Chemistry of Ocean Water Water on earth:
advertisement
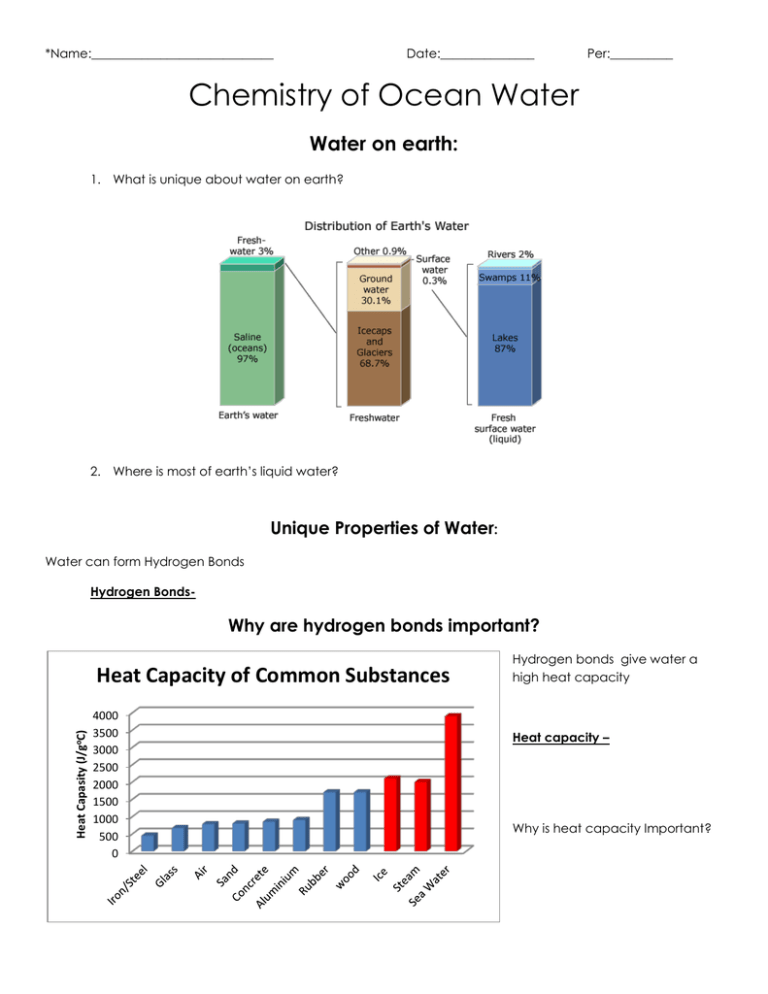
*Name:_____________________________ Date:_______________ Per:__________ Chemistry of Ocean Water Water on earth: 1. What is unique about water on earth? 2. Where is most of earth’s liquid water? Unique Properties of Water: Water can form Hydrogen Bonds Hydrogen Bonds- Why are hydrogen bonds important? Heat Capasity (J/goC) Heat Capacity of Common Substances 4000 3500 3000 2500 2000 1500 1000 500 0 Hydrogen bonds give water a high heat capacity Heat capacity – Why is heat capacity Important? Hydrogen bonds give water a high viscosity Viscosity of Common Liquids Viscosity – 6 Viscosity 5 4 3 2 Why is water high viscosity important? 1 0 Hydrogen bonds allow ice to float on liquid water Why does ice float on water? Why is ice floating important? Salt in Sea water? Salt – SalinityThe average salinity of Ocean Water is:_____________. This means in 1000Lb’s of water there Are _________ lb of salt Why are the oceans filled with salt water? Charged Particle Chloride (-1) Sodium (+1) Sulfate (-2) Magnesium (+2) Calcium (+2) Potassium (+1) Bicarbonate (-1) Bromide (-1) Borate (-1) Strontium (+1) Fluoride (-1) Other Composition of Sea Water Calcium (+2) 1% Potassium (+1) 1% Magnesium (+2) 4% Sulfate (-2) 8% Chloride (-1) 55% Sodium (+1) 31% % in sea water 55.03 30.59 7.68 3.68 1.18 1.11 0.41 0.19 0.08 0.04 0.003 0.001 Rule of Constant Composition – What is an experience in your life where the amount may change but the proportions of what makes it remains the same? Comparitive % Of Gases % in atmosphere % in surface water % in total ocean 83 78.08 48 36 21 15 11 N2 6 O2 How do gasses get in Sea water? What gas is in highest concentration in sea water? 0.03 CO2 0.89 1 0 Trace Gasses Oxygen gas in sea water: Explanation of Oxygen Graph: Dissolved Oxygen at the surface: Dissolved Oxygen in the middle layer: Oxygen Minimum Zone - Dissolved Oxygen in the depths: Carbon dioxide in sea water: Why is carbon dioxide (CO2) concentration so much more in ocean water? What does CO2 do in sea water? What is ocean acidification and what are its effects? Ocean acidification- 1. 2. 3. 4. 5. Nitrogen gas in sea water What are the two ways Nitrogen becomes dissolved in sea water? What is Eutrophication and what are its effects? Eutrophication – Effects of eutrophication: Temperature & Salinities Effects on Water Temperature and salinity combine to create 3 main lays of the ocean. Each of these layers has a distinct density Surface layer – Intermediate layer – Permanent (main) Thermocline – Deep layer – Light & water Any light we see is __________________. Any light we don’t see is ________________________. What is white light? What is black? Why does deep water appear blue? Why do smaller common prey organisms tend to be red? Sound & water What speed/frequency has the ability to travel the farthest? What two physical characteristics of water affect the speed of sound? Where is the slowest sound through water just taking temperature into consideration? Where is the slowest sound through water just taking pressure into consideration? What happens to the speed of sound through water when you take both temperature and pressure into consideration? The SOFAR Zone -