard under current regulations ✦ The only way for this to be effective is to make QC phantom and manual a requirement Having a single phantom with a unified QC manual and program solves many problems Tests designed to be: ✦ User friendly ✦ Organized to maximize efficiency ✦ Provide data to reflect performance of systems Remember, the above tests still have to undergo two more reviews 15 3/14/12 Many Thanks... ✦ Eric Berns, PhD ✦ Penny Butler, MS 16