Basic Radiation Interactions, Definition of Dosimetric Quantities, and Data Sources J.V. Siebers
advertisement

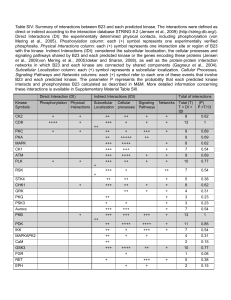
Basic Radiation Interactions, Definition of Dosimetric Quantities, and Data Sources J.V. Siebers Virginia Commonwealth University Richmond, Virginia USA 2009 AAPM Summer School Learningg Objectives j 1. 2 2. ©JVS: 2009 AAPM SS To review T i andd describe d ib the th bbasics i off radiation interactions for understanding radiation dosimetry To review definitions of quantities required for understanding radiation dosimetry Constants Units Conversions ©JVS: 2009 AAPM SS ©JVS: 2009 AAPM SS Scope Radiation Types Ionizing Interactions can remove atomic orbital electrons Particulate -electron -positron -proton -neutron p - alpha - etc. ©JVS: 2009 AAPM SS Non-Ionizing Electromagnetic Types yp of ionizingg radiation Di tl iionizing Directly i i radiation di ti Direct interactions via the Coulomb force along a particles track Charged particles electrons positrons protons heavy charged particles ©JVS: 2009 AAPM SS Direct Ionization Coulombic Interaction e A charged particle exerts electromagnetic forces on atomic electrons ©JVS: 2009 AAPM SS Energy transfer can result in the ejection of an electron (ionization) Indirectlyy Ionizingg Radiation Uncharged particles that must first transfer energy to a charged particle which can then further ionize matter Two T step t process Examples Electromagnetic El t ti radiations: di ti x- or γ-rays Neutrons ©JVS: 2009 AAPM SS Indirectly Ionizing Radiation Ph t l t i Eff Photoelectric Effectt e- h ©JVS: 2009 AAPM SS Ejected Ej t d electrons further ionize matter Radiant Energy gy R R – Total energy energy, excluding rest mass, mass carried by particles Photons: E = hν = hc/λ Electrons + other CPs: kinetic energy T ©JVS: 2009 AAPM SS Energy imparted ε imparted ε - Energy Rin R out Q Q Rin e- mass to energy conversion i resulting li from interactions or radioactive decay if(m→E), Q>0 e- h h if(E→m), Q<0 Rin c Rin u Rout c Rout u Q ©JVS: 2009 AAPM SS Rout D ©JVS: 2009 AAPM SS Dose d dm Gyy Energy deposited per unit mass 1 Gy = 1 J/kg Knowledge of D is the object of dosimetry Equilibrium Part 1: R di ti E Radiation Equilibrium ilib i h e- Rin Rout h e- ee- Rin Q Rout RE ©JVS: 2009 AAPM SS h h dQ d D Q dm dm RE Radiation Sources S Radioactive decay Accelerated charged particles Direct X-ray generators Atomic energy transitions Alpha-decay Beta-decay Electron capture Isomeric transitions Characteristic X-rays X rays Auger electrons Interaction products ©JVS: 2009 AAPM SS Radioactive Decayy General balance equations A Z P A AR Z ZR D ZARR R Q Q M ©JVS: 2009 AAPM SS P MD MR Q ©JVS: 2009 AAPM SS Radioactive Decay Activity dN A N dt At A0 e t1 2 ©JVS: 2009 AAPM SS ln 2 t Radioactive Decay α A Z P A 4 Z 2 D 24 He Q α ‘s have short range / A Z P A Z 1 A Z P A Z 1 D 10 Q D 10 Q Neutrino ( , ) results in spectrum p of energies g Emax and E are tabulated ( , ) are non-ionizing Electron Capture ZA P 10 e Z A1 D v Q Can occur when energetically prohibited Followed by characteristic x-rays or Auger electron so e c Transition a s o Isomeric A * Z P ZA P 00 Q decay from meta-stable state Internal Conversion ZA P* 01 e ZA P 01 e Q Competes with isomeric transition Results in ejection of atomic electron ©JVS: 2009 AAPM SS ©JVS: 2009 AAPM SS β+ Electron Capture ©JVS: 2009 AAPM SS O N 1.732 1 732 MeV M V 15 8 15 7 0 1 0 0 O 10 e 157 N 00 1.732 MeV 15 8 ©JVS: 2009 AAPM SS Accelerated Charged g Particles Di t use Direct Electrons, protons, … Indirect via production of electromagnetic radiation Synchrotron radiation Bremmstrahlung ©JVS: 2009 AAPM SS Synchrotron Radiation h Magnetic Field eSynchrotron image courtesy of http://www-project.slac.stanford.edu/ssrltxrf/spear.htm ©JVS: 2009 AAPM SS Bremmstrahlung h brems e- ©JVS: 2009 AAPM SS Atomic Energy gy Transition Characteristic x-ray xray h ©JVS: 2009 AAPM SS Atomic Energy Transition Auger Electron e- ©JVS: 2009 AAPM SS Quantifying Q y g Radiation Fields Th far Thus f R ε D ©JVS: 2009 AAPM SS Radiation Fluence dN da pparticles m 2 ©JVS: 2009 AAPM SS N is number of particles crossing i sphere h surrounding P with crosssectional area da Integrated over all directions and energies Single particle type Equivalent q definition of fluence l nTracks V ©JVS: 2009 AAPM SS l = particle track length through a volume l need not be straight Volume can be irregular U f l ffor M Useful Monte t Carlo applications Energy gy Fluence Definition dR da J m 2 Poly-energetic E E E dE Diff Differential ti l energy fluence fl E E d dE ©JVS: 2009 AAPM SS Mono-energetic E Attenuation d nt dl l 0 e l ©JVS: 2009 AAPM SS nt 1 m l 0 e Attenuation coefficient µ represents t th the iinteraction t ti ((removal)l) off primaries from the beam No consideration is given to what occurs as a result of the interaction l Secondary particles Energy-to-mass conversion … To remove density dependence, tabulated as µ/ρ [ 2/g] [cm / ] ©JVS: 2009 AAPM SS TERMA Total Energy Release per unit MAss J * TERMA kg Describes loss of radiant energy from uncharged primaries as they interact in material Energy lost can be absorbed locally or at a distance * For poly-energetic spectra E ©JVS: 2009 AAPM SS TERMA E E E dE J kg Aside: Photon Interactions ©JVS: 2009 AAPM SS To understand what happens with the radiant energy removed, understand the interactions (e.g. γ interactions) Photon interactions contributing to µ Rayleigh σ = Rayleigh + Compton scattering τ = photo-electric κ = pair production η = photo-nuclear ©JVS: 2009 AAPM SS m -1 Rayleigh y g S Scatteringg Elastic coherent scattering of the photon byy an atom Important for low energy photons Contributes C t ib t < 20% to t ttotal t l attenuation tt ti coefficient ©JVS: 2009 AAPM SS Compton Scattering e- h ©JVS: 2009 AAPM SS h NA Z e A cm2 g Compton p ©JVS: 2009 AAPM SS Photoelectric Effect e- h ©JVS: 2009 AAPM SS Te h Eb TA Photo-electric Z 34 h 23 τ increases when shell can participate in reaction ©JVS: 2009 AAPM SS Au Pair Production e- h pair e+ Tavail Te Te h 2 me c 2 ©JVS: 2009 AAPM SS mo c 2 T di radian Triplet Production Tavail h 2me c h ee- triplet e+ ©JVS: 2009 AAPM SS h 2me c T 3 2 2 Photo-nuclear interactions (γ n) (γ (γ,n), (γ,Xn), Xn) (γ,p), (γ p) … BE (Binding Energies) result in thresholds >~ 10 MeV Cross-section is small (η<0.1µ) Neutrons are ppenetratingg ©JVS: 2009 AAPM SS ©JVS: 2009 AAPM SS Pb attenuation coefficient ©JVS: 2009 AAPM SS Relative importance of interactions ©JVS: 2009 AAPM SS Summary photon interactions ©JVS: 2009 AAPM SS Energy transferred to charged particles per-interaction i t ti nonr general tr Rin u Rout u Q Average ©JVS: 2009 AAPM SS = = = photo compton pair tr ni ni tr l 0 e Recall Attenuation coefficient µ represents the interaction (removal) of primaries from the beam No consideration is given to what occurs as a result of the interaction l Secondary particles Energy-to-mass conversion … To remove density dependence, tabulated as µ/ρ [cm2/g] ©JVS: 2009 AAPM SS Mass-energy transfer coefficient Describes the transfer of energy to charged particles ti l tr tr h ©JVS: 2009 AAPM SS KERMA Kinetic Energy Release per unit MAss d tr KERMA K dm tr ©JVS: 2009 AAPM SS * J kg The transfer of radiant energy from uncharged primaries to charged particles as they interact in a material Energy transferred can be absorbed locally or at a distance *Mono-energetic, integrate for poly-energetic Net energy transfer Ruru R trnet trnettr trRout Routuu Rur Rout Rin inuu R out uQ Q nonr nonr r r Accounts for portion of kerma is radiated away T’ Te- h brems hv Compton example h Te- trnet Te hvbrems h ©JVS: 2009 AAPM SS Mass energy absorption coefficient Mass-energy R di ti loss Radiative l ffraction ti g g 1 trnet tr M Mass-energy absorption b ti coefficient ffi i t en tr 1 g ©JVS: 2009 AAPM SS Kerma Components K Kc Kr C lli i K Collision Kerma d trnet Kc dm en Kc * Portion of kerma that remains collisional energy losses (non-radiative) Radiative Kerma ©JVS: 2009 AAPM SS Portion of kerma (transported elsewhere) by radiative losses Exposure and W Exposure Historical Hi t i l radiation di ti unitit Ionization density in air Related to air collision kerma by mean energy required to produce an ion pair e X K c air W air W ev 33.97 ip e air ©JVS: 2009 AAPM SS dQ C X dm kg C kg 1.602 1019 ( J eV ) 1 ip J 33.97 1.602 electron 19 C 1 602 10 ( C electron ) Aside Indirectly ionizing radiation How many ionization events can be initiated by a 10 keV photo-electron? 1 ipp ? ip 10 10 eV 294 ip 33.97 eV 3 ©JVS: 2009 AAPM SS Equilibrium Part 2: Charged Particle Equilibrium h e- ee- e- e- Rin c Rout c h Rin c Rin u Rout c Rout u Q Rin u Rout u Q ... trnet ©JVS: 2009 AAPM SS CPE CPE d d trnet D Kc dm dm CPE CPE Charge g pparticles e-, e+, p, α, … Sources Accelerated beams Radioactive decay Reaction products Coulomb force interaction ©JVS: 2009 AAPM SS (e,γ) , … (n,p), … ((e,e), ) … Inverse square dependence Semi-continuous Semi continuous rather than discrete interactions Results in energy loss and directional change Interaction can be classified by impact parameter CP interactions b = impact parameter a = atomic t i radius di n = nuclear radius undisturbed incident trajectory b b>>a Soft, atomic interaction b~a Hard, knock-on interaction b<<a Nuclear interactions possible ©JVS: 2009 AAPM SS a Stopping S pp g ppower E Energy loss l per unitit path-length th l th dE S dx MeV cm S dE dx MeV cm 2 g S Separate t components t bby interaction i t ti S ©JVS: 2009 AAPM SS Scol S rad Stopping S pp g ppower formulations Basedd on B B Bethe-Bloch, th Bl h Heitler, H itl … Electrons: ICRU 37 2 2 S 1 Z 2 2 ln F 2 r m c N e e A 2 2 2 A Collisional 2 I m c e S rad 2 e r NA 2 Z E me c 2 B r 13 A 137 Material dependent terms ©JVS: 2009 AAPM SS T me c 2 v c 2 F 1 2 1 2 1 ln 2 8 ©JVS: 2009 AAPM SS Water--electrons ©JVS: 2009 AAPM SS Material Comparisons Electron stopping powers ©JVS: 2009 AAPM SS Stopping S pp g ppower formulations Scol Protons/ Heavy charged particles: ICRU 49 4 r me c N A 2 e 2 1 2 Z 2 1 2me c 2 2Wm z ln 2 A 2 I 1 2 C 2 B1 B2 Z 2 With Wm, the maximum energy gy that can be transferred to an electron in a single collision 2me c 2 2 Wm 2 1 ©JVS: 2009 AAPM SS 2 me 1 me 1 2 2 M M 1 Material dependent terms ©JVS: 2009 AAPM SS Water--protons p ©JVS: 2009 AAPM SS Recall KERMA Transfer of radiant energy from uncharged primaries to charged particles as they interact in a material d tr K dm K Emax tr ( E ) E E 0 E dE K Kc Kr d trnet Kc d dm ©JVS: 2009 AAPM SS Kc Emax en ( E ) E E 0 E dE CEMA Converted Energy per unit MAss Describes D ib energy transfer f from f primary i charged h d particles i l to secondary charged particles (δ-rays) Energy gy transferred can be absorbed locallyy or at a distance Defined in ICRU 60 Charged particle analog to KERMA dE J C=dEc/dm C c dm kg Emax ©JVS: 2009 AAPM SS CC =integral(). 0 E E Scol E dE CEMA example p Thin slab CP Φ Energy loss dE Sc t t ©JVS: 2009 AAPM SS Fluence Φ of incident mono-energetic charged particles CEMA C δCPE constant S/ρ straight particle paths When δ-ray equilibrium exists, CEMA = dose Sc J kg Restricted CEMA Excludes E l d energy llosses to t energetic ti (E (E>Δ) Δ) δ-rays (aka knock-on electrons) Such δ-rays are added to the fluence Φ’ C E E L E dE E E E E col E E ©JVS: 2009 AAPM SS Restricted Stopping Power L ©JVS: 2009 AAPM SS Scoll dEke k dx Includes energy transfers only up to energy Δ Excludes energy losses from to energetic (E>Δ) δ-rays Δ is chosen with respect to the distance the δ-rays can travel in the material of interest Restricted est cted C CEMA C Emax E E L E dE Energy loss for Ee > ∆ ©JVS: 2009 AAPM SS lim lim Emax lim lim Emax L C C Scol 0 E E S col E dE Track end term & electrons generated outside volume Path Length and Range Variations in energy loss and scattering result in different paths through a material ( & different maximum penetration distances) p = total distance traveled byy a pparticle w/o relation to direction R = average path length CSDA Range RCSDA To 0 ©JVS: 2009 AAPM SS 1 dE S (E) g cm 2 Range Rt = average depth of penetration in the original particle direction R50 = range g at 50% max dose Rp = practical or extrapolated range, intersection of tangent @R50 with brems tail ©JVS: 2009 AAPM SS Range-Energy Relationships Incident energy E0 2.33R50 Average energy at depth (Harder’s Formula) depth E Eo 1 R p ©JVS: 2009 AAPM SS Equilibrium Part 3: CPE Revisited For an external beam beam, if no attenuation, CPE exists beyond Dmax But, e- production due to attenuation T True CPE cannott exist i t for external beam ©JVS: 2009 AAPM SS Equilibrium Part 4: Transient Charged Particle For external F t l beams D( x) K c ( x) TCPE ©JVS: 2009 AAPM SS Neutron Interactions ….see text t t ©JVS: 2009 AAPM SS Problem #5 E h problem Each bl give i ©JVS: 2009 AAPM SS Problem #5 , ©JVS: 2009 AAPM SS , ©JVS: 2009 AAPM SS Thank you for your attention ©JVS: 2009 AAPM SS