VSEPR What shape are your molecules in?
advertisement
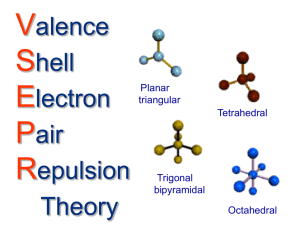
VSEPR What shape are your molecules in? Background you need… Lewis structures How many bonds do each element make? What can expand? Bonding (covalent) Polarity Electronegativity and determining bond type Resonance v. Isomers Formal charge Let’s review now….. Lewis Structures Remember that Lewis structures want a full outer shell Remember that for a given Lewis structure, the number of electrons around the atoms must equal the total number of electrons individually assigned. Ex: C has 4, H has 1, so CH4 must have 8 total Isomers Same formula, different arrangement of atoms Physically break bonds and MOVE atoms Resonance Structures Have the same alignment of atoms, but different bonding (electrons ONLY are moved, both in bonds and lone pairs) Determining formal charge Formal charge can be determined by: Normal number of electrons in outer shell [(1/2 the number of bonded electrons) + lone electrons] _____________________________________ = formal charge Example: N in NH4 FC =5- [(1/2 of 8)+ 0]= +1 Formal charge and stability The most “happy” molecules tend to have no formal charges However, molecules may be “happy” if they have not NET charge on them (if there is 1+ and 1-, so a net of +1 + (1)=0) Resonance structures that are the best have a minimal formal charge and a full octet around each atom What is VSEPR? Valence Shell Electron Pair Repulsion Theory Why? The shape of molecules influences their characteristics: Things like polarity which influence things like boiling point, melting point, which dictate their nature (solid, liquid or gas at room temperature) The parent geometries: all others come from these Steric Number The number of “things” sprouting off of an atom These can be either Bonds Of any order (1, 2, or 3) Or Lone pairs of electrons Steric Number Examples Ex #1: CH4 There are 4 H’s branching off , so the steric number is 4 SN=4 Ex #2: H2O SN= 4 Explain why Ex #3: CO2 SN= 2 Explain why General Formulas All molecules with a shared general formula have a shared geometry we use them to help note shape Formulas are typically written with A’s, X’s, and E’s The letters stand for: A= the central atom X *= the number of atoms attached to the central atom E= the number of lone pairs of electrons attached to the central atom *Some sources use A’s, B’s, and E’s General Formula Examples Ex #1: CH4 AX4 Ex #2: H2O AX2E2 Ex #3: CO2 AX2 Linear AX2 Trigonal planar AX3 Tetrahedral AX4 Pyramidal (Trigonal or tetrahedral) Tetrahedral parent shape 1 lone pair of electrons AX3E Bent Tetrahedral parent shape 2 lone pair of electrons AX2E2 When determining polarity it is important to look at the dipole moments- do they cancel out? Trigonal bipyramidal AX5 Seesaw a.k.a. Teeter-totter Trigonal bipyramidal parent shape 1 lone pair of electrons AX4E T-shaped Trigonal bipyramidal parent shape 2 lone pair of electrons AX3E2 Linear Trigonal bipyramidal parent shape 3 lone pair of electrons AX2E3 Octahedral AX6 Square pyramidal Octahedral parent shape 1 lone pair of electrons AX5E Square planar Octahedral parent shape 2 lone pair of electrons AX4E2 T-shaped Octahedral parent shape 1 lone pair of electrons AX3E3 Summary of shapes ID these VSEPR shapes… Sweet drill and practice web site Given generic shapes to ID: http://www.chemistry-drills.com/VSEPR1.php?q=1 Given molecules to draw out: Basic: http://www.chemistrydrills.com/VSEPR-1.php?q=2 Advanced: http://www.chemistrydrills.com/VSEPR-1.php?q=3