Thermodynamic assessment of the Pd–Y binary system
advertisement

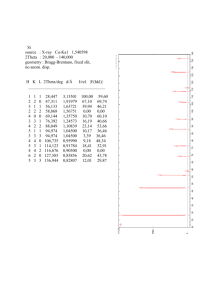
MATEC Web of Conferences 5, 04032 (2013) DOI: 10.1051/matecconf/20130504032 c Owned by the authors, published by EDP Sciences, 2013 Thermodynamic assessment of the Pd–Y binary system S. Kardellass, N. Selhaoui, A. Iddaoudi, M. Ait Amar, R. Karioui, and L. Bouirden Laboratory of Thermodynamics and Energy (L.T.E), Faculty of science, BP. 8106, University IbnZohr, Agadir, Morocco Abstract. The Pd–Y system was critically assessed using the CALPHAD technique. The solution phases (liquid, b.c.c., f.c.c. and h.c.p.) were modeled using the Redlich–Kister equation. The intermetallic compounds Pd3 Y and PdY, which have homogeneity ranges, were treated as the formula (Pd, Y)0.75 (Pd, Y)0.25 and (Pd, Y)0.5 (Pd, Y)0.5 by a two-sublattice model with a mutual substitution of Pd and Y on both sublattices. The optimization was carried out in two steps. In the first treatment, Pd3 Y and PdY are assumed to be stoichiometric compounds; in the second treatment they are treated by a sublattice model. The parameters obtained from the first treatment were used as starting values for the second treatment. The calculated phase diagram and the thermodynamic properties of the system are in satisfactory agreement with the experimental data. 1. INTRODUCTION The rare earth elements exhibiting a good combination of magnetic, optical, electrical and thermal properties have been paid increasing attention and widely used to prepare new high performance functional materials, such as permanent magnet, magnetic refrigerant and hydrogen storage material [1–3]. The CALPHAD technique, which has been recognized to be an important tool to significantly reduce time and cost during the development of materials, can provide a clear guidance for the materials design [4–6]. In order to develop the thermodynamic database of the rare earth alloys for reliable predictions of liquidus, phase fraction, equilibrium and non-equilibrium solidification behavior, etc. The thermodynamic assessment of the Pd–Y binary system was carried out by means of the CALPHAD method. 2. REVIEW OF EXPERIMENTAL DATA The phase diagram of the Pd–Y system was first measured by Loebich and Raub [7]. They found seven intermetallic compounds in the Pd–Y system and called them Pd3 Y, Pd2 Y, Pd5 Y4 , PdY, PdY3 , Pd3 Y2 and Pd2 Y5 , where Pd3 Y, PdY and Pd5 Y4 melted congruently and Pd3 Y2 , Pd2 Y and Pd2 Y3 formed by peritectic reactions. Later, Pd5 Y4 was identified to be Pd4 Y3 by Palenzona and Iandelli [8]. Pd7 Y and PdY3 were found to exist by Sanjines-Zeballos et al. [9] and Takao et al. [10], respectively. The results obtained have been taken by Massalski [22] to draw a phase diagram. Kuentzler and Loebich [11] presented a revised phase diagram and discussed the stability of the existing ordered structures. The homogeneity range of the intermetallic compound Pd3 Y and the solubility of Y in f.c.c.(Pd) were measured by Loebich and Raub [7] and Harris and Norman [12]. No appreciable solid solubility of Pd in Y was observed [7]. Loebich and Raub [7] indicated the presence of polymorphic transformation and a small range of homogeneity for PdY (51–52 at.% Y). Considering, however, the subsequent work of Kuentzler and Loebich [11] and Palenzona and Cirafici [13], the homogeneity range of PdY should be 50–51 at.% Y and the maximum melting point attributed to the ideal 50 at.% Y composition [14]. 3. THERMODYNAMIC MODELS 3.1. Pure elements The Gibbs energy function Gi (T) =◦ Gi − HiSER (298.15 K) (1) (298.15) for the element i (i = Pd, Y) in the phase ϕ (ϕ = Liquid, HCP A3, BCC A2 and FCC A1) is described by an equation of the following form: Gi (T) = a + bT + cT ln T + dT2 + eT3 + fT7 + gT−1 + hT−9 . (2) Where HSER (298.15 K) is the molar enthalpy of the i element i(i = Pd, Y) at 298.15 K in its standard element reference (SER) state, FCC A1 for Pd and HCP A3 for Y. In this article, the Gibbs energy functions are taken from the SGTE compilation of Dinsdale [15]. 3.2. Solution phases The solution phases [(FCC A1) Pd, HCP A3 (αY), BCC A2 (βY) and liquid] were modeled as substitutional solutions according to the polynomial Redlich–Kister model [16]. The Gibbs energy of one mol of formula unit of phase ϕ is expressed as the sum of the reference part ref ϕ G , the ideal part id G ϕ , and the excess part exc G ϕ : Gϕm = refG ϕ + idG ϕ + excG ϕ . (3) As used in the thermo-calc software [17]: ref ϕ G ϕ (T) = (0 GPd (T) − HSER Pd (298.15 K))xPd ϕ + (0 GY (T) − HSER Y (298.15 K))xY (4) This is an Open Access article distributed under the terms of the Creative Commons Attribution License 2.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Article available at http://www.matec-conferences.org or http://dx.doi.org/10.1051/matecconf/20130504032 MATEC Web of Conferences id G ϕ = RT (xPd ln xPd + xY ln xY ) (5) Where R is the gas constant, T the temperature, in Kelvin, Warning: xPd and xY are the fraction of elements Pd and Y, respectively. The excess terms of all the solution phases were modeled by the Redlich-Kister [16] formula: exc ϕ ϕ G ϕm (T ) = xPd xY [0 L Pd,Y (T ) + 1 LPd,Y (T )(xPd − xY ) ϕ + L Pd,Y (T )(xPd − xY )2 + · · · ] i LϕPd,Y (T ) = ai + bi T. (6) (7) ϕ Where i LPd,Y (T) is the interaction parameter between the elements Pd and Y, which is evaluated in the presented work, ai and bi are the coefficients to be optimized. 3.3. Stoichiometric compounds and intermediate phases The Gibbs energy of the stoichiometric compounds Ap Bq is expressed as follows: 0 G A p Bq = p 0 q 0 GA + G B + a + bT. p+q p+q (8) Where 0 G A and 0 G B are the Gibbs energy of the pure elements Pd and Y, respectively, a and b are parameters to be determined. The Pd3 Y (C14 Laves, isotypic with AuCu3 ) and PDY are treated by a two-sublattice model [18, 19]. The Gibbs energy function per mole (m) of the formula unit is the following: G Pd3Y m = 0 0 yPd yPd G Pd:Pd + yPd yY 0 G Pd:Y + yY yPd G Y:Pd 0 + yY yY G Y:Y + p RT (yPd Ln yPd + yY Ln yY ) + p RT (yPd Ln yPd + yY Ln yY ) yY yPd + pyPd n L Pd,Y:Pd (yPd − yY )n n + yY n n + qyPd yY yPd n L Pd:Pd,Y (yPd − yY )n n + yY n L Y:Pd:Y (yPd − occupied by element i or j · L i·Pd·Y and L PdY· j are the interaction parameters between Pd and Y in the second or first sublattice, when the other sublattice is occupied by element i or j. 0 Gi, j , Li:Pd,Y and LPd,Y: j were evaluated in the present work. 4. RESULTS AND DISCUSSIONS L Pd,Y:Y (yPd − yY )n Figure 1. Comparison of the Pd-Y calculated phase diagram with the experimental data. (b) Enlarged section of (Fig. 1). yY )n n G PdY = yPd 0 G Pd:Y + yY:Y 0 G Y:Y + RT (yPd Ln yPd ) m + yY Ln yY ) n L Pd,Y:Y (yPd − yY )n + yY n Where yi and y j are the site fraction of component i and j(i, j = Pd, Y) located on sublattice I and II, respectively, and the parameter 0 Gi: j represents the Gibbs free energy of the compound phase when the two sublattices are The evaluation of the phase diagram was carried out with the computer Thermo-Calcsoftoware [17] which makes it possible to optimize the formation Gibbs energies of the system phases by means of the least squares method using simultaneously thermodynamic and phase diagram data. The calculated phase diagram is shown in Fig. 1. It is compared with the numerous experimental data in Fig. 1. A good agreement is noted. All the parameters were evaluated and listed in Table 1. For the intermetallic compounds Pd3 Y, Pd4 Y3 , PdY ht, the coefficients a and b in Eq. (7), were adjusted according to the enthalpiesof formation measured by [22]. The calculated invariant equilibria in the Pd-Y system are listed in Table 2, where the largest uncertainty is about 4 ◦ C in the invariant temperature of the reaction Liq ↔ Pd2 Y5 + Pd2 Y3 In the reaction Liq ↔ Pd2 Y5 + Pd2 Y3 , the uncertainty in the invariant temperature is about 13 ◦ C [11]. In view of the estimated experimental 04032-p.2 REMCES XII Table 1. The optimized thermodynamic parameters of the Pd-Y system. Phase Liquid Thermodynamic models (Pd,Y)1 BCC A2 HCP A3 FCC A1 Pd7 Y (Pd,Y)1 (Va)3 (Pd,Y)1 (Va)5 (Pd)0.875 :(Y)0.125 Parameters in SI L = −344376.073 + 21.686T 1 liq L = −209832.431 + 54.045T No excess term No excess term 0 FCC A1 L = −640143 + 17.95T 7Y 298 FCC A1 GPd HPd − 0.125 298 HYHCP A3 Pd:Y − 0.875 0 liq FCC A1 = 0.875GPd + 0.125GYHCP A3 − 54709 + 10.815T Pd3 Y (Pd)0.75 :(Y)0.25 0.75 GPd:pd Pd Y0.25 FCC A1 − HPd = 80000 + GHSERPd Pd Y0.25 − 0.75HPd 0.75 GPd:Y FCCA1 − 0.25 HYHCP A3 =+ 0.25 GHSERPd + 0.75 GHSERY − 88887.7 + 6.135T Pd0.75 Y0.25 GY:Pd FCCA1 − 0.75HPd − 0.25HYHCP A3 =+ 75000 + 0.75GHSERPd + 0.25 GHSERY + 88887.7 − 6.135T Pd Y GY:Y0.75 0.25 Pd2 Y (Pd)0.667 :(Y)0.333 Pd2 Y GPd·Y Pd3 Y2 bt FCCA1 − 0.6HPd − 0.4HYHCP A3 FCC A1 = 0.6HPd + 0.4HYHCP A3 − 90425 + 2.08T (Pd)0.6 :(Y)0.4 (Pd)0.571 :(Y)0.429 = −103331.7 − 0.86T FCC A1 =+ 0.667 298 HPd + 0.333 298 HYHCP A3 − 90302.78 + 3.7T (Pd)0.6 :(Y)0.4 Pd Y bt Pd4 Y3 = 75000 + GHSERY FCC A1 − 0.667 298 HPd − 0.333 298 HYHCP A3 3 2 GPd·Y Pd3 Y2 ht − 0 Pd0.75 Y0.25 LPd:Y,Pd HYHCP A3 Pd Y ht 3 2 GPd·Y FCCA1 − 0.6HPd − 0.4HYHCP A3 FCC A1 = 0.6GPd + 0.4HYHCP A3 − 90107.45 + 1.84T PdY bt (Pd)0.5 :(Y)0.5 Pd Y HCPA3 FCCA1 4 3 GPd·Y − 0.571HPd − 0.429HY FCC A1 = 0.571GPd + 0.429GYHCP A3 − 90509 + 1.493T Pd 0.5 GPd·Y Y0.5 FCCA1 − 0.5HPd HCPA3 − 0.5HY =+ 0.5 GHSERPd + 0.5 GHSERY − 91389.8748 + 3.16T PdY ht (Pd)0.5 :(Y)0.5 Pd Y GY·Y0.5 0.5 − HYHCP A3 = 153095.5 + GHSERY 0 Pd0.5 Y0.5 LPd·Y:Y Pd0.5 Y0.5 GPd·Y = −141542.47 FCCA1 − 0.5HPd − 0.5HYHCP A3 =+ 0.5 GHSERPd + 0.5 GHSERY − 91389.8743 Pd2 Y3 (Pd)0.4 :(Y)0.6 Pd2 Y5 (Pd)0.286 :(Y)0.714 +3.16T Pd GY:Y0.75 Y0.25 0 Pd0.5 Y0.5 LPd,Y:Y PdY3 (Pd)0.25 :(Y)0.75 Pd2 Y3 GPd·Y − HYHCP A3 =+ 183383.5 − 7.7T + GHSERY = −162924.4 FCCA1 − 0.4HPd − 0.6HYHCP A3 FCC A1 = 0.4GPd + 0.6GYHCP A3 − 81146.17 + 7.23T Pd Y FCCA1 2 5 GPd:Y − 0.286HPd − 0.714HYHCP A3 FCC A1 = 0.286GPd + 0.714GYHCP A3 − 57513.8 + 3.47T PdY FCCA1 GPd:Y3 − 0.25HPd HCPA3 − 0.75HY FCC A1 = 0.25GPd + 0.75GYHCP A3 − 50238.5 + 2.914T 04032-p.3 MATEC Web of Conferences Table 2. Invariant reactions in the Pd-Y system. Reaction Present work T(K) X(Y) Liq + BCC A2 ↔ HCP A3 1751 0.980 1185 0.746 Liq + HCP A3 ↔ PdY3 Liq ↔ Pd2 Y5 + PdY3 1183 0.734 Liq ↔ Pd2 Y5 1187 Liq ↔ Pd2 Y5 + Pd2 Y5 1178 0.685 Liq + PdY ht ↔ Pd2 Y5 1233 0.649 Liq ↔ PdY ht 1723 Liq ↔ PdY ht + Pd4 Y3 1658 0.453 Liq ↔ Pd4 Y3 1683 Liq + Pd4 Y3 ↔ Pd3 Y2− ht 1546 0.376 Liq ↔ Pd3 Y2− ht + Pd2 Y 1519 0.367 Liq + Pd3 Y ↔ Pd2 Y 1578 0.342 Liq ↔ Pd3 Y 1973 Liq ↔ Pd3 Y + FCC A1 1478 0.147 FCC 0.132 Pd3Y 0.225 FCC A1 + Pd2 Y ↔ Pd7 Y FCC 777 0.101 Pd3Y 0.224 Pd3 Y2− ht ↔ Pd3 Y2− bt 1318 0.369 PdY− ht + Pd2 Y3 ↔ PdY− b 1171 − PdY− ht ↔ Pd4 Y3 + PdY− b 1149 − Ref [7] T(K) X(Y) 1751 0.980 1181 0.745 1168 0.730 1188 − 1176 0.700 1233 0.660 1723 0.500 1658 0.470 1683 − 1547 0.380 1518 0.370 1578 0.340 1973 0.250 1478 0.150 Figure 3. Calculated Pd-Y phase diagram when the liquid phase is suspended. 777 0.105 1318 1173 1143 − 0.520 0.510 Figure 4. Gibbs energy of mixing of the liquid phase at different temperature. at high temperatures. A terminal solid solutions and a two-phase domain existing between them are found to be stable (Fig. 3). Figure 4 shows the evolutions of Gibbs energy for the liquid phase asa function of temperature (T) with our optimization, when T is increasing up to 6000 K, the Gibbs energyfor the liquid phase decreases. The reference states were Pd and Y liquids. Figure 2. Calculated and measured enthalpies of formation of the intermetallic compounds. 5. CONCLUSIONS errors (about 1–2 at. %), all experimental invariant reaction compositions in the Pd–Y system are well reproduced. The assessed enthalpies of formation of the intermetallic compounds compared with experimental measurements are plotted in Fig. 2. The calculated enthalpies agree well with the experimental data [20–22]. As mentioned by [23] in order to check that the optimized thermodynamic parameters of the intermetallic compounds are satisfactory, we verified that when the liquid phase is suspended during the calculation of the Pd-Y phase diagram, the stoichiometric phases disappear All of the experimental phase equilibria and thermodynamic data of the Pd-Y system from the available literature have been critically evaluated. Within the regime of CALPHAD technique, the Redlich–Kister polynomial, the sublattice-compound energy model and the temperature dependant expression were adopted to describe the solution phases, the non-stoichiometric phases and the stoichiometric compounds, respectively. A set of self-consistent thermodynamic parameters has been obtained, which can reproduce satisfactorily most of the experimental data on thermodynamic properties and phase diagram within experimental uncertainties. 04032-p.4 REMCES XII References [1] K.H.J. Buschow, Rep. Progr. Phys. 1977; 40: 1179– 1256. [2] L. Schlapbach, A. Züttel, Nature. 2001; 414: 353– 358. [3] V.K. Pecharsky, K.A. Gschneidner, Phys. Rev. Lett. 1997; 78: 4494–4497. [4] L. Kaufman, H. Bernstein, Computer Calculation of Phase Diagram, Academic Press, New York, 1970. [5] N. Saunders, A.P. Miodownik, CALPHAD – A Comprehensive Guide, Pergamon Press, Oxford, UK, 1998. [6] H. Lukas, S.G. Fries, B. Sundman, Computational Thermodynamics—TheCalphad Method, Cambridge University Press, Cambridge, UK, 2007. [7] Q. Loebich Jr., E. Raub, J. Less-Common Met. 1973; 30: 47–62. [8] A. Palenzona, A. Iandelli, J. Less-Common Met. 1974; 34: 121–124. [9] R. Sanjines-Zeballos, B. Chabot, E. Parthe, J. LessCommon Met. 1980; 72: 17–20. [10] K. Takao, Y. Sakamoto, M. Yoshida, J. LessCommon Met. 1989; 152: 115–125. [11] R. Kuentzler, O. Loebich, J. Less-Common Met. 1985; 106: 335–348. [12] I.R. Harris, M. Norman, J. Less-Common Met. 1968; 15: 285–298. [13] A. Palenzona, S. Cirafici, Thermochim. Acta.1975; 12: 267–275. [14] G. Borzone, G. Cacciamani, R. Ferro, CALPHAD 1990; 14: 139–149. [15] AT. Dinsdale, SGTE data for pure elements. Calphad 1991; 15: 317–425. [16] O. Redlich, AKister. IndEng Chem. 1948; 40: 345–8. [17] B. Sundman, B. Jansson, JO. Andersson. The Thermo-Calc databank system. CALPHAD. 1985; 9: 153–90. [18] M. Hillert, L.I. Staffansson, Acta Chem. Scand. 1970; 24: 3618. [19] B. Sundman, J. Agren, J. Phys. Chem. Solids. 1981; 42: 297–301. [20] S. Paasch, H.-J. Schaller, Ber. Bunsenges. Phys. Chem. 1983; 87: 812. [21] R.A. Alqasmi, S. Paasch, H.J. Schaller, J. Alloys Comp. 1999; 283: 173–177. [22] N. Selhaoui, O. J. Kleppa, J. Alloys Comp. 1993; 191. [23] S.L. Chen, S. Daniel, F. Zhang, Y.A. Chang, W.A. Oates, R. Schmid-Fetzer, J. Phase Equilib. 2001; 22: 373. [24] Binary Alloy Phase Diagrams, Second Edition, Ed. T.B. Massalski, ASM International, Materials Park, Ohio. 1990; 3: 3065–3068. 04032-p.5