Atmospheres Terrestrial planet atmospheres 96% CO2 4% N2
advertisement
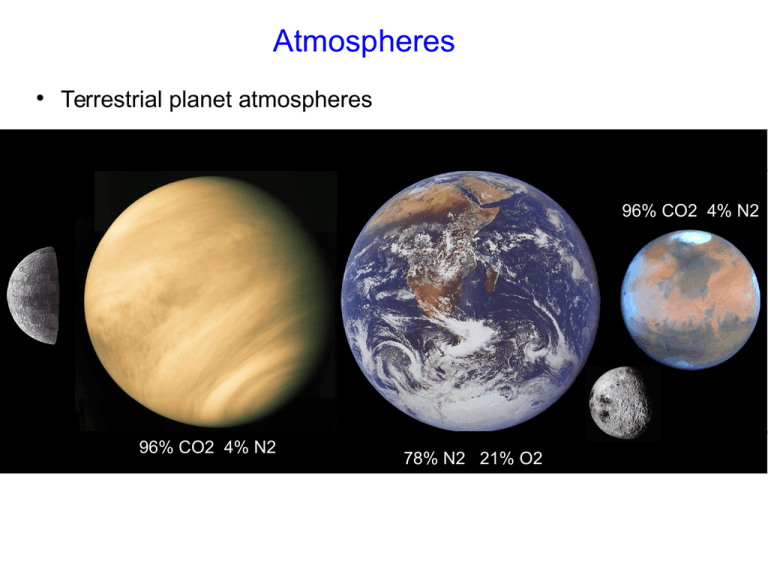
Atmospheres ● Terrestrial planet atmospheres 96% CO2 4% N2 96% CO2 4% N2 78% N2 21% O2 “Atmospheres” ● Jovian worlds “Atmospheres” ● Jovian worlds “Atmospheres” ● Jovian worlds Atmospheres 90+% N2 + CH4 Detecting Pluto's Atmosphere via Stellar Occultation Consider a single, completely elastic, gas atom... ● Estimate velocity vs. kT... 1 3 2 m v = kT 2 2 ● √ 3kT v= m Consider a particle launched from the surface at this speed, how high does it get? v initial t= g 2 initial 1 2 v x = gt = 2 2g On Earth, presuming a nitrogen molecule, this particle bounces up to an altitude of about 10 kilometers – pretty consistent with the thickness of the lower atmosphere. Atmospheric Retention and Escape Velocity ● Reference the previous equation to consider escape to infinity. Find T such that v escape 3kT = α m √ v escape = √ 2GM planet R As opposed to the “average” velocity, particles that can escape are in the high-velocity “tail” of the Maxwell-Boltzmann distribution (α= 5-6) Atmospheric Retention and Escape Velocity ● The retention condition then is that the mean thermal velocity be several times (α) lower than the escape velocity. – Different molecules/atoms have different escape conditions. – Heavy molecules can stick around. Hydrogen is most likely to escape. – T, of course, is a function of distance from the Sun. More distant objects have a better chance of holding on to an atmosphere. T = 2 G M planet mmolecule 2 3 α k R planet Atmospheric Retention and Escape Velocity ● ● ● Molecules escape from the “exosphere” - the region of the atmosphere where particle trajectories are unlikely to collide. For Earth the temperature of this layer is about 1000K. One can calculate an escape criterion based on the mean molecular weight of the species, µ. m molecule = μ m H T ex M μ>7.1 1000K M earth ( ● )( −1 R ) (R ) earth The Earth readily holds on to N2 and O2. Hydrogen atoms escape easily. T = 2 G M planet m molecule 2 3 α k R planet Escape from the Exosphere ● The Exosphere/Thermosphere is the region of the Earth's atmosphere that is so tenuous that gas atoms are on ballistic trajectories (few collisions). Gas escapes to space from this environment, which, energetically, is much warmer than the surface. The Earth's Geocorona ● ● The Lyman-alpha transition of hydrogen atoms scatters sunlight to make visible the escaping hydrogen in Earth's atmosphere. The outer boundary (edge of the exosphere) is established where solar radiation pressure exceeds the gravitational influence of the Earth. The Lunar “Atmosphere” ● At a density of 10-14 that of Earth's, the Moon has a transient atmosphere maintained by radioactive decay (helium, argon) and sputtered by the solar wind and cosmic rays (potassium, sodium). http://nightsky.jpl.nasa.gov/download-view.cfm?Doc_ID=483 http://www.nasa.gov/mission_pages/LADEE/news/lunar-atmosphere.html#.UoomszyJAjA The Solar Wind ● ● ● At the solar photospheric temperature of 6000K hydrogen is secure from escape. However, through a process that is not yet well understood, solar magnetic fields heat the upper atmosphere substantially. The escape velocity from the surface of the Sun is 620 km/s and the tenuous outer solar atmosphere escapes. v proton T = 160 km/ s 6 10 K ( ) 1 2 The Solar Wind ● ● The wind is tenuous – about 107 atoms/m3 or 10-21 kg/m3 measured 1AU from the Sun. The mean velocity is about 400 km/s – consistent with the thermal energy of the escaping gas – and is constant with increasing distance from the Sun since it is well above escape velocity. – ● Given the above two facts the density must fall off as 1/r2 The mass flux is about 108 kg/s 2 Ṁ =4 π r v ρ v proton T = 160 km/ s 6 10 K ( ) 1 2 The Heliopause ● The mass flux of the solar wind represents momentum. – As long as this momentum dominates the momentum/inertia of the surrounding (interstellar) medium the solar wind flows unimpeded. – At some point the solar wind's density falls to the point that the interstellar medium begins to dominate – the heliopause. – In reality things are more complicated due to shocks, magnetic fields, and other complexities The Heliopause ● The mass flux of the solar wind represents momentum. – As long as this momentum dominates the momentum/inertia of the surrounding (interstellar) medium the solar wind flows unimpeded. – At some point the solar wind's density falls to the point that the interstellar medium begins to dominate – the heliopause. – In reality things are more complicated due to shocks, magnetic fields, and other complexities Solar Wind Interaction with Earth's Magnetic Field ● ● ● Unimpeded, the solar wind would interact directly with Earth's outer atmosphere and erode it. The low pressure of the martian atmosphere is likely a consequence of this effect (or maybe not). The Earth's magnetic field deflect (charged) solar wind protons. Martian Atmospheric Loss ● ● All evidence for liquid flowing water on the surface of Mars points to an era when Mars had a substantially thicker atmosphere. Simply accounting for the Maxwell velocity distribution Mars should be able to hold on to a thick CO2 atmosphere, however solar wind stripping may play a significant role in planets with marginally bound atmospheres and weak magnetic fields. The Mars Atmosphere and Volatile EvolutionN experiment (launching today) aims to characterize Mars' atmospheric composition. It will orbit in a 6000 x 125 km ellipse dipping into the Martian exosphere each orbit with instruments that can directly sample atmospheric composition. It will correlate atmospheric composition and structure with solar activity. Hydrostatic Equilibrium and Scale Height ● Each layer of atmosphere must support the force/weight of the atmosphere above. The differential force (that is pressure) across any layer (dr) of the atmosphere is: G M (r )ρ dP = −ρ g dr = − dr 2 r ● The ideal gas law relates P and ρ P= ρk T μ m proton g μ mp dP ∫ P =− k T ∫ dr for constant g near a planetary surface, i.e. a simple plane-parallel atmosphere Hydrostatic Equilibrium and Scale Height ● Each layer of atmosphere must support the force/weight of the atmosphere above. The differential force (that is pressure) across any layer (dr) of the atmosphere is: G M (r )ρ dP = −ρ g dr = − dr 2 r ● The ideal gas law relates P and ρ P= ρk T μ m proton −Δ r P (r)=P o exp H ( ) kT H= g μ m proton Atmospheric “scale height” Conditions in the Solar Core ● Integrate the same equation, but accounting for the change in gravitational acceleration (and density) with radius. G M (r )ρ −dP = ρ g dr = dr = 2 r ● ● 4 3 G π r ρ(r ) ρ(r ) 3 ( ) r 2 dr Uh oh, this could get messy (they'll tell you all about it in graduate school) In the meantime.. 2 dP ≃ P central = g ρ = G M sun M sun 2 dr R sun R sun 4 π R 3 sun 3 ( ) P central ≃ 3 G M sun 4π R 4 sun Temperature in Solar Core ● Appeal to the ideal gas law (not a bad approximation since temperatures are so high we are dealing with individual elementary particles) dP ≃ P central = g ρ = G M ρ 2 dr R R ¿ ¿ ¿ P = k T ≃ G M sun ρ m particle R G M sun m particle Tc = R sun k P= ρk T μ m proton Temperature in Solar Core ● Appeal to the ideal gas law (not a bad approximation since temperatures are so high we are dealing with individual elementary particles) dP ≃ P central = g ρ = G M ρ 2 dr R R ¿ ¿ P= ρk T μ m proton ¿ P = k T ≃ G M sun ρ m particle R G M sun m particle Tc = R sun k P.E. = 12 million K = k skin crawling physics moment... the central temperature of the Sun is simply related to the gravitational potential energy of a single proton at the Sun's surface The Virial Theorm ● In a self-gravitating system in equilibrium kinetic and gravitational energy are equi-partitioned such that 2∗K.E. = − P.E. Why isn't Jupiter a Star ● Jupiter is 1/1000th the mass of the Sun and 1/10th the radius, so it's central temperature should be roughly 1/100th that of the Sun or about 100,000K – far too low for nuclear fusion. G M sun m particle M Tc = α R sun k R ● But the real question is why can't it become a star... – As it radiates away thermal energy it should be able to contract, giving up gravitational potential energy and since M is constant and R is getting smaller – get hotter. In fact getting hot enough to light its nuclear furnace stabilizing the configuration. ● ● The description above is exactly how a star “ignites” and achieves long term stability. Any self-gravitating sphere of hydrogen should be able to get hot enough eventually to achieve hydrogen fusion, however.... Degeneracy Pressure ● ● ...the scenario on the previous slide would be true if simple gas pressure were the only pressure in play. At high densities, that is small volume per individual particle, quantum mechanical degeneracy pressure (the desire of an electron not to be spatially confined) begins to dominate gas pressure and stops contraction. ΔxΔ p ≃ ℏ ● ● Gas pressure supports the Sun. Degeneracy pressure intervened long ago to halt the collapse of Jupiter and limit the core temperature. You'll learn about white dwarfs and neutron stars (related phenomena) in detail next semester. Brown Dwarfs ● So, degeneracy pressure intervened to prevent Jupiter from becoming a star, but such was not the case for the Sun. – ● ● ● Somewhere in between these two masses (0.001Msun and 1.0 Msun) lies the transition between brown dwarfs and stars. Brown dwarfs are star-like self gravitating balls of hydrogen where degeneracy pressure has thwarted achieving the required internal temperature necessary for thermonuclear fusion (a few million degrees) Object with masses lower than 0.08Msun (80 times the mass of Jupiter) are brown dwarfs – which cool steadily after an initially hot formation. Mind stretcher – the luminosity of a star has to do with it's configuration – not with the fact that nuclear burning is happening inside. The energy release from nuclear burning maintains the configuration... Back Down to Earth ● The temperature structure of the atmosphere is dictated by heating, cooling and bulk motion. Convection in the Troposphere ● Heating vs. cooling dictates the temperature profile at any layer of the atmosphere. – ● Heating vs. cooling translates to absorption vs. ease of radiation. Sunlight heating the surface dumps the most energy into the atmosphere. – The atmosphere itself radiates poorly so bulk motion of the atmosphere is required to get the energy out → convection. Convection in the Troposphere ● ● ● If a parcel of atmosphere is displaced upward it will find itself in a region of different pressure, temperature, and, in particular, density. In moving to a region of different pressure the parcel expands doing work against its environment and cooling – adiabatic expansion. The parcel is the equivalent of a hot air balloon, if it's new density is less than the local density it will be buoyant and continue to rise. Cloud Decks ● ● Clouds condense at the altitude where the temperature, set by adiabatic convection hits the condensation (dew) point. Ever notice that the cloud base for cumulus is lower when it is more humid? Temperature Profile in the Troposphere ● ● ● ● Adiabatic cooling thus sets the temperature profile in the troposphere due to convection. Temperature profiles can get complicated at night as temperature inversion turns off convection and the lower atmosphere can stratify. The vertical temperature profile is set by the “adiabatic lapse rate” which depends on the amount of water in the atmosphere. – Dry adiabatic lapse rate = 9.8C / kilometer – 5o F/thousand feet – Wet adiabatic lapse rate = 5C / kilometer – 3oF/thousand feet Clouds form at the level where the cooling crosses the dew point. More about Convection Convection occurs when the vertical temperature profile is less steep than the adiabatic lapse rate. − A rising parcel of air finds itself less dense than its surroundings and is thus buoyant. Convection is an extremely efficient conveyor of energy. − Once turned on, convection can easily carry the excess energy from the “hot” side of the fluid to the “cold”. − Convective atmospheres have their temperature profile set exactly to the adiabatic profile. No matter how much energy you put in at the bottom, convection can deal with the excess. Jupiter and low-mass (less massive than about 0.3Mo) are fully convective on the interior. − Their internal temperature profiles are adiabatic.





