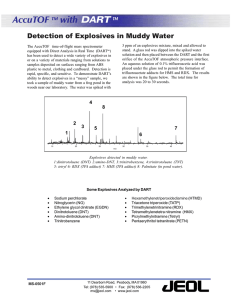
Trace detection of explosives using a membrane-based displacement immunoassay * Sina Y. Rabbany
advertisement

Journal of Immunological Methods 246 (2000) 69–77 www.elsevier.nl / locate / jim Trace detection of explosives using a membrane-based displacement immunoassay a, a a Sina Y. Rabbany *, William J. Lane , William A. Marganski , Anne W. Kusterbeck b , Frances S. Ligler b b a Bioengineering Program, Hofstra University, 104 Weed Hall, Hempstead, New York, NY 11549, USA The Center for Bio /Molecular Science and Engineering, Naval Research Laboratory, Washington, DC 20375, USA Received 26 April 2000; received in revised form 10 August 2000; accepted 6 September 2000 Abstract A compact membrane-based displacement immunoassay has been designed for rapid detection of explosive compounds 2,4,6-trinitrotoluene (TNT) and hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) at high femtomole levels. The system consists of activated porous membranes, onto which either TNT or RDX antibodies are immobilized, that are inserted into microreactor columns, incorporated into a flow system. The assay is prepared by saturating the immobilized antibody binding sites with labeled antigen. Target analyte is introduced upstream of the microreactor, while the displacement of labeled antigen is monitored downstream using a fluorometer. The concentration of displaced labeled antigen detected is proportional to the concentration of the target analyte introduced into the system. This system provides a reusable and reagentless sensor, suitable for continuous monitoring of explosives, with an operating lifetime of over 50 positive samples. Multiple assays were performed in approximately 5 min at different flow rates, using membranes saturated with varying antibody concentrations. The membrane-based format exhibited a detection limit of approximately 450 fmol for TNT and RDX (100 ml of 1 ng / ml solution) in laboratory samples. 2000 Elsevier Science B.V. All rights reserved. Keywords: Biosensor; Explosives; RDX; TNT; Flow immunosensor; Immobilized IgG 1. Introduction Explosives pose major security and environmental risks and hence there is a growing need for sensors capable of accurate and rapid on-site detection (Steinfeld and Wormhoudt, 1998). Specifically, tech- *Corresponding author. Tel.: 11-516-463-6672; fax: 11-516463-4939. E-mail address: eggsyr@hofstra.edu (S.Y. Rabbany). nologies for detecting easily concealable plastic explosives frequently used in terrorist attacks are essential to protect both human life and property (Fainberg, 1992). Additionally, environmental contamination by explosives, a serious problem at former munitions manufacturing facilities, has prompted regulatory agencies to mandate extensive monitoring (Van Emon and Lopez-Avila, 1992). Explosives can readily enter groundwater supplies from contaminated soil and hence pose environmental remediation concerns. For example, the explosive 2,4,6-trinitrotoluene (TNT) is classified as toxic by 0022-1759 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved. PII: S0022-1759( 00 )00301-X S.Y. Rabbany et al. / Journal of Immunological Methods 246 (2000) 69 – 77 70 the EPA at concentrations above 2 ng / ml (Environmental Protection Agency, 1989). There are several existing laboratory based methods to measure explosives in solution. The conventional methods include high performance liquid chromatography (HPLC) (Walsh et al., 1993), gas chromatography–mass spectroscopy (GC / MS) (Yinon and Zitrin, 1993), fluorimetric methods (Lapat et al., 1997), and capillary electrophoresis (CE) (Northrop et al., 1991). Promising technologies for trace detection include surface acoustic wave (SAW) devices (Weisch et al., 1996), ion mobility spectroscopy (IMS) (Garofolo et al., 1992), and fast-neutron transmission spectroscopy (FNTS) (Overley et al., 1995). Radioimmunoassays have also been used, but the issue of radioactive waste disposal is problematic. Fluorescent immunoassays have emerged as a potential alternative to these techniques (Rabbany et al., 1994), since they are well suited to on-site monitoring. The production of homogenous monoclonal antibodies against low molecular weight substances has advanced the development of highly specific immunological assays. Several immunological techniques for detection of explosives such as TNT have been published (Whelan et al., 1993; Dosch et al., 1998). Efforts toward the detection of analytes in the femtomole (Vellom et al., 1984) and attomole range (Narang et al., 1997) have also been documented in the literature. To this end, a biosensor has been developed for rapid, sensitive and specific detection of low molecular weight analytes using a fluorescent dye-labeled antigen in a continuous flow displacement format (Kusterbeck et al., 1990). Unlike ELISA-type systems, this immunoassay configuration does not require incubation, washing steps, or the introduction of reagents following sample loading (Wemhoff et al., 1992). Most importantly, multiple samples can be rapidly analyzed using a single aliquot of immobilized antibody. The kinetics of the displacement of fluorophorelabeled antigen from the immobilized antibody in the membrane-base immunoassay are represented by the following reaction utilizing the law of mass action: kd AbAg * 1 Ag áAbAg 1 Ag * kr where AbAg * is the complex of immobilized antibody and labeled antigen; Ag is the unlabeled antigen added during each sample injection; AbAg is the resulting complex of immobilized antibody and unlabeled antigen; and Ag * is the labeled antigen displaced from the antibody and detected downstream; k d and k r are the displacement and rebinding rate constants, respectively. Thus the rate of displacement equals k d [AbAg * ][Ag], whereas the rate of rebinding equals k r [AbAg][Ag * ]. The rate of complex formation is proportional to the concentration of both reacting species and the combination of the rate of the dissociation /(re)association reactions: → Ab 1 Ag Ab /Ag * ← → Ab 1 Ag AbAg ← In this displacement assay, the detected fluorescence signal relies primarily on the labeled antigen dissociation rate in conjunction with the competitive binding of the labeled and unlabeled antigen to the antibody. Fig. 1 provides a schematic diagram of the membrane-based continuous flow immunoassay. A more detailed description is found elsewhere (Rabbany et al., 1994). We have recently reported on the development of this membrane-based displacement flow immunoassay for the detection of TNT (Rabbany et al., 1998). The membrane-based assay provides a multisample detection approach, while offering sensitivity, reproducibility, and the flexibility to be adapted toward detection of different biomolecules. A commercial membrane-based flow displacement immunosensor, the Flow Assay Sensing and Testing device (F.A.S.T. 2000) is now available from Research International, Inc. (Woodenville, Washington). This device has been demonstrated during fieldtesting to be effective in performing on-site analyses of both TNT and RDX (Kusterbeck and Charles, 1998). We have continued to explore options that increase the detection limit and specificity of our membrane assay using the two explosives 2,4,6-trinitrotoluene (TNT) and hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). In particular, the effect of variables such as flow rate, antibody density, and membrane storage time are characterized. S.Y. Rabbany et al. / Journal of Immunological Methods 246 (2000) 69 – 77 71 Fig. 1. Schematic diagram representing the membrane-based displacement flow immunoassay. Immobilized antibody is saturated with labeled antigen. Unlabeled antigen is introduced, and a proportionate amount of labeled antigen is displaced from the immobilized antibody-binding sites and subsequently detected downstream. 2. Materials and methods 2.1. Monoclonal antibodies and analytes The monoclonal antibodies with a specificity for TNT (IgG 11B3) were generated specifically for our use by Perimmune (Rockville, MD). The monoclonal anti-RDX (IgG 50518) and the RDX analog were procured from Strategic Diagnostics, Inc. (Newark, DE). Analytical standards of 2,4,6-trinitrotoluene (TNT), a single ring aromatic compound that is widely-utilized military explosive, and hexahydro1,3,5-trinitro-1,3,5-triazine (RDX), a commonly used plastic explosive, were obtained from Radian International LLC (Austin, TX). All analytes were purchased as dilute solutions dissolved in acetonitrile. 2.2. Antibody immobilization Porous Immunodyne ABC membranes (Pall Corp., Port Washington, NY) of a 0.45 mm pore size were cut into circular disks with a diameter of 6.0 mm and height of 150 mm. The membrane consisted of a nylon mesh which was modified to incorporate unspecified surface reactive sites that facilitated the covalent binding of proteins, water, and other compounds containing nucleophilic groups. The membranes were incubated for 90 min in 10 ml of antibody solutions ranging in concentrations from 1.9 to 8.6 nmol / ml. After immobilizing the antibody, the membranes were placed into 100 ml of a 5 mg / ml Hammarsten casein solution containing 0.01% Triton-X-100 for one h in order to block any remaining binding sites on the membrane. 2.3. Labeled antigen saturation To prepare for saturation with labeled antigen and to remove non-specifically-bound proteins, the membranes were washed three times with 100 ml of phosphate buffer solution (PBS) containing 0.01% Triton X-100. The membranes were then dispensed into a small, disposable, flow-through column (Isolab Inc., Akron, OH) with a 50–100 ml head volume. The available antibody binding sites were saturated with 50 ml of a 30 mM fluorophore-labeled antigen 72 S.Y. Rabbany et al. / Journal of Immunological Methods 246 (2000) 69 – 77 (CY5-TNB or CY5-RDX) solution and incubated at room temperature overnight. The synthesis of the CY5-labeled analogs of TNT and RDX were reported previously (Shriver-Lake et al., 1995; Bart et al., 1997). 2.4. Membrane incorporation into the flow system The membrane-based displacement immunoassay was conducted in the laboratory flow system format consisting of a Rabbit-Plus peristaltic pump (Rainin Instruments, Emeryville, CA) and a fluorescence detector (Model 821-FP; Jasco Inc., Easton, MD) equipped with a 12 ml flow cell and a 150 W xenon light source. A 10 mM PBS solution containing 2.5% ethanol and a surfactant, 0.01% Tween-20, were pumped through the flow system to remove nonspecifically-bound fluorophore-labeled antigen and to ensure solubility of any TNT and RDX in the samples. 2.5. Explosive detection The experiments were run at flow rates ranging from 0.1 to 2.0 ml / min, while the assay eluant was monitored at an excitation of 635 nm and an emission of 661 nm. When the background fluorescence stabilized, 100 ml samples of analyte diluted with the buffer solution were introduced into the flow using a Rheodyne five-way valve sample injector. Analyte injections, which ranged from 0.01 to 10 000 ng / ml, were made in triplicate. Data are expressed as mean6S.E. A Hewlett Packard integrator (Palo Alto, CA) was used to record and quantify the displaced labeled antigen. 3. Results 3.1. Flow rate comparison To examine the effect of flow rate on signal intensity, a repetitive displacement assay was conducted using separate membranes for TNT and RDX. Fig. 2 depicts the signal intensity as the flow rates were decreased from 1.0 to 0.25 ml / min. The signal intensities measured for three repetitive 100 ml injections of a 37.5 ng / ml RDX or TNT solution are shown (Table 1). An increase in signal intensity is shown to correspond to a decrease in flow rate. A paired t-test determined that the signal intensities Fig. 2. TNT (h) and RDX (j) repetitive displacement assay. Membranes were coated using 4.4 nmol / ml antibody. Triplicate 100 ml injections of 37.5 ng / ml analyte were loaded at flow rates of 1.0, 0.5, 0.25 ml / min. The signal intensities differ for the flow rates tested with a significance of P,0.001. S.Y. Rabbany et al. / Journal of Immunological Methods 246 (2000) 69 – 77 73 Table 1 Relationship between flow rate and average signal intensity a Flow rate (ml / min) TNT signal intensity310 3 mean6S.E. RDX signal intensity310 3 mean6S.E. 0.25 0.50 1.0 765621 304611 24867 14016146 644617 212614 a Signal intensities measured for three repetitive 100 ml injections of a 37.5 ng / ml TNT or RDX solution. differ for the flow rates tested with a significance of P,0.001. 3.2. Dose–response curve The sensitivity of the membrane-based displacement immunoassay was examined by introducing various analyte concentrations to the immobilized antibody-labeled antigen complex at flow rates of 1.0, 0.50 and 0.25 ml / min. Standard curves showing the effect of flow rate on signal intensity are depicted in Fig. 3. The signal intensity is observed to increase proportionally with a decrease in the flow rate at each particular analyte injection. The inset illustrates the same data in the lower concentration range of 0.1–10 ng / ml. A similar study was conducted using RDX antibodies. Fig. 4 illustrates the effect of flow rate (0.1, 0.25, 0.5 ml / min) on signal intensity using a membrane saturated at 4.4 nmol / ml. A comparable relation was observed. Samples of phenylalanine (1000 ng / ml) were introduced as a negative control. The detected fluorescence signal was negligible compared to the background signal level for both assays. The detection threshold of 1 ng / ml determined experimentally, was defined at the lowest concentration whereby reproducible signals can be measured. This value is significantly higher than the theoretical limit which is defined as the mean of negative control63 standard deviations. Fig. 3. Effect of flow rate on dose response. Dose response curves illustrating the integrated areas of the peaks obtained upon injecting TNT in a 100 ml samples onto a membrane coated with an antibody density of 3.9 nmol / ml. These curves were generated at flow rates of 0.25 ml / min (j), 0.5 ml / min (d) and 1.0 ml / min (m). The inset illustrates the same data at the lower concentration range of 1 to 10 ng / ml. The mean values and standard errors for three to five sample injections are shown. 74 S.Y. Rabbany et al. / Journal of Immunological Methods 246 (2000) 69 – 77 Fig. 4. Dose response curves showing the integrated areas of the peaks obtained upon injecting 100 ml samples of RDX solution, onto a membrane coated with an antibody density of 4.4 nmol / ml. These curves were generated at flow rates of 0.1 ml / min (j), 0.25 ml / min (d) and 0.5 ml / min (m). The mean values and standard errors for triplicate injections are shown. 3.3. Immobilized antibody density The effect of TNT antibody density on signal intensity is illustrated in Fig. 5. Fig. 5A shows the data obtained at flow rate of 1.0 ml / min for three membranes that had been prepared using antibody solutions of 1.9, 3.9 and 7.8 nmol / ml, respectively. The data for the flow rates of 0.5 and 0.25 ml / min are shown in Figs. 5B and C. The signal intensity has the lowest magnitude at the highest antibody concentration. However, discernable differences in the three densities was observed only at the lowest flow rate. The effect of RDX antibody density (4.4 and 8.75 nmol / ml), at the flow rate of 0.25 ml / min, on signal intensity is shown in Fig. 6. For increasing concentrations of loaded antigen (RDX), corresponding increases were observed in the total displaced labeled antigen, Ag * . The inset depicts the same relationship as the concentration of the labeled RDX is increased to 1200 ng / ml. Membranes could be stored in PBS at 48C for extended periods (up to 8 months) and used in later assays, thought lower displacement levels were observed. In general, signal intensities for stored membranes were decreased as the concentrations of the injected RDX were increased. 4. Discussion Primary requirements of an immunoassay for detection of explosives are specificity (to avoid false positive or false negative responses to interferents), a low detection threshold (to identify targets at low concentrations), and a rapid response time (to shorten analysis time and reduce costs). We have developed a membrane-based displacement immunoassay for the detection of explosives that addresses all three of these requirements. This portable system, which requires no reagent addition during the assay (i.e., samples are only diluted with PBS to adjust pH), is able to process a large number of samples in a relatively short period of time. It can detect trace amounts of explosives over a large range of sample concentrations without eliciting a response to false targets. Previously, we demonstrated the high specificity exhibited by a membrane-based displacement system for TNT (Rabbany et al., 1998). In an effort to improve the signal produced by the membrane- S.Y. Rabbany et al. / Journal of Immunological Methods 246 (2000) 69 – 77 Fig. 5. Comparison of the effects of antibody density on the signal intensity. Dose response curves obtained at a flow rates of 1.0 ml / min (A), 0.5 ml / min (B), and 0.25 ml / min (C) are shown for membranes coated with antibody solutions of 1.9 nmol / ml (j), 3.9 nmol / ml (d) and 7.8 nmol / ml (m). The mean values and standard errors for three to five sample injections are shown. based immunoassay, this study investigated parameters such as flow rate and antibody density for two major explosives. In this study, the membrane-based immunoassay can detect TNT and RDX standards as low as 1 ng / ml. This corresponds to a detection threshold (in mole fraction) of 81 parts per trillion (ppt) for TNT and 79 parts per trillion (ppt) for RDX. The detection threshold and signal intensity can be adjusted by changing the flow rate and antibody density. For example, a decrease in flow rate increased the signal intensity because of an increase in the interaction time between the injected analyte and the immobilized antibody-labeled antigen complex. The sensor’s detection threshold was improved with lowering the antibody density. Antibody density also influenced the signal intensity at the lower flow rates. To 75 improve the design of the membrane-based displacement immunoassay, elucidating the relationship between antibody density and flow rate is critical. Under flow conditions, the membranes with different antibody density may be affected to a different extent by the unoccupied binding sites, thus effecting the degree of reassociation of the displaced labeled antigen. Detection thresholds as low as five parts per billion were measured with an alternative explosive detection system, Method 4054 and Method 4051 (US Environmental Protection Agency), for both explosives diluted in laboratory samples. However, our membrane-based immunoassay possesses a detection threshold approximately three orders of magnitude less than these two other methods, making it more appropriate for application in the field. Because, it is well known that field-environmental samples may contain complex mixtures that may, in fact, effect the response of the assay. Such degradation should be considered when using the membrane assay. With respect to analytical precision and accuracy, the membrane-based displacement immunoassay performance has been demonstrated to be well correlated to the two alternative explosive detection systems, Method 4050 and Method 4051, which are approved by the Environmental Protection Agency (EPA) for the detection of TNT and RDX, respectively (US Environmental Protection Agency). In these assays, an enzyme conjugate of TNT or RDX competes with TNT or RDX present in samples of unknown concentration for a binding site on immobilized TNT or RDX antibody. The storage or shelf-life of biochemical reagents used in test kits is also critical to the wide-spread utilization of the system in the field. The stability of the membrane-based immunoassay was examined by preparing membranes and then generating a calibration curve after a long storage period. The detection threshold remained fairly low, approximately 20 ng / ml, and the dynamic range remained high over a broad range of analyte concentrations, approximately 1–600 ng / ml, even with the negative effects of a long storage period. Further studies are warranted to determine whether the reduced signal intensity produced by the stored membrane was due to reduced antibody activity or the requirement for 76 S.Y. Rabbany et al. / Journal of Immunological Methods 246 (2000) 69 – 77 Fig. 6. Dose response curves illustrating the integrated areas of the peaks obtained upon injecting RDX in 100 ml of samples onto a membrane coated with antibody solutions of 4.4 nmol / ml (j) and 8.75 nmol / ml (d). These curves were generated at a flow rate of 0.25 ml / min The inset illustrates the same dose repose curve over a larger concentration range. The mean values and standard errors for two to four sample injections are shown. more extensive washing. The latter may have reduced the amount of labeled antigen available for displacement. It is well known that field environmental samples contain complex mixtures that may, in fact, affect the assay. This effect is further intensified because no extraction or pretreatment is done on a sample prior to analysis. Though previous studies with environmental samples showed minimal problems with matrix interferents (Bart et al., 1997), degradation of response should be considered when analyzing complex mixtures. To validate assay response, tests should always be run at each site with appropriate blanks, controls, and standards prepared in the field matrix. Finally, for environmental applications, it is critical that determinations of the target at the regulatory threshold be reliable. Data from initial field studies using the membrane-based immunoassay showed that environmental samples from some sites may decrease the sensitivity of the assay and make accurate analyses more difficult (Kusterbeck and Charles, 1998). The results described in this study demonstrate significant improvement in detection limits and provide a way to overcome difficulties often seen with complex environmental matrices. In conclusion, rapid, reliable and sensitive methods for the detection of explosives in the field are needed. To comply with this need, a membranebased displacement immunoassay was developed which controls the detection threshold by changes in flow rate while reducing sample volume. Another advantage of our displacement immunoassay is that negative assays have minimal impact on the system. For example, often 70–90% of soil samples analyzed during a site investigation for explosives do not contain detectable levels of contamination. Using a field screening method for site characterization and laboratory methods for the verification would significantly decrease the number of samples analyzed using the much more expensive analytical methods and significantly reduce costs. Acknowledgements This work was supported by a grant to Hofstra University from the Naval Research Laboratory. The S.Y. Rabbany et al. / Journal of Immunological Methods 246 (2000) 69 – 77 authors wish to thank Drs. David Holt, Joe Pancrazio, and Lenny Tender for their review of the manuscript. The views expressed are those of the authors and do not necessarily represent those of the US Navy or the Department of Defense. References Bart, J.C., Judd, L.J., Hoffman, K.E., Wilkins, A.M., Kusterbeck, A.W., 1997. Application of portable immounosensor to detect the explosives TNT and RDX in groundwater samples. Environ. Sci. Technol. 31, 1505. Dosch, M., Weller, M.G., Buckmann, A.F., Niessner, R., 1998. Homogeneous immunoassay for the detection of trinitrotoluene (TNT) based on the reactivation of apoglucose oxidase using a novel FAD-trinitrotoluene conjugate. Fresenius’ J. Anal. Chem. 361, 174. Environmental Protection Agency, 1989. Health Advisory for TNT. Criteria and Standard Division, Office of Drinking Water, Washington, DC. Fainberg, A., 1992. Explosives detection for aviation security. Science 255, 1531. Garofolo, F., Migliozzi, V., Roio, B., 1992. Rapid Commun. Mass Spectrometry 8, 527. Kusterbeck, A.W., Wemhoff, G.A., Charles, P.T., Yeager, D.A., Bredehorst, R., Vogel, C.W., Ligler, F.S., 1990. Continuous flow immunoassay for rapid and sensitive detection of small molecules. J. Immunol. Meth. 135, 191. Kusterbeck, A.W., Charles, P.T., 1998. Field demonstration of a portable flow immunosensor. Field Analytical Chemistry and Technol. 2 (6), 341. Lapat, A., Szekelyhidi, L., Hornyak, I., 1997. Spectrofluorimetric determination of 1,3,5-trinitro-1,3,5-triazacyclohexane (hexogen RDX) as a nitramine type explosive. Biomed. Chromatogr. 11, 102. Narang, U., Gauger, P.R., Ligler, F.S., 1997. Capillary-based displacement flow immunosensor. Anal. Chem. 69, 1961. Northrop, D.M., Martrie, D.E., MacCrehan, W.A., 1991. Separation and identification of organic gunshot and explosive 77 constituents by micellar electrokinetic capillary electrophoresis. Anal. Chem. 63, 1038. Overley, J.C., Chmelik, M.S., Rasmussen, R.J., Sieger, G.E., Schofield, R.M., Lefevre, H.W., 1995. Results of blind tests for explosives in luggage using fast-neutron transmission spectroscopy (FNTS). Proc. 2nd Explosive detection Tech. Symposium. 305. Rabbany, S.Y., Donner, B.L., Ligler, F.S., 1994. Optical immunosensors. Crit. Rev. Biomed. Eng. 22, 307. Rabbany, S.Y., Marganski, W.A., Kusterbeck, A.W., Ligler, F.S., 1998. Kinetics of antibody binding at solid–liquid interfaces in flow. Biosensors and Bioelectronics 13, 939. Shriver-Lake, L.C., Breslin, K.A., Charles, P.T., Conrad, D.W., Golden, J.P., Ligler, F.S., 1995. Detection of TNT in water using fiber-optic biosenser. Anal. Chem. 67, 2431. Steinfeld, J.I., Wormhoudt, J., 1998. Explosives detection: a challenge for physical chemistry. Ann. Rev. Phys. Chem. 49, 203. US Environmental Protection Agency, SW-846 December, 1996, US EPA Method 4050, and 4051. Van Emon, J.M., Lopez-Avila, V., 1992. Immunological methods for environmental analysis. Anal. Chem. 64, 79A. Vellom, D., Hinkley, J., Loucks, A., Egghart, H., DeLuca, M., 1984. Analytical applications of bioluminescence and chemiluminescence. Proc. Int. Symp. 3rd. Birmingham, UK, p. 133. Walsh, M.E., Jenkins, T.F., Schnitker, P.S., Elwell, J.W., Stutz, M.H., 1993. Evaluation of SW-846 method 8330 for characterization of sites contaminated with residues of high explosives. US Army Cold Regions Research and Engineering Laboratory (Special Report 93). Weisch, W., Klein, C., von Schickfus, M., Hunklinger, S., 1996. Development of a surface acoustic wave immunosensor. Anal. Chem. 68, 2000. Wemhoff, G.A., Rabbany, S.Y., Kusterbeck, A.W., Ogert, R.A., Bredehorst, R., Ligler, F.S., 1992. J. Immunol. Meth. 156, 223. Whelan, J.P., Kusterbeck, A.W., Wemhoff, G.A., Bredehorst, R., Ligler, F.S., 1993. Continuous flow immunosensor for detection of explosives. Anal. Chem. 65, 3561. Yinon, J., Zitrin, S., 1993. Modern Methods and Applications in Analysis of Explosives. Wiley, New York.