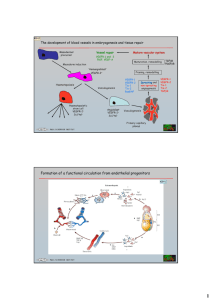
VASCULAR ENDOTHELIAL GROWTH FACTOR-B IN PHYSIOLOGY AND DISEASE
advertisement

Physiol Rev 94: 779 –794, 2014 doi:10.1152/physrev.00028.2013 VASCULAR ENDOTHELIAL GROWTH FACTOR-B IN PHYSIOLOGY AND DISEASE Maija Bry, Riikka Kivelä, Veli-Matti Leppänen, and Kari Alitalo Wihuri Research Institute and Translational Cancer Biology Program, University of Helsinki, Helsinki, Finland Bry M, Kivelä R, Leppänen V-M, Alitalo K. Vascular Endothelial Growth Factor-B in Physiology and Disease. Physiol Rev 94: 779–794, 2014; doi:10.1152/physrev.00028.2013.— Vascular endothelial growth factor-B (VEGF-B), discovered over 15 years ago, has long been seen as one of the more ambiguous members of the VEGF family. VEGF-B is produced as two isoforms: one that binds strongly to heparan sulfate in the pericellular matrix and a soluble form that can acquire binding via proteolytic processing. Both forms of VEGF-B bind to VEGF-receptor 1 (VEGFR-1) and the neuropilin-1 (NRP-1) coreceptor, which are expressed mainly in blood vascular endothelial cells. VEGF-B-deficient mice and rats are viable without any overt phenotype, and the ability of VEGF-B to induce angiogenesis in most tissues is weak. This has been a puzzle, as the related placenta growth factor (PlGF) binds to the same receptors and induces angiogenesis and arteriogenesis in a variety of tissues. However, it seems that VEGF-B is a vascular growth factor that is more tissue specific and can have trophic and metabolic effects, and its binding to VEGFR-1 shows subtle but important differences compared with that of PlGF. VEGF-B has the potential to induce coronary vessel growth and cardiac hypertrophy, which can protect the heart from ischemic damage as well as heart failure. In addition, VEGF-B is abundantly expressed in tissues with highly active energy metabolism, where it could support significant metabolic functions. VEGF-B also has a role in neuroprotection, but unlike other members of the VEGF family, it does not have a clear role in tumor progression. Here we review what is hitherto known about the functions of this growth factor in physiology and disease. L I. II. III. IV. INTRODUCTION THE VASCULAR ENDOTHELIAL... VEGF-B SUMMARY 779 779 781 789 I. INTRODUCTION The blood circulatory system is the first organ system to develop, starting in the third week of development in the human embryo when passive diffusion of nutrients and waste products becomes insufficient for development (118). Members of the vascular endothelial growth factor (VEGF) family are major regulators of blood and lymphatic vessel development. Vasculogenesis, referring to the initial formation of the primitive vascular plexus, begins when hemangioblast progenitors of mesodermal origin migrate and differentiate to form primary blood islands, from which both hematopoietic and endothelial cells are formed (114). The primitive vascular plexus is subsequently remodeled into a network consisting of arteries, veins, and capillaries of different sizes, and the vessels are stabilized by recruited mural cells, such as pericytes and smooth muscle cells. Angiogenesis, the process of blood vessel formation from preexisting vessels, occurs through either sprouting or intussusception (splitting) in development and in various disease processes (reviewed in Ref. 30; see also Ref. 113). VEGF, the archetypal angiogenic growth factor, was first identified as a permeability-inducing factor secreted by tumor cells (121) and later as a growth factor for vascular endothelial cells (ECs) (46, 86). Other members of the VEGF family were subsequently characterized, but VEGF-B has remained one of the more ambiguous growth factors, as efforts to discover angiogenic effects for it in vivo have given mainly negative results. The aim of this review is to summarize what is currently known about VEGF-B to endeavor to elucidate the main functions for this growth factor in physiology and disease. We first briefly review what is known about the other members of the VEGF family and their receptors before concentrating on VEGF-B. II. THE VASCULAR ENDOTHELIAL GROWTH FACTOR FAMILY The VEGF family consists of five secreted dimeric glycoprotein growth factors in mammals: VEGF (or VEGF-A), VEGF-B, VEGF-C, VEGF-D, and placenta growth factor (PlGF) (for reviews, see Refs. 64, 125). As members of the platelet-derived growth factor (PDGF)/VEGF superfamily, VEGFs contain a VEGF/PDGF homology domain with eight conserved cysteine residues involved in inter- and intramolecular disulfide bond formation. The VEGF ligands bind with differing specificities to three mainly endothelial transmembrane tyrosine kinase receptors, VEGFR-1/fms-like 0031-9333/14 Copyright © 2014 the American Physiological Society 779 BRY ET AL. tyrosine kinase 1 (Flt1), VEGFR-2/human kinase insert domain receptor (KDR)/mouse fetal liver kinase 1 (Flk1), and VEGFR-3/fms-like tyrosine kinase 4 (Flt4) (for recent reviews, see Refs. 77, 123) (FIGURE 1A). Neuropilins (NRP)-1 and NRP-2, which transduce semaphorin signals mainly in neuronal cells, also provide coreceptor functions for the various VEGFRs in endothelial cells (reviewed in Ref. 106). which has hampered its application to therapeutic angiogenesis. VEGF and VEGFR-1 are upregulated in hypoxia via hypoxia inducible factor (HIF)-1␣-mediated transcription (50, 110, 119). Nutrient and oxygen deprivation also induce the potent metabolic regulator PGC-1␣ (peroxisome proliferator-activated receptor-␥ coactivator-1␣), which can induce VEGF (and VEGF-B) independently of HIF-1␣ in skeletal muscle (8, 18), highlighting the close coordination of blood supply and regulation of tissue metabolism. VEGF is essential for vasculature development, as mice lacking even a single VEGF allele die during embryogenesis as a result of impaired angiogenesis and blood island formation (23, 45). VEGF binds to VEGFR-1 and VEGFR-2 (35, 111), as well as to NRP-1 and NRP-2 (55, 130). PlGF was isolated from a human placental cDNA library shortly after the discovery of VEGF (for a review, see Ref. 38). PlGF exists as four isoforms in humans, but only one (PlGF-2) in mice. PlGF is expressed abundantly in the placenta, and at lower levels in for example the heart, lungs, and skeletal muscle (for reviews, see Refs. 34, 38). PlGF is very similar to VEGF-B in many respects, but their effects on angiogenesis and arteriogenesis seem to differ considerably. PlGF binds to heparan sulfate and to the same receptors as VEGF-B, namely, VEGFR-1 and NRP-1 (95, 104). PlGF gene-targeted mice are viable, but their angiogenesis and arteriogenesis are impaired in ischemia, inflammation, and wound healing, as well as in the hypoxic brain (24, 51). PlGF deficiency was shown to impair adipose tissue growth at least in part by inhibiting angiogenesis (90). Unlike In addition to functioning as a mitogen for ECs, VEGF also regulates endothelial cell survival (6, 15, 16). Downstream signaling by VEGFR-2, following VEGF binding, receptor dimerization, and autophosphorylation of several tyrosine kinase residues, involves numerous pathways, including the phospholipase C (PLC)-␥/protein kinase C (PKC) pathway, resulting in the activation of the c-Raf-MEK-MAP kinase cascade and cell proliferation (58, 135, 144). Activation of the PI3K-Akt signal transduction pathway stimulates cell survival and migration and eNOS activation (54). Administration of VEGF results in robust angiogenesis in various tissues (reviewed in Ref. 44), but VEGF is also a potent inducer of vascular permeability and inflammation (98), A PlGF VEGF-B VEGF-C VEGF-D VEGF CCBE1+Protease S S S S sVEGFR-1 NRP-1 NRP-1 VEGFR-1 NRP-2 VEGFR-2 VEGFR-3 B 1 2 3 4 5 6A 6B Vegfb 7 exon FIGURE 1. The VEGF family, receptor interactions, and structure of the VEGF-B gene. A: specific binding of VEGFs to their endothelial receptors. The dashed line indicates proteolytic processing that is required before VEGF-C and human VEGF-D can bind to VEGFR-2. Proteolysis activates VEGF-C and VEGF-D. Recent data show that the collagen- and calcium-binding EGF domains 1 (CCBE1) protein promotes proVEGF-C binding to VEGFR-3 followed by proteolytic cleavage by a disintegrin and metalloprotease with thrombospondin motifs-3 (ADAMTS3), resulting in the mature form of VEGF-C and increased VEGFR-3 signaling (69a). B: schematic structure of the Vegfb gene and its alternatively spliced transcripts. Shown are exons (numbered) and introns with the alternative splice acceptor (SA) sites that produce the VEGF-B167 and VEGF-B186 isoforms, where exon 6A is lacking from VEGF-B167 mRNA. The arrowhead indicates the site of proteolytic processing of VEGF-B186. Red, sequence encoding the VEGF homology domain. 167 SA: 186 780 Physiol Rev • VOL 94 • JULY 2014 • www.prv.org VEGF-B IN PHYSIOLOGY AND DISEASE VEGF-B, PlGF is able to stimulate angiogenesis and collateral vessel growth in the ischemic heart and limb with similar efficiency as VEGF (92). PlGF also increases vessel permeability (20, 99) and inflammation (103, 120). However, in a recent report, intracranial PlGF administration using an adeno-associated virus (AAV) vector strongly stimulated angiogenesis and arteriogenesis in the brain without significant inflammation or edema (52). PlGF has been reported to stimulate the migration and survival of endothelial cells (4, 153) and the proliferation of smooth muscle cells (13). It has been reported in numerous studies that PlGF can make heterodimers with VEGF, and some results have suggested that it regulates intermolecular crosstalk between VEGFR-1 and VEGFR-2, in part via VEGF/PlGF heterodimer formation (21, 104). However, PlGF also seems to be capable of inducing unique signals downstream of VEGFR-1 compared with VEGF and stronger VEGFR-1 tyrosine phosphorylation than VEGF-B (7, 9, 82). PlGF may mediate most of its arteriogenic effects via recruitment of growth factor-secreting monocytes that express VEGFR-1 (108). Much remains to be learned about the mechanisms of PlGFinduced vascularization. Interestingly, in the mouse heart, PlGF was recently shown to induce myocardial angiogenesis and cardiac hypertrophy through a nitric oxide (NO)dependent mechanism via the Akt/mTORC1 pathway (69). However, in another recent study, PlGF only secondarily supported pressure overload-induced cardiac hypertrophy through a paracrine mechanism via endothelial cells and fibroblasts to induce capillary growth and fibroblast proliferation (3). A. VEGFR-1 VEGFR-1 is composed of an extracellular ligand binding part, a transmembrane domain, intracellular tyrosine kinase (TK) domain, and carboxy-terminal region (126). The extracellular part consists of seven immunoglobulin-like (Ig) domains. VEGF and PlGF bind to the second and third Ig domains of VEGFR-1 and induce productive receptor dimers for autophosphorylation in the TK domain (35, 136). Mice lacking VEGFR-1 produce an excess of ECs and a disorganized vasculature and die in utero at E8.5–9 (48, 49). This suggests that at least during the development of the vascular system, VEGFR-1 is mainly a negative regulator of angiogenesis because of its high affinity for VEGF, as it can sequester VEGF away from actively signaling VEGFR-2 homodimers. Similarly, VEGFR-1 deletion in adult mice resulted in endothelial cell proliferation and increased vessel density in the lungs, heart, retina, brain, kidneys, and liver and protected against myocardial infarction (MI), at least in part via upregulation of VEGFR-2 signaling (63). This is consistent with the fact that mice engineered to express only a truncated form of VEGFR-1 lacking the TK domain responsible for signaling are viable and display no obvious vascular defects (62). The tyrosine kinase activity of VEGFR-1 in cultured endothelial cells is weak, and its downstream signaling is poorly understood (reviewed in Ref. 124). On the other hand, in addition to ECs, VEGFR-1 is expressed at least in monocytes/macrophages, and activation of its TK domain is required for their activation and migration in response to ligand (11, 31). VEGFR-1 is also expressed in neuronal cells, with implications in glial cell development and survival (28, 79, 143). A soluble form of the VEGFR-1 extracellular domain (sVEGFR-1), which is able to neutralize VEGF (74), plays a role in regulating local guidance of emerging vessel sprouts (26), and it has been shown to be involved in the pathogenesis of preeclampsia (87, 105a, 112). Interestingly, VEGFR-1 is expressed also in the endothelium of coronary arteries in adult mouse and fetal human heart, whereas VEGFR-2 is not, suggesting that VEGFR-1 could play a role in coronary vessel development (70, 75, 105). VEGFR-1 mRNA was also detected in cultured neonatal rat cardiomyocytes; however, the expression level was three orders of magnitude lower than in cultured endothelial cells (149). VEGFR-1 can heterodimerize with VEGFR-2 and thus modify signaling through VEGFR-2 homodimers (32). It is currently not known if the expression pattern of VEGFR-1 is changed in pathological conditions in, e.g., the heart or adipose tissue. B. Neuropilins The neuropilins, NRP-1 and NRP-2, were originally identified as receptors for semaphorins that mediate repulsive signals during neuronal axon guidance (27, 78). NRP-1 and NRP-2 lack cytoplasmic enzyme activity, but they function as coreceptors, complexing with other transmembrane receptors such as the endothelial VEGFRs (reviewed in Refs. 106, 148). VEGF isoforms bind with differing specificities to NRP-1 and NRP-2. NRP-1, which binds also VEGF-B and PIGF (93, 95), is essential for the formation of the vasculature, as mice lacking NRP-1 develop vascular defects and die at E13.5 (73). In the heart, NRP-1 is expressed along with VEGFR-1 in coronary vessels, myocardial capillaries, and epicardial vessels (72, 105). Interestingly, NRP-1 is also expressed in the developing myocardium and endocardium in mouse embryos at E12.5 (93). III. VEGF-B A. Structure and Processing VEGF-B (previously also known as VRF, VEGF-related factor), first discovered in 1996, has a structure very similar to that of VEGF, and mouse VEGF-B shares ⬃43% amino acid sequence identity with mouse VEGF164 (56, 101). The Vegfb gene is highly conserved in mammals with ⬃88% homology between the mouse and human growth factors at the amino acid sequence level (101). A primitive form of Vegfb with only 26% homology to human Vegfb may be Physiol Rev • VOL 94 • JULY 2014 • www.prv.org 781 BRY ET AL. present in frogs, but Vegfb has not been identified in zebrafish (117). Vegfb consists of seven exons and generates two isoforms because of the existence of alternative splice acceptor sites in exon 6 (56, 102) (FIGURE 1B). VEGF-B167 has a highly basic heparin-binding carboxy terminus, whereas VEGF-B186 contains a hydrophobic carboxy terminus and is modified by O-glycosylation and proteolytic processing (102). VEGF-B167 thus binds tightly to heparan sulfate proteoglycans on the cell surface and in the extracellular matrix, whereas at least the uncleaved VEGF-B186 is freely diffusible. The molecular masses of homodimers of VEGF-B167 and VEGF-B186 are 42 and 60 kDa, respectively. Both isoforms are simultaneously expressed in various tissues and bind to VEGFR-1 (and sVEGFR-1) as well as to NRP-1, but not to the major mitogenic endothelial receptors VEGFR-2 and VEGFR-3 (93, 100). Proteolytic processing of VEGF-B186 is required for its binding to NRP-1 (93). In culture, VEGF-B is also able to form heterodimers with VEGF (101), but covalent heterodimers of this type have not been demonstrated in vivo. Unlike VEGF, VEGF-B expression does not seem to be directly regulated by hypoxia (41), although hypoxia appeared to induce VEGF-B in the mouse retina in a recent report (128). VEGF-B has a wide tissue distribution in mice, being most abundant in tissues with high metabolic activity such as the myocardium, skeletal muscle, and vascular smooth muscle, as well as in brown adipose tissue, brain, kidney, and parietal cells of the stomach (1, 22, 80, 101). This suggests a role for VEGF-B in coordinating the crosstalk between angiogenesis and metabolism. Analysis of VEGF-B mRNA in normal C57Bl/6J mouse tissues by quantitative RT-PCR confirmed that VEGF-B expression levels were the highest in the heart and skeletal muscle (FIGURE 2A) (see also Ref. 59). Slow-twitch aerobic muscles such as soleus and the red part of the gastrocnemius had higher levels of VEGF-B than fast-twitch glycolytic muscles, such as tibialis anterior and the white part of the gastrocnemius, further supporting the idea that VEGF-B is involved in aerobic energy metabolism. The levels of VEGF-B in brown adipose tissue were between those found in slow and fast muscles, while in visceral white adipose tissue the levels were lower, although still higher than in most other tissues. Staining of VEGF-B gene-targeted VegfblacZ/lacZ mouse hearts (14) for -galactosidase shows strong VEGF-B expression in the cardiomyocytes (FIGURE 2B). Analysis of VEGF-B mRNA expression in healthy human tissues using the MediSapiens database consisting of hundreds of microarrays of various human tissues (www.medisapiens.com) revealed the highest expression levels in the heart, adipose tissue, skeletal muscle, and blood vessels, which also expressed high levels of VEGFR-1 and NRP-1 (FIGURE 3, A AND B). The adipose tissue samples from humans mainly represent biopsies from sub- 782 cutaneous depots, which may have different expression levels than visceral fat. Interestingly, VEGF-B and PlGF had very different expression patterns (FIGURE 3C). VEGF, which is required during embryonic development, showed the broadest expression across different tissues compared with VEGF-B and PlGF. B. Differences Between VEGF-B and PlGF Although VEGF-B and PlGF transduce their signals via VEGFR-1 and NRP-1, their effects on blood vessel growth, permeability, and tissue inflammation differ considerably (20). However, both growth factors are dispensable for normal embryonic development. Like many other receptor tyrosine kinases, the VEGFRs are activated by ligand-induced dimerization, followed by tyrosine autophosphorylation of the intracellular kinase domain to generate downstream signaling (83). For ligand binding, the VEGFRs share a conserved interface in Ig domain (D) 2, while D3 provides additional interactions (29, 67, 84, 85, 142). The membrane-proximal domains 4 –7 (D4 –7) facilitate dimerization through homotypic contacts formed between D4 –5 and D7 (FIGURE 4A) (85, 116, 145). VEGFRs show a hingelike rigid body motion in D2–3 (19, 84), suggesting that ligand-induced D2–3 reorientation initiates the cascade of structural rearrangements behind the homotypic interactions and receptor activation. Crystal structure analysis shows that VEGF-B, VEGF, and PlGF interact with VEGFR-1 D2 in a similar manner (29, 67, 68, 142) (FIGURE 4B). VEGF and PlGF also require D3 of VEGFR-1 for high-affinity binding (29, 33). However, D3 does not provide additional affinity for VEGF-B binding to VEGFR-1 (7). The receptor specificity of VEGF ligands is determined by an amino-terminal alpha helix and three peptide loops. VEGF-B fails to efficiently induce productive VEGFR-1 dimerization and signaling as a result of the unique structure of VEGF-B loop 1 (L1; FIGURE 4, C AND D). Importantly, swapping L1 from PlGF to VEGF-B conferred the angiogenic properties of PlGF to the resulting chimera and vice versa. Since L1 in VEGFs interacts only with D3 (19, 84), the poor ability of VEGF-B to activate VEGFR-1 compared with PlGF can be explained by inadequate L1-D3 interactions. These results also suggest that, at least in endothelial cells, the effects of VEGF-B occur through inhibition of the interaction of other ligands with VEGFR-1 (see also FIGURE 6), thus inducing more efficient and spatially controlled interaction of VEGF with the highly mitogenic VEGFR-2. How VEGF-B and PlGF signaling interactions are regulated in macrophages, which also express VEGFR-1 and NRP-1 (25, 31), remains to be studied. C. Role in Angiogenesis Although initial reports indicated that VEGF-B is able to stimulate EC growth in vitro (101), this may have been due Physiol Rev • VOL 94 • JULY 2014 • www.prv.org VEGF-B IN PHYSIOLOGY AND DISEASE A 1.2 mRNA expression of VEGF-B relative to the heart (=1) 1 0.8 0.6 0.4 nc re as ey Pa dn Ki le en r Sp ve Li ng Lu n ai Br h ac om St W AT T BA A hi te G TA W ed G A us R le So H ea rt 0.2 Skeletal muscle β-gal B VegfblacZ/lacZ WT FIGURE 2. VEGF-B expression levels are the highest in the heart, skeletal muscle, and brown adipose tissue in mice. A: quantitative RT-PCR of VEGF-B mRNA expression in 3-mo-old mouse tissues relative to the heart (set to 1). Note that expression levels are higher in “red” muscles than in “white” muscles. Red and white GA indicate red and white parts of gastrocnemius muscle. TA, tibialis anterior muscle; BAT, brown adipose tissue; WAT, white adipose tissue. B: -galactosidase (-gal) staining of gene-targeted VegfblacZ/lacZ mouse hearts showing VEGF-B expression in the cardiomyocytes. WT, wild-type control heart. Scale bar: 50 m. to heterodimer formation with endogenous VEGF, as the ability of recombinant VEGF-B to stimulate sprouting angiogenesis directly is poor in most tissues. VEGF-B did not stimulate vessel growth when delivered into muscle or periadventitial tissue via adenoviral vectors (17, 115), and transgenic overexpression of VEGF-B in the skin increased blood vessel density only minimally, although it increased capillary diameter to some extent (72). VEGF-B did not improve vascular growth in the ischemic limb in two studies (81, 88), although opposite results have also been published (127, 140). On the other hand, VEGF-B overexpressed in endothelial cells of transgenic mice was claimed to potenti- ate, rather than initiate, angiogenesis (97). Overexpression of VEGF-B was also shown to aggravate pathological retinal and choroidal neovascularization in mice (152) and was proposed to act as a survival factor for ECs, regulating the expression of vascular pro-survival genes via both NRP-1 and VEGFR-1 (150). D. Effects of VEGF-B in the Heart Mice deficient of VEGF-B are viable and fertile and display only mild phenotypes in the heart. This is manifested as an atrioventricular conduction abnormality characterized by a Physiol Rev • VOL 94 • JULY 2014 • www.prv.org 783 BRY ET AL. A 2000 1500 1000 B VEGF-B VEGFR-1 NRP-1 C PlGF VEGF-B Mesenchymal stem cell Placenta Kidney Pancreas Liver Stomach Adrenal gland Pituitary gland PNS ganglion Cerebellum Cerebrum Lung Skin Connective tissue Adipose tissue Bone Skeletal muscle Heart Blood vessel Spleen Lymph node Hematopoietic stem cell Reticulocyte Blood dendritic cell Blood monocyte 500 VEGF-A Connective tissue Placenta Liver Spleen Kidney Pancreas Adrenal gland Blood vessel Mesenchymal stem cell Adipose tissue Heart Skeletal muscle Reticulocyte Pituitary gland Placenta Adipose tissue Heart Mesenchymal stem cell Kidney Lymph node Spleen Adrenal gland Blood vessel Skeletal muscle Blood dendritic cell Pancreas Connective tissue Reticulocyte Pituitary gland FIGURE 3. Comparison of the RNA expression levels of VEGF-B and its receptors, PlGF and VEGF, in healthy human tissues. A: box plot analysis of VEGF-B mRNA expression levels (arbitrary units) over a range of healthy human tissues extracted from the MediSapiens database (http://www.medisapiens.com). Note the highest VEGF-B expression levels in adipose tissue, heart, skeletal muscle, blood vessels, and reticulocytes. The y-axis defines the expression level, while the x-axis shows the tissue type. B: comparison of the expression of VEGF-B with its receptors, showing that among the tissues with high VEGF-B expression, adipose tissue, heart, skeletal muscle, and blood vessels also highly express VEGFR-1 and NRP-1. C: comparison of the expression patterns of VEGF-B and its closest relatives PlGF and VEGF. Note that the expression patterns of VEGF-B and PlGF, which bind to the same receptors, are very different, whereas the metabolically active tissues (adipose tissue, heart, and skeletal muscle) express abundant VEGF-B and VEGF. Darkest shades of blue indicate lowest relative expression, and darkest shades of red indicate the highest relative expression. prolonged PQ interval in one strain (2), or as a smaller heart size with slightly dysfunctional coronary vasculature and impaired recovery after myocardial ischemia in another (14). The latter mouse strain also showed some resistance to development of pulmonary hypertension and vascular remodeling during chronic hypoxia (141). VEGF-B-deficient rats had no obvious vascular or developmental defects (75). After experimental MI, larger infarct scars were detected in cardiac sections from the VEGF-B gene-targeted rats, although heart function was not significantly affected (75). Collectively, the results from VEGF-B gene-deleted mice and rats suggest a role for VEGF-B in the heart in pathological settings. 784 Interestingly, VEGF-B expression is spatially and temporally correlated with the commencement and progression of coronary endothelial growth in the heart, suggesting that it could play a role in coronary vessel development (14). However, no obvious defects have been reported in mouse embryos lacking VEGF-B. Importantly, however, antibodies against VEGF-B were found to inhibit coronary artery development in the quail embryo (138, 139). An excess of VEGF-B activates the Akt/mTORC1 and Erk1/2 MAPK pathways in the heart (FIGURE 6) (75). Activation of Erk1/2 occurred at least in part through VEGFR-2 phosphorylation, an indirect result of VEGF-B binding to Physiol Rev • VOL 94 • JULY 2014 • www.prv.org VEGF-B IN PHYSIOLOGY AND DISEASE A B D2’ D1’ PIGF/VEGF-B D2 D1 PIGF D2 D2’ D3 D3’ VEGFR-1 C αN αN D5’ L1 D5 L2 D αN D7’ D7 VEGFR ECD L3 L1 L2 Variable Conserved FIGURE 4. Comparison of PlGF and VEGF-B structures. A: a model of the ligand-induced VEGFR extracellular domain dimerization with the crystal structure of the PlGF/VEGFR-1 D2 complex (PDB code 1RV6; colored in cyan) superimposed with a model of the VEGF-C/VEGFR-3 extracellular domain (ECD) complex (85). Ligandinduced homotypic interactions occur in the VEGFR membrane-proximal Ig-like domains D5 and D7 (85, 145). B: PlGF and VEGF-B show similar modes of binding to VEGFR-1 D2 (29, 67). A top view is shown of the superpositioning of the VEGF-B/VEGFR-1 D2 (PDB code 2XAC) and the PlGF/VEGFR-1 D2 complex structures with VEGF-B and PlGF shown in ribbon diagram and colored in orange and cyan, respectively, and the VEGFR-1 D2 from the PlGF complex in cartoon and colored in gray. VEGFR-1 D2 from the VEGF-B complex is not shown. C: the same as in B from a side view with the other PlGF monomer shown as a surface model. The receptor-binding epitopes: loops 1 to 3 (L1 to L3) and the amino-terminal alpha-helix (␣N) are labeled. PlGF and VEGF-B show a major difference in L1 and L3 conformations. D: a color-coded conservation pattern between PlGF and VEGF-B in the VEGF homology region. A representative set of mammalian PlGF and VEGF-B amino acid sequences were aligned using ClustalW, and the rate of conservation was calculated and plotted on PlGF in C using Consurf server. Cyan through dark red is used for labeling from variable to conserved amino acids. As shown by Anisimov et al. (7), PlGF and VEGF-B show significant differences in L1 and L3. VEGFR-1, which increased the availability of VEGF for activation of VEGFR-2 (75). The Erk1/2 activation via VEGFR-2 is likely responsible for the coronary vessel growth and arterialization observed in the VEGF-B overexpressing hearts. Importantly, blocking VEGFR-2 signaling was able to reduce the vessel enlargement seen in VEGF-B overexpressing mice. On the other hand, in contrast to the hypertrophy induced by PlGF (69), blocking NO signaling did not affect the VEGF-B-induced cardiac hypertrophy (75). Importantly, the VEGF-B-induced hypertrophy remained physiological even in old rats, and did not advance into heart failure (75). E. VEGF-B as a Therapeutic Factor in Cardiac Disease Several studies have indicated a role for VEGF-B in coronary vasculature and in cardioprotection. VEGF-B levels decreased following experimentally induced MI in rats as well as in heart failure resulting from transverse aortic constriction (65, 151). Myocardial expression levels of VEGF-B were decreased also in human heart disease (75). Low VEGF-B plasma levels were found to accurately predict left ventricular dysfunction and remodeling after MI, Physiol Rev • VOL 94 • JULY 2014 • www.prv.org 785 BRY ET AL. suggesting that VEGF-B could be used as a prognostic biomarker with stronger predictive value than troponin T (36, 37). Interestingly, the opposite was true for PlGF, as increased levels of PlGF predicted heart failure (36). However, to date, studies about the kinetics of VEGF-B after ischemic damage in the myocardium are lacking. VEGF-B does not seem to induce capillary angiogenesis in most studies, but mainly enlargement of myocardial capillaries and growth of coronary arteries (20, 72). In addition, VEGF-B counteracts apoptosis of cardiomyocytes and improves cardiac contractility in animal models of human heart disease (149). However, the responsible mechanisms and signaling between endothelial cells and cardiomyocytes in these settings are still largely unknown. Therapeutic vectors expressing VEGF-B have shown promise in several experimental models of myocardial ischemia or heart failure (TABLE 1). An overdose of VEGF-B186 via transient adenoviral delivery into the pig myocardium enlarged myocardial vessels after acute infarction, which was inhibited by administration of either soluble VEGFR-1 or soluble NRP-1, but not by blocking VEGFR-2 signaling or nitric oxide production (81). Adenoviral delivery of VEGF-B167 activated the PI3K/Akt pathway, enlarged capillaries and ameliorated angiotensin II-induced diastolic dysfunction in rats (122). Adenoviral delivery of VEGF-B186 was equally capable of enlarging myocardial capillaries in mice (66). However, AAV-mediated administration of VEGF-B167 preserved cardiac contractility and prevented cardiomyocyte apoptosis after experimental MI in rats without significant vascular effects (149). Similar results were achieved in dogs subjected to tachypacing-induced development of dilated cardiomyopathy, where AAV-VEGF-B administration delayed the progression towards heart failure (107). In these studies, it is important to note that adenoviral vectors me- diate very high but transient levels of expression and induce a strong inflammatory response, whereas AAVs mediate steady, long-term expression without significant inflammation. Transgenic overexpression of VEGF-B induced cardiac hypertrophy as well as capillary enlargement in both mice and rats (20, 72, 88). In rats, VEGF-B also caused a striking growth of the epicardial and subendocardial coronary vessels, which importantly occurred without increasing inflammation or vascular permeability, in contrast to other members of the VEGF family (FIGURE 5) (20). The coronary arterial growth induced by VEGF-B led to increased functional coronary reserve, protecting the heart from ischemic damage caused by coronary artery ligation (75). Systemic delivery of an AAV-VEGF-B vector to adult rats reproduced both the vascular and hypertrophic phenotypes, an important consideration for possible therapeutic strategies. However, the effects of VEGF-B overexpression required 2–3 wk before becoming apparent, so it is unlikely that VEGF-B induced vascular changes would be beneficial during acute myocardial infarction. However, VEGF-B could improve long-term recovery from ischemia and counteract the development of heart failure. Indeed, a VEGF-B186 adenovirus was shown to improve systolic function in progressive left ventricular hypertrophy caused by transverse aortic constriction in mice (65). Thus the above studies and others have implicated the heart as a specific target for VEGF-B. F. Metabolic Effects of VEGF-B Interestingly, expression of VEGF-B and a nuclear-encoded mitochondrial gene and protein cluster set appear to be Table 1. Experimental models of VEGF-B therapy in the heart Vector/Transgene/Isoform Species Effect Transgene (␣MHC-VEGF-B167) Mouse Transgene (␣MHC-genomic VEGF-B) Rat Adenovirus (VEGF-B186) Adenovirus (VEGF-B186) Adenovirus (VEGF-B167) Pig Mouse Rat Cardiomyocyte hypertrophy; myocardial capillary enlargment; ischemia protection ex vivo (72) Cardiomyocyte hypertrophy; myocardial capillary enlargement; expansion of coronary arteries; no increased inflammation or vascular leakage (20); increased functional coronary reserve; ischemia protection in vivo (75) Enlargement of myocardial vessels after myocardial infarction (81) Capillary enlargement (66) Capillary enlargement and improved cardiac function in angiotensin II-induced diastolic dysfunction (122) Increased density of capillaries and arterioles in infarct zone (88) Increased capillary density and cardiomyocyte area in remote myocardium after ischemia (137) Antiapoptotic effect on cardiomyocytes after myocardial infarction; cardiomyocyte hypertrophy; no significant vascular effects (149) Delayed progression of heart failure during tachypacing-induced cardiomyopathy; antiapoptotic effect on cardiomyocytes (107) Improves systolic function following transverse aortic constriction; antiapoptotic effect on cardiomyocytes; capillary enlargement (65) Adenovirus or protein infusion (VEGF-B167) Mouse Adenovirus (human VEGF-B167) Mouse AAV2-VEGF-B167 Rat AAV9-VEGF-B167 Dog AAV9-VEGF-B186 Mouse 786 Physiol Rev • VOL 94 • JULY 2014 • www.prv.org VEGF-B IN PHYSIOLOGY AND DISEASE A WT LV B WT coordinately regulated, which is not the case for the other VEGF family members (59, 96). VEGF-B expression in skeletal muscle is upregulated by the transcription factor PGC-1␣ (18, 134), as has been previously shown for VEGF (8). In addition, VEGF-B expression was induced in skeletal muscle by voluntary exercise in both mice and humans (18, 76). As PGC-1␣ is the major regulator of mitochondrial biogenesis and oxidative metabolism, these findings underline the close interplay between oxygen delivery by the vasculature and target tissue metabolism. VEGF-B deletion was recently reported to lead to decreased expression of fatty acid transport proteins (FATPs) FATP-3 and FATP-4 in endothelial cells. This correlated with a decreased amount of lipid droplets in cardiomyocytes and skeletal muscle fibers, leading to increased white adipose tissue mass and body weight in old mice (59). Further analysis demonstrated that VEGF-Bdeficient mice fed a high-fat diet or crossed with diabetic db/db mice have improved insulin sensitivity, glucose homeostasis, and blood lipid profiles (60). This was accompanied by reduced lipid storage in muscle, heart, and TG LV TG FIGURE 5. Cardiac arteriogenesis in VEGF-B transgenic rats. A myocardium-specific VEGF-B transgene induced impressive growth of coronary arteries and their branches in rats, without increasing inflammation or vascular leakage, as shown by Bry et al. (20) and Kivelä et al. (75). A: shown are representative immunofluorescence images of capillaries (green) and smooth muscle cells (yellow) in the myocardium of a VEGF-B transgenic (TG) and control (WT) rat. LV indicates the left ventricle. Scale bar: 200 m. B: 3-dimensional micro-computed tomography (CT) images of the coronary artery tree in WT and VEGF-B TG rats. pancreas, but not in the liver, and increased weight gain on high-fat diet. VEGFR-1 and its tyrosine kinase activity as well as NRP-1 were needed for the VEGF-B-mediated effects on fatty acid transport across the endothelium (59). These results suggested that endothelial growth factors play a role in tissue lipid homeostasis and could provide a promising novel target for the treatment of metabolic and cardiovascular diseases. The functional importance of angiogenesis and vascular endothelial regulation in adipose tissues has gained increased attention recently. Interestingly, both repression (91) and overexpression (40, 131) of VEGF were reported to protect against high-fat diet-induced obesity and/or insulin resistance. The expression of VEGF-B and FATPs 1– 4 were increased in white adipose tissue of VEGF-deficient mice, via an unknown mechanism (91). These mice demonstrated lower body weight and resistance to high-fat dietinduced obesity, together with upregulation of brown adipose tissue-specific genes in white adipose tissue. In another study, the expression of VEGF-B and FATP-3 in brown adipose tissue was reduced after cold exposure, but mech- Physiol Rev • VOL 94 • JULY 2014 • www.prv.org 787 BRY ET AL. anistic experiments were not conducted (12). VEGF-B was also shown to affect FATP-1 and FATP-4 levels in a neuroblastoma cell line (147). In rats, however, we found no difference in fatty acid uptake between VEGF-B transgenic, gene-deleted, or wild-type hearts, although VEGF-B administration increased the levels of FATP-4 mRNA (75). In fact, fatty acid and triglyceride levels were actually reduced in the rat hearts overexpressing VEGF-B. On the other hand, VEGF-B overexpression led to an adjustment of cardiomyocyte metabolic pathways in favor of glucose oxidation and macromolecular biosynthesis. These metabolic changes may have contributed to the ischemia protection seen in this model, where cardiomyocyte mitochondria were protected against ischemia-reperfusion injury (75). Thus it is clear that the metabolic effects of VEGF-B are not as simple as has been previously suggested, and much remains to be learned about the specific role of VEGF-B in metabolic regulation. In summary, there are several studies describing the role of vascular growth factors in adipose tissue homeostasis. Some studies have reported changes in VEGF-B expression by therapeutic drugs, such as rosiglitazone, which stimulates peroxisome proliferator-associated receptor-␥ (53), but so far no study has investigated the role of VEGF-B in adipose tissue regulation. Based on the gene expression data from both mice and humans, VEGF-B and its receptors are highly expressed in both white and brown adipose tissue, suggesting that VEGF-B could have a function also in these tissues. An important question is whether VEGF-B acts in a paracrine manner via the endothelium, directly on the adipocytes, or also via VEGFR-1 expressing macrophages, which are important in adipose tissue regulation especially in pathological settings (94). G. VEGF-B-Induced Neuroprotection VEGF-B is also expressed in the central nervous system (1). Interestingly, VEGF-B-deficient mice showed impaired recovery from cerebral ischemic injury (132), and administration of VEGF-B stimulated neurogenesis in adult mice (133). Furthermore, VEGF-B186 protected primary motor neurons against degeneration in culture (109). Accordingly, mice lacking VEGF-B developed a more severe form of motor neuron degeneration when crossed with SOD1 mutant mice, a model of amyotrophic lateral sclerosis. Importantly, administration of VEGF-B186 into the ventricles of the brain prolonged the survival of mutant SOD1 rats, and the in vitro and in vivo effects of VEGF-B were dependent on the tyrosine kinase activity of its receptor VEGFR-1 in motor neurons. VEGF-B was furthermore upregulated in an in vitro model of Parkinson’s disease induced by the neurotoxin rotenone, and the addition of exogenous VEGF-B167 improved neu- 788 ronal survival (43). In a preclinical in vivo Parkinson’s disease model, exogenous VEGF-B186 showed neuroprotective effects, likely due to the partial protection of dopaminergic fibers in the striatum and partial rescue of the dopaminergic neurons in the caudal subregion of the substantia nigra (42). This neuroprotective effect was further investigated in a follow-up study, where VEGF-B expression was confirmed in the substantia nigra pars compacta of human patients with Parkinson’s disease (147). VEGF-B and VEGFR-1 are expressed in dorsal root ganglion neurons that innervate the hindlimb skin (39). Mice lacking VEGF-B or functional VEGFR-1 developed more retrograde degeneration of sensory neurons in a distal neuropathy model, whereas mice overexpressing VEGF-B186 or VEGFR-1 selectively in neurons were protected against the distal neuropathy. Exogenous VEGF-B186 was shown to be protective by directly affecting sensory neurons and not the surrounding vasculature (39). Inhibition of apoptosis was suggested as a possible mechanism for the VEGF-B-induced neuroprotection (89). VEGF-B treatment also rescued neurons from apoptosis in the retina and brain in mouse models of ocular neurodegenerative disorders and stroke, respectively. The neuroprotective effect of VEGF-B seems to be directly mediated by VEGFR-1 expressed by the neuronal cells and not via improved angiogenesis (39, 109). As a possible protection mechanism, inhibition of apoptosis by VEGF-B has also been proposed in cardiac ischemia (149). However, further studies are needed to clarify this as a potential downstream mechanism of VEGF-B - VEGFR-1 signaling. H. VEGF-B in Cancer It has been proposed that VEGF-B could play a role early in tumor development at the stage of adenoma formation, and VEGF-B expression is upregulated in for example ovarian, colorectal, renal, and prostate cancer (57, 61). However, only one study has addressed the role of VEGF-B in tumor development using a genetic model (5). This study analyzed the effects of VEGF-B overexpression versus deficiency in the context of pancreatic endocrine adenocarcinoma in transgenic RIP1-Tag2 mice. Interestingly, ectopic expression of VEGF-B reduced tumor growth, whereas mice lacking VEGF-B presented with larger tumors. The mechanism of VEGF-B action, however, remained unsolved, but the tumor vasculature and inflammation, the usual suspects, were not affected by the presence or absence of VEGF-B. The role of PlGF in tumor angiogenesis is also controversial (10, 47, 146). PlGF blocking antibodies were reported to inhibit the growth of medulloblastomas and tumors that express VEGFR-1 in tumor cells in addition to endothelial cells (129, 146). However, it is not known whether the expression levels of VEGF-B in the tumors affect the efficacy of anti-PlGF therapies. Physiol Rev • VOL 94 • JULY 2014 • www.prv.org VEGF-B IN PHYSIOLOGY AND DISEASE IV. SUMMARY Because VEGF-B is dispensable during embryonic development and even high VEGF-B expression levels induced by viral delivery do not induce adverse effects, such as inflammation or vascular leakage, VEGF-B seems to possess a wide therapeutic window, which would be important for its possible use in human diseases. The idea that a vascular growth factor could also regulate tissue metabolism may sound unexpected at first; however, it is logical that both oxygen delivery by blood vessels and oxygen usage by mitochondria would be coordinated to be able to match nutrient/oxygen supply and demand. The close interplay between angiogenesis and mitochondrial biogenesis and oxidative metabolism will likely be the focus of numerous studies in the near future, and VEGF-B is one potential candidate molecule in this crosstalk. VEGF-B still remains an enigmatic vascular growth factor, but recent studies have indicated that VEGF-B can regulate blood vessel and cardiomyocyte growth in the heart, tissue metabolism, and cell survival (FIGURE 6). In addition to its effects on endothelial cells, studies in the central nervous system provided evidence that VEGF-B can directly affect neurons expressing VEGFR-1. Overall, VEGF-B seems to be important for tissue protection, whether it be in the context of heart failure or neuron degeneration. The possible role of VEGF-B in coronary artery development is an open question, but since gene-deleted mice and rats are viable and fertile and born in normal Mendelian ratios, this has not been studied in detail. EC VEGF-B VEGFR-1 VEGF NRP-1 PF PF O2 and Nutrients VEGFR-1 VEGFR-2 VESSEL SIZE ARTERIALIZATION pERK pERK , pAkt APOPTOSIS pAMPK mTOR Malonyl-CoA pS6K1 FASN pS6 FATTY ACID SYNTHESIS HYPERTROPHY PROTEIN SYNTHESIS CMC FIGURE 6. A schematic model of the main effects of VEGF-B overexpression in the heart. VEGF-B, expressed by cardiomyocytes, binds to VEGFR-1 expressed in vascular endothelial cells and NRP-1 expressed in both endothelial cells and cardiomyocytes. Elevated levels of VEGF-B can displace VEGF from VEGFR-1, therefore increasing VEGF availability for VEGFR-2, which can induce capillary growth and arteriogenesis via increased Erk1/2 signals (9, 63, 75). Endothelial-cardiomyocyte crosstalk, which employs paracrine factors (PF), matrix molecules, and small-molecular-weight components, is essential for the coordination of physiological heart adaptation to various stresses. The vascular endothelium is also central as a gateway for the transport of nutrients and oxygen to the cardiomyocytes. Shown in the lower part of the figure are some of the signaling pathways and downstream effects of VEGF-B overexpression in the heart, leading to increased fatty acid and protein synthesis, cardiomyocyte hypertrophy, and decreased apoptosis (75, 107, 122, 149). AMPK, AMPactivated protein kinase; FASN, fatty acid synthase; mTOR, mammalian target of rapamycin; S6K, ribosomal protein S6 kinase. Physiol Rev • VOL 94 • JULY 2014 • www.prv.org 789 BRY ET AL. ACKNOWLEDGMENTS pendent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC1alpha. Nature 451: 1008 –1012, 2008. We thank Drs. Seppo Ylä-Herttuala and Ulf Eriksson for collaboration; however, the views presented in this review are the sole responsibility of the authors. We apologize that a number of valuable contributions could not be cited because of space restrictions. M. Bry and R. Kivelä contributed equally to this article. Address for reprint requests and other correspondence: K. Alitalo, Wihuri Research Institute, Biomedicum Helsinki, PO Box 63 (Haartmaninkatu 8), FI-00014 University of Helsinki, Helsinki, Finland (e-mail: kari.alitalo@ helsinki.fi). GRANTS The authors’ studies were supported by Academy of Finland Grants 265982, 272683, 273612, and 273817; the Sigrid Juselius Foundation; European Research Council Grant ERC-2010-AdG-268804, Marie Curie Actions FP7/ 2007-2013REA 317250; Leducq Foundation Grant 11CVD03; and the Finnish Foundation for Cardiovascular Research. 9. Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U, Dewerchin M, Dombrowski S, Stanimirovic D, Van Hummelen P, Dehio C, Hicklin DJ, Persico G, Herbert JM, Communi D, Shibuya M, Collen D, Conway EM, Carmeliet P. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med 9: 936 –943, 2003. 10. Bais C, Wu X, Yao J, Yang S, Crawford Y, McCutcheon K, Tan C, Kolumam G, Vernes JM, Eastham-Anderson J, Haughney P, Kowanetz M, Hagenbeek T, Kasman I, Reslan HB, Ross J, Van Bruggen N, Carano RA, Meng YJ, Hongo JA, Stephan JP, Shibuya M, Ferrara N. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell 141: 166 –177, 2010. 11. Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87: 3336 –3343, 1996. 12. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200 –205, 2011. 13. Bellik L, Vinci MC, Filippi S, Ledda F, Parenti A. Intracellular pathways triggered by the selective FLT-1-agonist placental growth factor in vascular smooth muscle cells exposed to hypoxia. Br J Pharmacol 146: 568 –575, 2005. 14. Bellomo D, Headrick JP, Silins GU, Paterson CA, Thomas PS, Gartside M, Mould A, Cahill MM, Tonks ID, Grimmond SM, Townson S, Wells C, Little M, Cummings MC, Hayward NK, Kay GF. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res 86: E29 –E35, 2000. 15. Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest 103: 159 –165, 1999. DISCLOSURES No conflicts of interest, financial or otherwise, are declared by the authors. 16. Benjamin LE, Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci USA 94: 8761– 8766, 1997. REFERENCES 17. Bhardwaj S, Roy H, Gruchala M, Viita H, Kholova I, Kokina I, Achen MG, Stacker SA, Hedman M, Alitalo K, Yla-Herttuala S. Angiogenic responses of vascular endothelial growth factors in periadventitial tissue. Hum Gene Ther 14: 1451–1462, 2003. 1. Aase K, Lymboussaki A, Kaipainen A, Olofsson B, Alitalo K, Eriksson U. Localization of VEGF-B in the mouse embryo suggests a paracrine role of the growth factor in the developing vasculature. Dev Dyn 215: 12–25, 1999. 2. Aase K, von Euler G, Li X, Ponten A, Thoren P, Cao R, Cao Y, Olofsson B, GebreMedhin S, Pekny M, Alitalo K, Betsholtz C, Eriksson U. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation 104: 358 –364, 2001. 3. Accornero F, van Berlo JH, Benard MJ, Lorenz JN, Carmeliet P, Molkentin JD. Placental growth factor regulates cardiac adaptation and hypertrophy through a paracrine mechanism. Circ Res 109: 272–280, 2011. 4. Adini A, Kornaga T, Firoozbakht F, Benjamin LE. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res 62: 2749 –2752, 2002. 5. Albrecht I, Kopfstein L, Strittmatter K, Schomber T, Falkevall A, Hagberg CE, Lorentz P, Jeltsch M, Alitalo K, Eriksson U, Christofori G, Pietras K. Suppressive effects of vascular endothelial growth factor-B on tumor growth in a mouse model of pancreatic neuroendocrine tumorigenesis. PLoS One 5: e14109, 2010. 6. Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1: 1024 –1028, 1995. 18. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463– 468, 2012. 19. Brozzo MS, Bjelic S, Kisko K, Schleier T, Leppänen VM, Alitalo K, Winkler FK, BallmerHofer K. Thermodynamic and structural description of allosterically regulated VEGFR-2 dimerization. Blood 119: 1781–1788, 2012. 20. Bry M, Kivelä R, Holopainen T, Anisimov A, Tammela T, Soronen J, Silvola J, Saraste A, Jeltsch M, Korpisalo P, Carmeliet P, Lemstrom KB, Shibuya M, Yla-Herttuala S, Alhonen L, Mervaala E, Andersson LC, Knuuti J, Alitalo K. Vascular endothelial growth factor-B acts as a coronary growth factor in transgenic rats without inducing angiogenesis, vascular leak, or inflammation. Circulation 122: 1725–1733, 2010. 21. Cao Y, Linden P, Shima D, Browne F, Folkman J. In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J Clin Invest 98: 2507–2511, 1996. 22. Capoccia BJ, Huh WJ, Mills JC. How form follows functional genomics: gene expression profiling gastric epithelial cells with a particular discourse on the parietal cell. Physiol Genomics 37: 67–78, 2009. 7. Anisimov A, Leppänen VM, Tvorogov D, Zarkada G, Jeltsch M, Holopainen T, Kaijalainen S, Alitalo K. The basis for the distinct biological activities of vascular endothelial growth factor receptor-1 ligands. Sci Signal 6: ra52, 2013. 23. Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Ebenhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435– 439, 1996. 8. Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-inde- 24. Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, 790 Physiol Rev • VOL 94 • JULY 2014 • www.prv.org VEGF-B IN PHYSIOLOGY AND DISEASE Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7: 575–583, 2001. 25. Carrer A, Moimas S, Zacchigna S, Pattarini L, Zentilin L, Ruozi G, Mano M, Sinigaglia M, Maione F, Serini G, Giraudo E, Bussolino F, Giacca M. Neuropilin-1 identifies a subset of bone marrow Gr1- monocytes that can induce tumor vessel normalization and inhibit tumor growth. Cancer Res 72: 6371– 6381, 2012. 26. Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell 17: 377–386, 2009. 27. Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19: 547–559, 1997. 28. Choi JS, Kim HY, Cha JH, Choi JY, Park SI, Jeong CH, Jeun SS, Lee MY. Upregulation of vascular endothelial growth factor receptors Flt-1 and Flk-1 following acute spinal cord contusion in rats. J Histochem Cytochem 55: 821– 830, 2007. 29. Christinger HW, Fuh G, de Vos AM, Wiesmann C. The crystal structure of placental growth factor in complex with domain 2 of vascular endothelial growth factor receptor-1. J Biol Chem 279: 10382–10388, 2004. 30. Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol 27: 563–584, 2011. 31. Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 271: 17629 –17634, 1996. 32. Cudmore MJ, Hewett PW, Ahmad S, Wang KQ, Cai M, Al-Ani B, Fujisawa T, Ma B, Sissaoui S, Ramma W, Miller MR, Newby DE, Gu Y, Barleon B, Weich H, Ahmed A. The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat Commun 3: 972, 2012. 42. Falk T, Yue X, Zhang S, McCourt AD, Yee BJ, Gonzalez RT, Sherman SJ. Vascular endothelial growth factor-B is neuroprotective in an in vivo rat model of Parkinson’s disease. Neurosci Lett 496: 43– 47, 2011. 43. Falk T, Zhang S, Sherman SJ. Vascular endothelial growth factor B (VEGF-B) is upregulated and exogenous VEGF-B is neuroprotective in a culture model of Parkinson’s disease. Mol Neurodegener 4: 49, 2009. 44. Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 29: 789 – 791, 2009. 45. Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380: 439 – 442, 1996. 46. Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 161: 851– 858, 1989. 47. Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131: 463– 475, 2007. 48. Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376: 66 –70, 1995. 49. Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development 126: 3015–3025, 1999. 50. Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604 – 4613, 1996. 51. Freitas-Andrade M, Carmeliet P, Charlebois C, Stanimirovic DB, Moreno MJ. PlGF knockout delays brain vessel growth and maturation upon systemic hypoxic challenge. J Cereb Blood Flow Metab 32: 663– 675, 2012. 33. Davis-Smyth T, Presta LG, Ferrara N. Mapping the charged residues in the second immunoglobulin-like domain of the vascular endothelial growth factor/placenta growth factor receptor Flt-1 required for binding and structural stability. J Biol Chem 273: 3216 –3222, 1998. 52. Gaal EI, Tammela T, Anisimov A, Marbacher S, Honkanen P, Zarkada G, Leppänen VM, Tatlisumak T, Hernesniemi J, Niemela M, Alitalo K. Comparison of vascular growth factors in the murine brain reveals placenta growth factor as prime candidate for CNS revascularization. Blood 122: 658 – 665, 2013. 34. De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med 44: 1–9, 2012. 53. Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab 295: E1056 –E1064, 2008. 35. De Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 255: 989 – 991, 1992. 36. Devaux Y, Azuaje F, Vausort M, Yvorra C, Wagner DR. Integrated protein network and microarray analysis to identify potential biomarkers after myocardial infarction. Funct Integr Genomics 10: 329 –337, 2010. 37. Devaux Y, Vausort M, Azuaje F, Vaillant M, Lair ML, Gayat E, Lassus J, Ng LL, Kelly D, Wagner DR, Squire IB. Low levels of vascular endothelial growth factor B predict left ventricular remodeling after acute myocardial infarction. J Card Fail 18: 330 –337, 2012. 54. Gerber HP, McMurtrey A, Kowalski J, Minhong Y, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3=-kinase/Akt signal transduction pathway. J Biol Chem 273: 30336 –30343, 1998. 55. Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J Biol Chem 275: 18040 –18045, 2000. 38. Dewerchin M, Carmeliet P. PlGF: a multitasking cytokine with disease-restricted activity. Cold Spring Harb Persect Med 2: 2012. 56. Grimmond S, Lagercrantz J, Drinkwater C, Silins G, Townson S, Pollock P, Gotley D, Carson E, Rakar S, Nordenskjold M, Ward L, Hayward N, Weber G. Cloning and characterization of a novel human gene related to vascular endothelial growth factor. Genome Res 6: 124 –131, 1996. 39. Dhondt J, Peeraer E, Verheyen A, Nuydens R, Buysschaert I, Poesen K, Van Geyte K, Beerens M, Shibuya M, Haigh JJ, Meert T, Carmeliet P, Lambrechts D. Neuronal FLT1 receptor and its selective ligand VEGF-B protect against retrograde degeneration of sensory neurons. FASEB J 25: 1461–1473, 2011. 57. Gunningham SP, Currie MJ, Han C, Turner K, Scott PA, Robinson BA, Harris AL, Fox SB. Vascular endothelial growth factor-B and vascular endothelial growth factor-C expression in renal cell carcinomas: regulation by the von Hippel-Lindau gene and hypoxia. Cancer Res 61: 3206 –3211, 2001. 40. Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, Roca C, Ramos D, Pujol A, Riu E, Ruberte J, Bosch F. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 61: 1801–1813, 2012. 58. Guo D, Jia Q, Song HY, Warren RS, Donner DB. Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J Biol Chem 270: 6729 – 6733, 1995. 41. Enholm B, Paavonen K, Ristimaki A, Kumar V, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Joukov V, Eriksson U, Alitalo K. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 14: 2475–2483, 1997. 59. Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, Klar J, Genove G, Pietras K, Stone-Elander S, Claesson-Welsh L, Yla-Herttuala S, Lindahl P, Eriksson U. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 464: 917–921, 2010. Physiol Rev • VOL 94 • JULY 2014 • www.prv.org 791 BRY ET AL. 60. Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsater H, Scotney P, Nyqvist D, Samen E, Lu L, Stone-Elander S, Proietto J, Andrikopoulos S, Sjoholm A, Nash A, Eriksson U. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature 490: 426 – 430, 2012. 79. Krueger J, Liu D, Scholz K, Zimmer A, Shi Y, Klein C, Siekmann A, Schulte-Merker S, Cudmore M, Ahmed A, le Noble F le Noble F. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development 138: 2111–2120, 2011. 61. Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PA, Robinson BA, Fox SB. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J Pathol 200: 183–194, 2003. 80. Lagercrantz J, Farnebo F, Larsson C, Tvrdik T, Weber G, Piehl F. A comparative study of the expression patterns for vegf, vegf-b/vrf and vegf-c in the developing and adult mouse. Biochim Biophys Acta 1398: 157–163, 1998. 62. Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA 95: 9349 –9354, 1998. 63. Ho VC, Duan LJ, Cronin C, Liang BT, Fong GH. Elevated VEGF receptor-2 abundance contributes to increased angiogenesis in VEGF receptor-1 deficient mice. Circulation 126: 741–752, 2012. 64. Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol 6: 209, 2005. 65. Huusko J, Lottonen L, Merentie M, Gurzeler E, Anisimov A, Miyanohara A, Alitalo K, Tavi P, Yla-Herttuala S. AAV9-mediated VEGF-B gene transfer improves systolic function in progressive left ventricular hypertrophy. Mol Ther 20: 2212–2221, 2012. 66. Huusko J, Merentie M, Dijkstra MH, Ryhanen MM, Karvinen H, Rissanen TT, Vanwildemeersch M, Hedman M, Lipponen J, Heinonen SE, Eriksson U, Shibuya M, Yla-Herttuala S. The effects of VEGF-R1 and VEGF-R2 ligands on angiogenic responses and left ventricular function in mice. Cardiovasc Res 86: 122–130, 2010. 67. Iyer S, Darley PI, Acharya KR. Structural insights into the binding of vascular endothelial growth factor-B by VEGFR-1(D2): recognition and specificity. J Biol Chem 285: 23779 –23789, 2010. 68. Iyer S, Scotney PD, Nash AD, Ravi Acharya K. Crystal structure of human vascular endothelial growth factor-B: identification of amino acids important for receptor binding. J Mol Biol 359: 76 – 85, 2006. 69. Jaba IM, Zhuang ZW, Li N, Jiang Y, Martin KA, Sinusas AJ, Papademetris X, Simons M, Sessa WC, Young LH, Tirziu D. NO triggers RGS4 degradation to coordinate angiogenesis and cardiomyocyte growth. J Clin Invest 123: 1718 –1731, 2013. 69a.Jeltsch M, Jha SK, Tvorogov D, Anisimov A, Leppänen VM, Holopainen T, Kivelä R, Ortega S, Karpanen T, Alitalo K. CCBE1 enhances lymphangiogenesis via ADAMTS3mediated VEGF-C activation. Circulation 2014 Feb 19 (Epub ahead of print). 70. Kaipainen A, Korhonen J, Pajusola K, Aprelikova O, Persico MG, Terman BI, Alitalo K. The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J Exp Med 178: 2077–2088, 1993. 81. Lahteenvuo JE, Lahteenvuo MT, Kivelä A, Rosenlew C, Falkevall A, Klar J, Heikura T, Rissanen TT, Vahakangas E, Korpisalo P, Enholm B, Carmeliet P, Alitalo K, Eriksson U, Yla-Herttuala S. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1and neuropilin receptor-1-dependent mechanisms. Circulation 119: 845– 856, 2009. 82. Landgren E, Schiller P, Cao Y, Claesson-Welsh L. Placenta growth factor stimulates MAP kinase and mitogenicity but not phospholipase C-gamma and migration of endothelial cells expressing Flt 1. Oncogene 16: 359 –367, 1998. 83. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134, 2010. 84. Leppänen VM, Prota AE, Jeltsch M, Anisimov A, Kalkkinen N, Strandin T, Lankinen H, Goldman A, Ballmer-Hofer K, Alitalo K. Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc Natl Acad Sci USA 107: 2425–2430, 2010. 85. Leppänen VM, Tvorogov D, Kisko K, Prota AE, Jeltsch M, Anisimov A, MarkovicMueller S, Stuttfeld E, Goldie KN, Ballmer-Hofer K, Alitalo K. Structural and mechanistic insights into VEGFR-3 ligand binding and activation. Proc Natl Acad Sci USA 110: 12960 –12965, 2013. 86. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306 –1309, 1989. 87. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672– 683, 2004. 88. Li X, Tjwa M, Van Hove I, Enholm B, Neven E, Paavonen K, Jeltsch M, Juan TD, Sievers RE, Chorianopoulos E, Wada H, Vanwildemeersch M, Noel A, Foidart JM, Springer ML, von Degenfeld G, Dewerchin M, Blau HM, Alitalo K, Eriksson U, Carmeliet P, Moons L. Reevaluation of the role of VEGF-B suggests a restricted role in the revascularization of the ischemic myocardium. Arterioscler Thromb Vasc Biol 28: 1614 – 1620, 2008. 89. Li Y, Zhang F, Nagai N, Tang Z, Zhang S, Scotney P, Lennartsson J, Zhu C, Qu Y, Fang C, Hua J, Matsuo O, Fong GH, Ding H, Cao Y, Becker KG, Nash A, Heldin CH, Li X. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest 118: 913–923, 2008. 72. Karpanen T, Bry M, Ollila HM, Seppanen-Laakso T, Liimatta E, Leskinen H, Kivelä R, Helkamaa T, Merentie M, Jeltsch M, Paavonen K, Andersson LC, Mervaala E, Hassinen IE, Yla-Herttuala S, Oresic M, Alitalo K. Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circ Res 103: 1018 –1026, 2008. 90. Lijnen HR, Christiaens V, Scroyen I, Voros G, Tjwa M, Carmeliet P, Collen D. Impaired adipose tissue development in mice with inactivation of placental growth factor function. Diabetes 55: 2698 –2704, 2006. 73. Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development 126: 4895– 4902, 1999. 91. Lu X, Ji Y, Zhang L, Zhang Y, Zhang S, An Y, Liu P, Zheng Y. Resistance to obesity by repression of VEGF gene expression through induction of brown-like adipocyte differentiation. Endocrinology 153: 3123–3132, 2012. 74. Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 90: 10705–10709, 1993. 92. Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med 8: 831– 840, 2002. 75. Kivelä R, Bry M, Robciuc MR, Rasanen M, Taavitsainen M, Silvola JM, Saraste A, Hulmi JJ, Anisimov A, Mayranpaa MI, Lindeman JH, Eklund L, Hellberg S, Hlushchuk R, Zhuang ZW, Simons M, Djonov V, Knuuti J, Mervaala E, Alitalo K. VEGF-B-induced vascular growth leads to metabolic reprogramming and ischemia resistance in the heart. EMBO Mol Med 6: 307–321, 2014. 76. Kivelä R, Silvennoinen M, Lehti M, Jalava S, Vihko V, Kainulainen H. Exercise-induced expression of angiogenic growth factors in skeletal muscle and in capillaries of healthy and diabetic mice. Cardiovasc Diabetol 7: 13, 2008. 93. Makinen T, Olofsson B, Karpanen T, Hellman U, Soker S, Klagsbrun M, Eriksson U, Alitalo K. Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1. J Biol Chem 274: 21217–21222, 1999. 94. Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab 17: 851– 859, 2013. 77. Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Persect Med 2: a006502, 2012. 95. Migdal M, Huppertz B, Tessler S, Comforti A, Shibuya M, Reich R, Baumann H, Neufeld G. Neuropilin-1 is a placenta growth factor-2 receptor. J Biol Chem 273: 22272–22278, 1998. 78. Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell 90: 753–762, 1997. 96. Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M, Patterson N, Lander ES, Mann M. Integrated analysis of 792 Physiol Rev • VOL 94 • JULY 2014 • www.prv.org VEGF-B IN PHYSIOLOGY AND DISEASE protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 115: 629 – 640, 2003. 97. Mould AW, Greco SA, Cahill MM, Tonks ID, Bellomo D, Patterson C, Zournazi A, Nash A, Scotney P, Hayward NK, Kay GF. Transgenic overexpression of vascular endothelial growth factor-B isoforms by endothelial cells potentiates postnatal vessel growth in vivo and in vitro. Circ Res 97: e60 –70, 2005. 114. Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol 11: 73–91, 1995. 115. Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholova I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Yla-Herttuala S. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res 92: 1098 –1106, 2003. 98. Nagy JA, Dvorak AM, Dvorak HF. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med 2: a006544, 2012. 116. Ruch C, Skiniotis G, Steinmetz MO, Walz T, Ballmer-Hofer K. Structure of a VEGFVEGF receptor complex determined by electron microscopy. Nat Struct Mol Biol 14: 249 –250, 2007. 99. Odorisio T, Schietroma C, Zaccaria ML, Cianfarani F, Tiveron C, Tatangelo L, Failla CM, Zambruno G. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. J Cell Sci 115: 2559 –2567, 2002. 117. Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev 89: 607– 648, 2009. 100. Olofsson B, Korpelainen E, Pepper MS, Mandriota SJ, Aase K, Kumar V, Gunji Y, Jeltsch MM, Shibuya M, Alitalo K, Eriksson U. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA 95: 11709 –11714, 1998. 118. Sadler TW. Langman’s Medical Embryology. Philadelphia, PA: Lippincott Williams & Wilkins, 2006, p. . 119. Schweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359: 843– 848, 1992. 101. Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, Orpana A, Pettersson RF, Alitalo K, Eriksson U. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci USA 93: 2576 –2581, 1996. 120. Selvaraj SK, Giri RK, Perelman N, Johnson C, Malik P, Kalra VK. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood 102: 1515–1524, 2003. 102. Olofsson B, Pajusola K, von Euler G, Chilov D, Alitalo K, Eriksson U. Genomic organization of the mouse and human genes for vascular endothelial growth factor B (VEGF-B) and characterization of a second splice isoform. J Biol Chem 271: 19310 – 19317, 1996. 121. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219: 983–985, 1983. 103. Oura H, Bertoncini J, Velasco P, Brown LF, Carmeliet P, Detmar M. A critical role of placental growth factor in the induction of inflammation and edema formation. Blood 101: 560 –567, 2003. 122. Serpi R, Tolonen AM, Huusko J, Rysa J, Tenhunen O, Yla-Herttuala S, Ruskoaho H. Vascular endothelial growth factor-B gene transfer prevents angiotensin II-induced diastolic dysfunction via proliferation and capillary dilatation in rats. Cardiovasc Res 89: 204 –213, 2011. 104. Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269: 25646 –25654, 1994. 123. Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153: 13–19, 2013. 105. Partanen TA, Makinen T, Arola J, Suda T, Weich HA, Alitalo K. Endothelial growth factor receptors in human fetal heart. Circulation 100: 583–586, 1999. 124. Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis 9: 225–231, 2006. 105a.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs I, del Monte I, Hilfiker-Kleiner D, Karumanchi SA, Avany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 485: 333–338, 2012. 106. Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J 411: 211–226, 2008. 107. Pepe M, Mamdani M, Zentilin L, Csiszar A, Qanud K, Zacchigna S, Ungvari Z, Puligadda U, Moimas S, Xu X, Edwards JG, Hintze TH, Giacca M, Recchia FA. Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy. Circ Res 106: 1893–1903, 2010. 108. Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res 92: 378 –385, 2003. 109. Poesen K, Lambrechts D, Van Damme P, Dhondt J, Bender F, Frank N, Bogaert E, Claes B, Heylen L, Verheyen A, Raes K, Tjwa M, Eriksson U, Shibuya M, Nuydens R, Van Den Bosch L, Meert T, D’Hooge R, Sendtner M, Robberecht W, Carmeliet P. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration. J Neurosci 28: 10451–10459, 2008. 110. Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9: 677– 684, 2003. 111. Quinn T, Peters K, DeVries C, Ferrara N, Williams L. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci USA 90: 7533–7537, 1993. 112. Rana S, Schnettler WT, Powe C, Wenger J, Salahuddin S, Cerdeira AS, Verlohren S, Perschel FH, Arany Z, Lim KH, Thadhani R, Karumanchi SA. Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens Pregnancy 32: 189 –201, 2013. 113. Risau W. Mechanisms of angiogenesis. Nature 386: 671– 674, 1997. 125. Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res 312: 549 –560, 2006. 126. Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M. Nucleotide sequence and expression of a novel human receptor type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 5: 519 –524, 1990. 127. Silvestre JS, Tamarat R, Ebrahimian TG, Le-Roux A, Clergue M, Emmanuel F, Duriez M, Schwartz B, Branellec D, Levy BI. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ Res 93: 114 –123, 2003. 128. Singh NK, Hansen DE 3rd, Kundumani-Sridharan V, Rao GN. Both Kdr and Flt1 play a vital role in hypoxia-induced Src-PLD1-PKCgamma-cPLA(2) activation and retinal neovascularization. Blood 121: 1911–1923, 2013. 129. Snuderl M, Batista A, Kirkpatrick ND, Ruiz de Almodovar C, Riedemann L, Walsh EC, Anolik R, Huang Y, Martin JD, Kamoun W, Knevels E, Schmidt T, Farrar CT, Vakoc BJ, Mohan N, Chung E, Roberge S, Peterson T, Bais C, Zhelyazkova BH, Yip S, Hasselblatt M, Rossig C, Niemeyer E, Ferrara N, Klagsbrun M, Duda DG, Fukumura D, Xu L, Carmeliet P, Jain RK. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 152: 1065–1076, 2013. 130. Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92: 735–745, 1998. 131. Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA 109: 5874 –5879, 2012. 132. Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Increased severity of cerebral ischemic injury in vascular endothelial growth factor-B-deficient mice. J Cereb Blood Flow Metab 24: 1146 –1152, 2004. 133. Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol 289: 329 –335, 2006. Physiol Rev • VOL 94 • JULY 2014 • www.prv.org 793 BRY ET AL. 134. Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1alpha-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS One 6: e28290, 2011. 145. Yang Y, Xie P, Opatowsky Y, Schlessinger J. Direct contacts between extracellular membrane-proximal domains are required for VEGF receptor activation and cell signaling. Proc Natl Acad Sci USA 107: 1906 –1911, 2010. 135. Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene 18: 2221–2230, 1999. 146. Yao J, Wu X, Zhuang G, Kasman IM, Vogt T, Phan V, Shibuya M, Ferrara N, Bais C. Expression of a functional VEGFR-1 in tumor cells is a major determinant of anti-PlGF antibodies efficacy. Proc Natl Acad Sci USA 108: 11590 –11595, 2011. 136. Tanaka K, Yamaguchi S, Sawano A, Shibuya M. Characterization of the extracellular domain in vascular endothelial growth factor receptor-1 (Flt-1 tyrosine kinase). Jpn J Cancer Res 88: 867– 876, 1997. 147. Yue X, Hariri DJ, Caballero B, Zhang S, Bartlett MJ, Kaut O, Mount DW, Wullner U, Sherman SJ, Falk T. Comparative study of the neurotrophic effects elicited by VEGF-B and GDNF in preclinical in vivo models of Parkinson’s disease. Neuroscience 258: 385–400, 2014. 137. Tirziu D, Chorianopoulos E, Moodie KL, Palac RT, Zhuang ZW, Tjwa M, Roncal C, Eriksson U, Fu Q, Elfenbein A, Hall AE, Carmeliet P, Moons L, Simons M. Myocardial hypertrophy in the absence of external stimuli is induced by angiogenesis in mice. J Clin Invest 117: 3188 –3197, 2007. 138. Tomanek RJ, Holifield JS, Reiter RS, Sandra A, Lin JJ. Role of VEGF family members and receptors in coronary vessel formation. Dev Dyn 225: 233–240, 2002. 139. Tomanek RJ, Ishii Y, Holifield JS, Sjogren CL, Hansen HK, Mikawa T. VEGF family members regulate myocardial tubulogenesis and coronary artery formation in the embryo. Circ Res 98: 947–953, 2006. 140. Wafai R, Tudor EM, Angus JA, Wright CE. Vascular effects of FGF-2 and VEGF-B in rabbits with bilateral hind limb ischemia. J Vasc Res 46: 45–54, 2009. 141. Wanstall JC, Gambino A, Jeffery TK, Cahill MM, Bellomo D, Hayward NK, Kay GF. Vascular endothelial growth factor-B-deficient mice show impaired development of hypoxic pulmonary hypertension. Cardiovasc Res 55: 361–368, 2002. 142. Wiesmann C, Fuh G, Christinger HW, Eigenbrot C, Wells JA, de Vos AM. Crystal structure at 1.7 Å resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell 91: 695–704, 1997. 148. Zachary IC. How neuropilin-1 regulates receptor tyrosine kinase signalling: the knowns and known unknowns. Biochem Soc Trans 39: 1583–1591, 2011. 149. Zentilin L, Puligadda U, Lionetti V, Zacchigna S, Collesi C, Pattarini L, Ruozi G, Camporesi S, Sinagra G, Pepe M, Recchia FA, Giacca M. Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB J 24: 1467–1478, 2010. 150. Zhang F, Tang Z, Hou X, Lennartsson J, Li Y, Koch AW, Scotney P, Lee C, Arjunan P, Dong L, Kumar A, Rissanen TT, Wang B, Nagai N, Fons P, Fariss R, Zhang Y, Wawrousek E, Tansey G, Raber J, Fong GH, Ding H, Greenberg DA, Becker KG, Herbert JM, Nash A, Yla-Herttuala S, Cao Y, Watts RJ, Li X. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA 106: 6152– 6157, 2009. 151. Zhao T, Zhao W, Chen Y, Liu L, Ahokas RA, Sun Y. Differential expression of vascular endothelial growth factor isoforms and receptor subtypes in the infarcted heart. Int J Cardiol 167: 2638 –2645, 2012. 143. Wittko-Schneider IM, Schneider FT, Plate KH. Brain homeostasis: VEGF receptor 1 and 2-two unequal brothers in mind. Cell Mol Life Sci 70: 1705–1725, 2013. 152. Zhong X, Huang H, Shen J, Zacchigna S, Zentilin L, Giacca M, Vinores SA. Vascular endothelial growth factor-B gene transfer exacerbates retinal and choroidal neovascularization and vasopermeability without promoting inflammation. Mol Vis 17: 492–507, 2011. 144. Xia P, Aiello LP, Ishii H, Jiang ZY, Park DJ, Robinson GS, Takagi H, Newsome WP, Jirousek MR, King GL. Characterization of vascular endothelial growth factor’s effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J Clin Invest 98: 2018 –2026, 1996. 153. Ziche M, Maglione D, Ribatti D, Morbidelli L, Lago CT, Battisti M, Paoletti I, Barra A, Tucci M, Parise G, Vincenti V, Granger HJ, Viglietto G, Persico MG. Placenta growth factor-1 is chemotactic, mitogenic, and angiogenic. Lab Invest 76: 517– 531, 1997. 794 Physiol Rev • VOL 94 • JULY 2014 • www.prv.org