Karen Lynne Waddell for the degree of Master of Science

AN ABSTRACT OF THE THESIS OF
Karen Lynne Waddell for the degree of Master of Science in Forest Science presented on August 29,1983.
Title: The Effect of Light Intensity on Douglas-fir Foliage
Quality:
Survival and Development of Western Spruce Budworm
Abstract approved:
Dr. Gary B. Pitman
Foliage from Douglas-fir(var. glauca) seedlings grown under two light intensities was bioassayed to examine palatability to the western spruce budworm (Choristoneura occidentalis Free.).
Samples were collected in Fall, Winter and early Spring to identify seasonal changes in host foliage, as evidenced by the response of spruce budworms.
Fall collected foliage had little effect on budworm survival and fitness.
Winter and Spring foliage grown in full sun produced significantly greater larval and pupal mortality, longer larval development times, lower pupal weights and reduced fecundity.
These results indicate the level of Douglas-fir foliar defenses was influenced by season and light intensity.
I suggest that the available carbon and energy resources decline in the shade due to lower photosynthetic activity.
This in turn reduces the biosynthetic substrates needed for the costly production of defensive
chemicals, and therefore, increases the foliage palatability to spruce budworm.
The dynamics of seasonal sinks for carbon and energy compounds may determine when and to what extent fol jar defenses are synthesized.
FOREST RESEARCH LABORATORY
LIBRARY
OREGON STATE UNIVERTY
The Effect of Light Intensity on Douglas-fir Foliage Quality:
Survival and Development of Western Spruce Budworm by
Karen Lynne Waddell
A THESIS submitted to
Oregon State University in partial fullfillment of the requirements for the degree of
Master of Science
Completed August 29, 1983
Commencement June 1984
Approved:
Associate Professor of Forestry in Charge ot Major
Department Head of Forest Science
Dean of Graduate School
Date thesis is presented August 29, 1983
Typed by Karen Lynne Waddell for Karen Lynne Waddell
TABLE OF CONTENTS
INTRODUCTION
MATERIALS AND METHODS
Seedling Source
Experimental Design
Laboratory Procedures
Statistical Analyses
RESULTS
Fall Sampling Period
Winter and Spring Sampling Periods
Budworm Mortality
Larval Development Time
Pupal Weights
Fecundity
DISCUSSION
Light Intensity
Seasonal Pattern
Implications to Budworm Populations
LITERATURE CITED
APPENDIX
Literature Review
Bibliography
Page
1
30
34
35
45
4
6
8
4
4
10
10
11
11
13
14
14
16
16
18
19
LIST OF TABLES
Table
1
2
3
4
5
6
7
Mean standard control development times, pupal weights, and fecundity measured on budworms fed control diets.
Mean percent budworm mortality(original scale) caused by foliage from the Fall (Oct) sample period.
The split-plot ANOVA tables for all ten variables measured on foliage collected during the Fall,
Winter and Spring sampling periods.
Mean percent larval and pupal mortality produced by toxic group foliage within light intensity treatments from the Winter sampling period.
Mean percent budworm mortality produced by toxic group foliage within light intensity treatments during Winter and Spring sampling periods.
Mean larval development times of spruce budworms feeding on Douglas-fir foliage grown under two light intensities.
Mean fecundity of spruce budworms fed foliage from two light intensities.
Page
22
23
24-25
26
27
28
29
THE EFFECT OF LIGHT INTENSITY ON DOUGLAS-FIR FOLIAGE QUALITY:
SURVIVAL AND DEVELOPMENT OF WESTERN SPRUCE BUDWORM
INTRODUCTION
The western spruce budworm (Choristoneura occidentalis
Free.) causes serious defoliation in coniferous forests of
North America (Carolin and Honing, 1972).
Douglas-fir
(Pseudotsuga menziesii (Mirb.)Franco), the principal economic host in the Northwest, exhibits differential genetic susceptibility to the budworm in laboratory bioassays (Perry and Pitman, 1983).
Expression of this genetic potential for resistance maybe influenced by existing environmental conditions (i.e. light, water and/or nutrients) within forest stands (Kogan, 1975).
Decreasing light quantity and quality can modify fundamental physiological processes of host plants resulting in a loss of pest resistance (Tingey and Singh, 1980;
Gerhold etal., 1966).
Species that thrive in full sun, such as Douglas-fir, have a higher light compensation point than tolerant species and respond to higher light intensity by increasing photosynthetic activity (Boardman, 1977; Bjorkman,
1973; Woodman, 1971; Krueger and Ferrell, 1965), producing a larger pool of available photosynthates (carbon) and high-energy compounds (ATP, NADPH2).
The allocation of these carbon and energy resources to various biochemical fractions is dynamic, depending on the strength of seasonal sinks.
2
Detailed studies have been conducted at different phenological stages of plant growth to determine the priority pattern for allocation among plant organs (Webb, 1977; Webb,
1975; Gordon and Larson, 1968; Shiroya et al., 1966) and biochemical fractions (Chung and Barnes, 1980; Mooney and Chu,
1974).
Miong the biochemical fractions, phenolics and terpenoids were found to take low priority during the growing season, becoming the major sink only after shoot, needle and root elongation have been satisfied.
These secondary chemicals
(allelochemics) often contribute to the defensive mechanism of plants by reducing foliage palatability (Rhoades and Cates,
1976), but are costly both energetically and biochemically to produce (Chew and Rodman, 1979; Rhoades, 1979; Wright, etal.,
1979; Herout, 1970).
Thus, assuming these defenses exist in
Douglas-fir, knowledge of their seasonal abundance under different light regimes can be valuable when studying plant-herbivore interactions.
It is not surprising then that in some species the accumulation and yield of allelochemics diminishes under low light intensities (Firmage, 1981;
Langenheim et al., 1981).
The spruce budworm prefers young current years foliage, but if an asynchrony occurs between bud flush and larval emergence from diapause, they are forced to feed on mature foliage or move to other hosts (Beckwith and Burnell, 1982;
Edit and MacGillivray, 1972).
Old foliage is known to reduce survival and fitness in both larval and pupal stages
3
(Perry and Pitman, 1983; Edit and Cameron, 1971; Heron, 1965;
Greenbank, 1963) presumably due to an accumulation of defensive chemicals.
Yearly fluctuations in budworm population dynamics may be influenced by the relative toxicity of mature foliage during these asynchronous feeding periods.
The objective of this study was to examine the survival and growth response of western spruce budworm, feeding on
Douglas-fir (var. glauca) foliage grown under two light intensities.
Seasonal differences in foliage palatability were also explored by collecting samples in Fall, Winter and Spring.
4
MATERIALS AND METHODS
Seedling Source
Douglas-fir (Pseudotsuga menziesii var. glauca) seeds were collected for a previous study in 1971 from wind pollinated trees in eastern Oregon on the Ochoco and Malheur National
Forests.
The Ochoco population is located near the town of
Prineville (PV) and the Malheur population near John Day (JO).
The seedlings are identified as a family, according to their population and half-sibling mother tree origin.
In 1979, ten seeds were potted in one gallon containers and grown at the
Forest Research Laboratory in Corvallis, Oregon for three years.
Beginning in 1980, the foliage toxicity level of each family was established by Perry and Pitnian (1983) with spruce budworm bioassays.
Toxicity level was determined by the percent mortality exhibited by the budworm larvae after feeding on diets containing one year old foliage.
Seedlings from the group of families with known toxicity levels were selected for this study.
Before budburst in March 1982, seedlings were transplanted to individual 20cm pots, fertilized and allowed to recover from transplant shock for a three month period.
Experimental Design
The three year old seedlings were divided into four toxic groups based on the family level of toxicity previously established in 1980 and identified as High (H), Medium
(MID-PV), Medium (MID-JO) and Low (L).
The H group was a combination of both PY and JO populations due to the lack of
5 sufficient high mortality seedlings.
The two medium mortality groups are made up of either PV or JD individuals, and the L group is comprised solely of PV families.
Initially, the groups were characterized by the following ranges in larval mortality: H(70-95%); MID-PV(40-55%); MID-JD(50-65%), and
L(l-20%).
The mortality level of old foliage was re-established for the present study (1982) and found to be consistantly lower than the 1980 values, but retained the sequence from high to low.
The final 1982 toxicity levels were identified as total budworm mortality (larval and pupal) caused by foliage pooled from all families in one group: H(57.5%);
MID-PV (35%); MID-JD(40%); and L(5%).
The experiment was set up as a randomized block split-plot design in space, with three blocks, four toxic groups
(subplots) and two light intensity treatments (65% shade cloth and full sun as the whole plots).
One subplot experimental unit consists of twenty seedlings from each toxic group (a total of six units per group).
Current years needles were plucked from all twenty seedlings and pooled to form one sample for each toxic group by light combination.
The shade treatment was randomly assigned to one half of a block, followed by the random assignment of one of the six experimental units representing each toxic group, to each shade treatment.
The shade cloth was applied June 1, 1982 and sampling began four months later, to allow sufficient time for a seedling response.
Foliage samples were taken on the following
dates: October 1, l982(Fall); January 1, 1983(Winter); and
March 1, 1983(Early Spring).
Laboratory Procedures
The pooled foliage samples were stored at -60°C until budworm diets were prepared for the bioassays.
Laboratory conditions and techniques for rearing a diapausing western spruce budworm colony were adopted from Robertson (1979) and
Lyon etal. (1972).
The budworm population used in this study had been reared for over fifty generations in the laboratory.
The temperature varied between 21-290C instead of the recommended 23-260C range due to the lack of a thermal control device in the growth chamber.
Bioassay diets consisted of a mixture of ground foliage with standard artificial diet ingredients (Lyon, et.al., 1972) in a 1:2 weight proportion (dry diet without agar:wet foliage).
A Polytron tissue homogenizer was used to puree the foliage in the volume of distilled water required by the standard recipe.
This mixture was then blended with the dry ingredients followed by the hot agar.
Four milliliters of the bioassay mixture was poured into 40 individual insect rearing cups approximately 1 cm3 in volume.
One 2nd-instar post-diapause spruce budworm larva was placed in each cup and sealed in with a clear plastic iron-on lid.
One group of 40 budworms tested each replicate of the treatment combinations.
The mean over all three blocks, then, consists of information on up to 120 budworms, which should average out the high
6
7 variation within a replicate.
Ten variables were recorded over the life span of the insect: larval, pupal and total mortality; larval and pupal development times for each sex; male and female pupal weights; and fecundity of mated pairs within a toxic group.
Larval mortality was reported only after evidence of feeding (i.e. frass) accompanied the dead larva.
This estimate of mortality is most likely very conservative, because other factors such as volatizing monoterpenes acting as feeding deterrents are not considered.
Larvae were replaced when death was the apparent result of ir6n burns or drowning in condensation droplets.
The post-diapause larval development time began when placed on bioassay diets and continued to the date of pupation.
Pupae were weighed within 24 hours of pupation, sexed according to the number of abdominal segments (females have four and males five), and observed for the length of development from the onset of pupation to the date of emergence.
Pupal mortality was recorded upon adult emergence failure.
Surviving pupae from each test diet were paired for mating and placed in 16 oz. Dixie@ containers.
The mating chambers contained moistened filter paper, waxpaper strips and were covered with clear plastic lids to observe pupal emergence and mating success.
Fecundity per female was determined by counting the number of eggs laid over a seven day period.
8
A control diet formulated from the standard recipe mixed with 2% dry weight of ground Douglas-fir (var. menziesii) foliage (non-toxic needles from current flush) was used to bioassay 100 budworms.
All bioassays were conducted at the same time, thus only one laboratory control was required.
The mortality observed on test diets was adjusted by removing control mortality with the following equation (after Retnakaran and Tomkins, 1982):
% Mort fpost feeding number
%pre feeding number pre feedinj number in control control)jX 100 post feeding number in
The other seven variables recorded on the control fed budworms were used as a standard, representing normal budworm survival and development under the existing laboratory conditions.
Statistical Analysis
The data was analyzed with the SPSS statistical software package (Hull and Nie, 1981) using the MANOVA procedure for a split plot design.
A split plot ANOVA was run on each of the ten variables independently, for each of the three sampling periods (Fall, Winter and Spring).
Where the F-test was significant, treatment means were further compared with Tukey's Multiple Comparison (HSD) and the
Least Significant Difference (LSD) tests to find where the differences lie.
Since the three mortality variables are percentage data containing values within the 0-30% range, an
arcsine transformation was used to spread out the tails of the distribution and improve norma1ity(p'=arcsinJ; Steel and
Torrie, 1980).
All ANOVA and multiple comparison tests utilized the transformed data set, thus sunnnary tables of means in the original scale do not contain standard error terms.
It is inappropriate to retransform variances and standard errors obtained from arcsin transformed data (Steel and Torrie, pg. 236, 1980).
9
10
RESULTS
Values for development times, pupal weights and fecundity were obtained from budworms fed the 2% control diet, and are considered standard control rates under the existing laboratory conditions (Table 1).
These values aid in the interpretation of treatments affects, especially when investigating extended or restricted development times.
Fall Sampling Period
Foliage collected from both light intensity treatments during the Fall (Oct. 1) sampling period produced few significant differences on any of the ten variables.
Three variables affected, however, included female pupal development times, male pupal weights, and fecundity all being significantly greater for budworms fed shaded foliage, irrespective of toxic group origin.
Development averaged 6.48
(+.08) days, mean weight was 130.4 (+4.3) mg and females laid an average of 234.6 (+24.7) eggs when fed shaded foliage, compared to 6.35 (+.lO) days, 111.9 (7.5) mg and 200 (+16.8) eggs/female on full sun foliage.
The only other significant
F-tests on Fall foliage were among toxic groups (subplots) averaged over whole plots, for each mortality variable.
Overall budworm mortality is very low on Fall foliage, and when investigating group means little distinction between toxic levels is apparent (Table 2).
The H and both MID-level groups are not different among themselves, but each generated significantly greater larval and total mortality than the L
11 toxic group.
Pupal mortality showed additional separations between toxic groups, as indicated in Table 2.
The L toxic group appears to establish itself as a weak budworm killer early in the season (Fall), whereas the other groups have not yet developed the ordered levels they are known to possess.
The MID-level toxicity groups from both populations tend to produce similar measurements, a pattern that is repeated throughout the other analyses on the later sampling times.
ANOVA's on the remaining variables measured in
October produced non-significant F-tests for all factors in the experiment.
Winter and Early Spring Sampling Periods
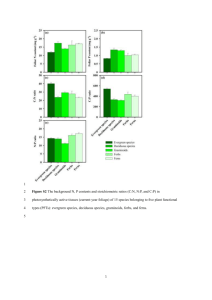
Spruce budworm responded significantly differet to foliage growing in full sun compared to 65% shade on many of the variables examined (Table 3).
The three mortality variables, larval development of both sexes, male and female pupal weights and fecundity were influenced either independently by light intensity or toxic group, or dependently with an interaction between the two.
Male pupal weights were only significant when budworms fed on foliage picked in early
Spring.
Pupal development time was not significantly affected by any treatment combination in either sampling period.
Budworni Mortality
The two-way table of means for mortality variables measured on Winter and Spring foliage is presented in Tables 4 and 5.
Budworm mortality observed on full sun foliage always
12 exceeded mortality caused by shaded foliage. There was no significant interaction between shade and light group for the larval and pupal mortalities recorded on budworms fed the
Winter foliage (Table 4).
Both light and toxic group were significant independently for these two variables, with full sun producing higher mortalities than shade and toxic groups differing as indicated by the letters (a-c).
Where an interaction exists for total mortality on Winter foliage and all three mortality variables measured on Spring foliage, mean separation within and between light treatments can be investigated (Table 5).
The most notable response attributable to the ingestion of foliage grown in full sun, is a significant increase in larval, pupal and total mortality in all but the L toxic group.
Within a particular light intensity at any time period, the MID-PV and MID-JD groups do not differ significantly from each other.
In general, shaded foliage tends to reduce expression of the previously identified toxicity for each group except L, creating a pattern of similar mortalities among the H, MID-PV and MID-JD groups.
Foliage grown in the full sun, however, develops the distinct toxicity pattern from high to low observed in the initial bioassays of one year old foliage (see Methods).
By Spring this pattern becomes obvious, with the greatest mortality response measured on budworms feeding on H group sun foliage, an intermediate mortality level developed on both MID-JD and PV groups, and the
lowest toxicity and highest survival was observed on the L group foliage.
Larval Development Time
There was significant interaction between both treatments within each sampling period for development of both sexes.
Larval development is always significantly longer when fed sun grown foliage, with certain toxic groups causing more extended growth periods than others (Table 6).
The treatment means should be compared with the standard development times found in
Table 1, to examine relative changes due to treatment effects.
Foliage collected during both sampling periods produced nearly identical results within each toxic group for each sex.
Shade foliage from MID-JD and L groups caused female development time to shorten approximately two and a half days below the standard 23.9 days for larval growth in controls, while sun foliage increased the time by more than three days
(Table 6).
The HIGH and MID-PV development times on shaded foliage were close to the standard 23.9 days, with a smaller one to two day increase on sun foliage.
Male development followed a similar pattern.
The H and
MID-PV times were close to the standard male 21.3 day period when sampled from the shade treatment, but increased up to 2 days when budworms fed on sun foliage.
The MID-JD and L foliage caused a restricted budworm growth period (18-19 days) in the shade, and an extended period in the sun (24-26 days).
To summarize both sampling periods, shaded foliage from
13
14 all toxic groups produced shorter larval development times for both sexes, compared to foliage grown in full sun.
Within either shade or sun treatments, the MID-PV and H groups and the
MID-JD and L groups responded similarily (relative to the normal development period observed on control fed budworms).
For both sexes, the MID-JO and L shaded foliage showed a considerable restriction in budworm development time and a substantial extension when grown in full sun.
Pupal Weights
Pupal weights were consistantly lighter when larvae were fed sun grown foliage.
Females normally weigh more than males with a mean on control diets of 181.76 mg compared to 120.74
for males (Table 1).
Females weighed significantly less on sun foliage from both Winter and Spring sampling periods with a mean of 170.55 mg and 171.1 mg, respectively, compared to 185.6
mg and 188.04 mg on shaded foliage.
The same trend occurred in males, with a mean of 127.7 mg and 129.6 mg for the two sampling periods in shade, compared to 111.57 and 113.97 mg on sun foliage.
No meaningful separations among toxic groups were observed.
When coupling these results with development times, a pattern of extended growth and low pupal weights develops for budworms fed sun grown foliage.
Fecundity
No differences in fecundity due to light intensity were detected on Winter foliage, but females laid significantly more eggs if larval development occurred on shaded foliage collected
15 in Spring (Table 7).
Certain toxic groups were significantly different in both sample periods as indicated by the letters in the table.
This reduction in fecundity supports the previous observations that sun grown foliage degrades the fitness attributes of western spruce budworms.
16
DISCUSSION
The adverse response exhibited by western spruce budworms fed on Winter and Spring foliage grown under high light intensity may indicate a qualitative and/or quantitative change in foliar defensive chemistry.
The direct impact on budworm fitness is evident in the decreased survival, extended development periods, lower pupal weights and reduced fecundity.
These are characteristic signs that food quality and/or palatability have declined (Rhoades, 1979).
Three factors need to be recognized in this study as determinants of foliage quality: the light intensity level, influencing photosynthetic activity; the sample month, reflecting seasonal resource allocation priorities; and the toxic group origin, signifying genotypic characteristics.
Family differences within each group are muted due to the pooling of foliage, but
I anticipate some genetic variability is being averaged out.
Since water and nutrient regimes were constant in all treatments, differential effects of the pooled foliage within any season or group are assumed to be induced by the light intensity level.
Light Intensity
The appearance of an elevated defense system in the
Douglas-fir foliage grown in full sun may be attributable to changes in photosynthetic activity.
Relatively, there should be greater photoassimilation rates in full light intensity compared to shaded conditions due to the high compensation
17 point of Douglas-fir var. glauca (Krueger and Ferrell, 1965), producing a larger pool of available carbon and energy-rich compounds for biosynthesis.
Since secondary chemicals used in defense are metabolically costly, requiring the same biosynthetic substrates and energy needed for structural growth, the final yield of allelochemics is expected to decline when resources are limiting.
Recently, both Firmage (1981) and Langenheimet al. (1981) found significantly lower yields of essential oils and terpenoids in plants grown under low light intensities.
Many others observed a loss in phenotypic resistance in cultivated plants when grown in shade (see refs. in Maxwell and
Jennings, 1980; Gerhold etal., 1966; and Fluck, 1963).
Cellular constraints were investigated by Croteau etal. (1972) who found evidence that monoterpene synthesis occurs at energy-poor sites and that production increased when energy-rich photosynthates were abundant.
In addition, if photoassimilates become limiting, catabolic turnover of monoterpenes may accelerate, releasing tied up carbon and energy for primary metabolic processes (Burbott and Loomis,
1969).
Shaded Douglas-fir may then be experiencing lower rates of allelochemic synthesis due to a restricted carbon and energy pool and/or accelerated catabolism of existing terpenoid constituents.
This creates an overall reduction in the accumulation of secondary chemical defenses in mature foliage,
18 evident in the greater survival and fitness of spruce budworm fed shaded foliage.
Seasonal Pattern
Current years foliage collected in October had little effect on budworm survival and fitness, indicating the lack of a functional defense system against this herbivore.
By January and March, a sharp increase in budworm mortality along with the distinct differences in larval development were evident between shade and sun, suggesting that mature foliage has accumulated an increased array or quantity of defensive allelochemics.
The change in foliage suitability is probably due to a late seasonal shift in the carbon and energy sink towards protection, supporting the data obtained by Mooney and Chu
(1974) and Chung and Barnes (1980).
Both reported the major sink or priority for available photosynthates and energy during the active growing season is structural tissues.
Generally, the seasonal progression of resource sinks for Douglas-fir begins with the rapidly dividing meristematic region in the buds and subsequent needle elongation (Webb, 1975; 1977; Allen and Owens, 1972).
Work on other conifer species (Gordon and
Larson, 1968) has shown that when needles have matured by late summer and begin to contribute rather than subtract from the photosynthate pool, stem cambial activity shifts to latewood formation, becoming the next major sink.
In the northwest, root activity is highest in the fall when it puts the major drain on available resources (Hermann, 1977; Webb. 1977).
19
It appears, then, that overall structural growth in
Douglas-fir is not curtailed until sometime in fall.
This suggests that the bulk of carbon is not available for allelochemic production until either late in the growing season or during the nongrowing season.
This would explain the lack of apparent defenses in October and the sharp increase by
January and March.
These insect bioassay results parallel the chemical results of Mooney and Chu (1974) and Chung and Barnes
(1980), who found a rapid increase in photosynthate allocated to protection (phenolics) during the nongrowing season.
Furthermore, photosynthesis can continue year round in the
Pacific Northwest due to the mild winter climatic conditions as shown by Emmingham and Waring (1977).
Douglas-fir can double its photosynthetic rate during cool, moist winters, and in some cases, have produced almost one-fourth of the total yearly photosynthates in the winter months alone (Helms, 1964).
The 1982-83 winter season was mild in Corvallis, with considerable rainfall, no snow and few days of freezing temperatures.
Photoassimilation was most likely enlarging the available resource pool throughout the winter, with a commensurate increase in foliar allelochemics.
Implications to Budworm Populations
The relative toxicity of mature foliage can be influential in budworm population dynamics, even though the preferred food is immature needles from the current years flush.
Spruce budworms diapause through the winter as 2nd instar larvae, and
20 emerge in the spring after a period of cold with a subsequent accumulation of heat units have been satisfied
(Thomas, 1976).
Initiation of actual emergence from the hibernaculum requires sufficiently high ambient air temperatures.
This is contrasted to the additional requirement of warm soil temperatures needed for buds to swell and flush
(Lavender etal., 1973; Cleary and Waring, 1969).
If budworm larvae are cued to emerge before buds flush in the spring due to a period of unseasonally high solar radiation, they must search for either staminate flowers or mature foliage as a food source.
It is during this asynchronous period that significant mortalities may result if mature foliage is the only food option.
This can directly determine the initial population size for that year.
These events were observed on western spruce budworm in north-central
Washington by Beckwith and Burnell (1982).
They reported a large proportion of 2nd instar larvae were mining old needles when bud expansion was delayed due to cold soil temperatures.
Later in the season, as abundance of the preferred food source declines, older instars may be forced onto mature foliage, causing a reduction in the final pupal weight and fecundity.
In addition, if the previous years buds were severely damaged by early budworm mining, lateral bud formation was probably destroyed, limiting the leaf area of the current bud flush.
Once again, larvae are eventually forced onto the only available food source, mature foliage.
When forests become dense and stagnant, they often are under severe competitive stress for light, water and/or nutrients.
If low light levels within a forest strata affect foliage quality of mature trees, similar to the response reported here by seedlings, we can expect dense stands to be more susceptible to budworm attack.
When light is abundant
(i.e. minimal shading from neighboring trees) however, mature foliage may accumulate defensive allelochemics and play a major role in the regulation of budworm populations.
21
Table 1.
Mean standard control weights and fecundity control diets (+sd.) development times, pupal measured on budworms fed
(1).
Sex
Female
Male
Development
(days)
Larval Pupal
23.9
6.4
(.57) (.70)
21.3
6.3
(.82) (.48)
Pupal Weights
181.76 mg
(4.46)
120.74 mg
(3.81)
Fecundity
229 eggs!
female
(22.83)
(1).
These values are considered standard under the existing laboratory conditions, and are averages of up to 100 budworms.
22
23
Table 2.
Mean percent budworm mortality(original scale) caused by foliage from the Fall (Oct) sample period (1).
Mortality
Variable
Larval
Pupal
Total
H
5.3
a
1.84 bc
4.37 a
Toxic Group.
(%)
MID-PV
6.15 a
4.40 ac
10.20 a
MID-JD
3.60 a
6.35 a
9.35 a
L
2.36 b
.45 b
1.65 b
(1) Letters (a-c) represent significance (p>.05) horizontally between toxic groups for each variable.
Identical letters indicate no difference between groups.
See methods for toxic groups definitions.
Table 3.
The split-plot ANOVA tables for dli ten variables measured on foliage collected during the
Fall, Winter and Spring sampling periods (1).
SOURCE
FALL
Block
Light
ErrorA
ToxGrp
Inter.
ErrorS
OF
3
3
12
2
1
2
MS
UIORT
6.47
35.35
4.47
62.48
1.03
5.96
WINTER
Block
Light
ErrorA
ToxGrp
Inter.
ErrorS
3
3
12
2
1
2
9.95
175.05
2.94
507.94
17.59
5.94
SIGNIF.
.409
.107
.001
.912
.228
.016
.000
.075
SPRING
Block
LIght
ErrorB
ToxGrp
Inter.
ErrorS
3
3
12
2
1
2
3.99
463.55
4.71
351.08
43.30
7.65
.542
.010
.000
.012
MS
PMORT
SIGNIF.
39.19
5.06
5.66
82.10
2.33
9.98
.126
.444
.003
.872
58.08
.217
300.91
.0495
16.08
276.36
.001
31.87
.284
22.38
18.54
.029
494.50
.001
.56
196.48
.000
103.47
.001
8.37
MS
TMORT
SIGNIF.
44.97
44.70
11.64
133.80
1.29
11.06
.206
.189
.001
.950
26.63
428.90
1.72
739.75
22.46
5.06
.061
.004
.000
.026
3.49
849.23
2.01
552.70
99.61
8.00
.365
.002
.000
.001
MS
LDEVF
SIGNIF.
.18
.74
.03
.02
.37
.21
.125
.034
.975
.207
.13
102.51
.04
1.11
12.00
.22
.250
.001
.017
.000
.07
143.08
.11
1.35
8.94
.14
.621
.001
.001
.000
MS
LDEVM
SIGNIF.
.26
.02
.02
.07
.15
.23
.055
.369
.821
.600
.14
103.75
.01
1.47
13.98
.13
.053
.000
.001
.000
.12
138.24
.10
1.56
7.23
.15
.438
.001
.001
.000
Table 3.
(Cont.) The split-plot ANOVA tables for all ten variables measured on foliage collected during the Fall, Winter and Spring sampling periods (1).
SOURCE
FALL
Block
Light
ErrorA
ToxGrp
Inter.
ErrorS
OF
3
3
12
2
1
2
MS
PDEVM
SIGNIF.
.0054
.0067
.0004
.0183
.0144
.0151
.071
.057
.348
.446
MS
PDEVF
SIGNIF.
.0067
.1067
.0017
.0144
.0100
.0081
.200
.015
.202
.338
MS
PWTF
SIGNIF.
20.82
.805
1100.26
.070
85.93
75.17
.526
34.06
.787
96.05
MS
PWTM
SIGNIF.
3.26
.848
2269.82
.007
15.71
31.31
.317
14.62
.621
23.95
MS
FECUND
SIGNIF.
202.71
.149
7869.88
.004
35.43
846.94
.106
202.13
.625
334.61
WINTER
Block
Light
(rrorA
ToxGrp
Inter.
Errorb
3
3
12
2
1
2
.0163
.0817
.0054
.0139
.0272
.0281
.250
.060
.692
.439
.0029
.1067
.0104
.0072
.0367
.0194
.781
.085
.775
.186
173.88
.200
1515.27
.027
43.37
109.38
.207
194.64
61.91
.065
86.13
.345
1953.01
45.39
.022
121.86
.256
61.75
.528
79.34
561.27
.493
653.13
.388
545.21
2642.62
.008
251.34
.626
417.10
SPRING
Block
Light
ErrorS
2
1
2
.0004
.0017
.0129
.969
.754
.0004
.0067
.0129
.969
.547
29.48
.394
1933.22
19.19
.010
1.97
.818
2196.51
.004
8.86
59.56
.589
3901.50
.021
85.08
ToxGrp
Inter.
ErrorB
3
3
12
.0028
.0050
.0156
.909
.810
.0022
.0044
.0100
.879;
.726
49.99
.008
50.28
.008
7.82
230.63
.000
125.65
.001
10.68
3603.04
.000
43.44
.703
90.80
(1) IMORT, PMORT, and NORT=larval, pupal and total mortality respectively.
development times for females and males.
PDEVM and PDEVF=pupal development times for males and females.
PWTF and PWTM=pupal weights for females and males.
LDEVF and LDEVMlarval
FECUND=fecundity of mated pairs.
DFdegrees of freedom, MSmean square and SIGNIFs1gnif1cance of the resultant F value.
26
Table 4.
Mean percent larval and pupal mortality produced by toxic group foliage within light intensity treatments from the Winter sampling period (1).
Mortality
Variable
LARVAL
Toxic
Group
H
MID-PV
MID-JD
L
Overall Means
WINTER SAMPLE PERIOD
Overall
Shade Sun Means
15.00
8.30
20.00
.51
10.95
28.50
16.70
22.60
2.40
17.60*
2l.80a
l2.50b
2l.30a
l.46c
PUPAL H
MID-PV
MID-JD
L
Overall Means
9.00
6.30
.72
.51
4.13
23.10
11.30
10.80
1.50
11.67*
16.lOa
8.8Oac
5.8Obc
l.Olb
(1) * indicates significance (p>.05) horizontally between light intensity means.
Letters a-c compare significance vertically between toxic group means.
No interaction was detected between treatments.
27
Table 5.
Mean percent budworm mortality produced by toxic group foliage within light intensity treatments during
Winter and Spring sampling periods (1).
Mortality Toxic
Variable Group
TOTAL
(Winter)
H
MID-PV
MID-JD
L
Mean
Shade
22.70c
14.lOa
20.lOac
l.lOb
14.50
Sun
45.00c
26.lOa
31 .30a
3.80b
26.50**
**
**
**
**
LARVAL
(Spring)
H
MID-PV
MID-JD
L
Mean
PUPAL
(Spring)
H
MID-PV
MID-JD
L
Mean
TOTAL
(Spring)
H
MID-PV
MID-JD
L
Mean l3.30a
7.4Oab
12.50a
2.40b
8.90
35.20c
20.90a
2l.70a
AA
**
*
4.00b
ns
20.45**
3.9Oab
7.20a
4.82ab
l.47b
4.35
26.90c
**
12.00a
*
17.00ac **
2.20b
ns
14.70**
16.70a
l4.lOa
16.60a
3.80b
12.80
5l.90c
30.40a
**
**
**
35.60a
5.50b
ns
30.85**
(1) *,**, and ns indicate P>.05, P>.01, and no significance respectively, between any two light treatments (horizontally).
The letters a-c compare significance (p>.05) vertically, between toxic groups within one light treatment.
There was significant interaction between treatments in all the above cases.
28
Table 6.
Mean larval development times of spruce budworms feeding on Douglas-fir foliage grown under two light intensities.
Sex
Female
Male
Toxic
Group
H
MID-PV
MID-JD
L
Mean
MEAN LARVAL DEVELOPMENT TIME
(days)
Shade
Winter
Sun
24.lOa
23.43ac
2l.3Obc
20.60b
22.36
25.07b
*
25.97ab **
27.97a
**
26.93ab *
26.49 **
Shade
Spring
Sun
21.93a
23.87b
21.lOc
21.03c
21.98
25.27a
**
25.90a
A
28.lOb
**
27.87b
**
26.79 **
H
MID-PV
MID-JD
L
Mean
21.33a
2l.47a
18.60b
18.50b
19.98
22.20a
*
23.83b
**
25.80c
**
24.7Ocb AA
24.70 **
20.00a
2l.73b
19.00c
18.60c
19.83
23.87a
23.90a
25.97b
24.80a
24.64 **
AA
**
**
**
(1) The ** indicate significance (p>.05
and p>.Ol) horizontally between light treatments, for individual toxic groups.
The letters a-c indicate significance vertically (p>.05) between toxic groups within a light treatment, where identical letters represent nonsignificance.
Interactions were significant between both factors for each sex and season
29
Table 7.
Mean fecundity (eggs/female) of spruce budworms fed foliage from two light intensities (1).
Toxic
Group
H
MID-PV
MID-JD
L
Means
WINTER
LIGHT INTENSITY
Shade Sun Means
188.5
142.3
140.6
166.1
159.4
165.8
150.7
124.0
145.3
146.5ns
177.2a
146.5ab
132.3b
l55.7ab
SPRING
LIGHT INTENSITY
Shade Sun Means
172.4
167.1
122.0
136.1
149.4
141.8
146.1
92.3
128.8
127.3*
157.la
156.6a
107.2b
132.5c
(1).
Letters a-c indicate significance (p>.05) vertically among toxic group means.
Winter light intensity was not significant.
No interaction was detected between factors.
* and ns indicate significance (p>.05) and nonsignificance horizontally.
30
LITERATURE CITED
Allen, G.S. and J.N. Owens.
1972.
The Life History of Douglasfir.
Can. For. Serv., Ottawa.
l39p.
Beckwith, R.C., and D.G. Burnell.
1982.
Spring larval dispersal of the western spruce budworm (Lepidoptera:
Tortricidae) in north-central Washington.
Envirn. Ento.
11:828-832.
Björkman, 0.
1973.
Comparative studies on photosynthesis in higher plants.
Photophysiol.
8:1-63.
Boardman, N.K. 1977.
Comparative photosynthesis of sun and shade plants.
Ann. Rev. Plant Physiol. 28:355-377.
Burbott, A.J. and W.D. Loomis.
1969.
Evidence of metabolic turnover in peppermint.
Plant Physiol. 44:173-179.
Carolin, V.M. and F.W. Honing.
1972.
Western Spruce Budworm.
USDA Forest Pest Leaflet, 53.
8p.
Chew, F.S. and J.E. Rodman.
1979.
Plant resources for chemical defense.
In: Herbivores: Their Interaction with
Secondary Plant Metabolites, G. Rosenthal and D.H. Janzen
(eds.).
pg 271-307.
Chung, H. and R.L. Barnes.
1980.
Photosynthate allocation in
Pinus taeda, II.
Seasonal aspects of photosynthate allocation to different biochemical fractions in shoots.
Can. J. For. Res.
10:338-347.
Croteau, R., A.J. Burbott and W.D. Loomis.
1972.
Biosynthesis of mono- and sesqui-terpenes in peppermint.
Phytochem.
11:2937-2948.
Edit, D.C. and M.D. Cameron.
1971.
Delayed budbreak and spruce budworm survival.
Bi-Monthly Res. Notes. 27:28-29.
Edit, D.C. and H.G. MacGillivray.
1972.
Resistance of seven fir species to spruce budworm and other insects.
Bi-Monthly Res. Notes.
28:2-3.
Emmingham, W.H. and R.H. Waring.
1977.
An index of photosynthesis for comparing forest sites in western Oregon.
Can.J. For. Res. 7:165-174.
Firmage, D.H.
1981.
Environmental influences on the monoterpene variation in Hedeoma drummondii.
Biochem.
System. and Ecol.
9:53-58.
31
Flück, H.
1963.
Intrinsic and extrinsic factors affecting the production of secondary plant products.
In: Chemical
Plant Taxonomy, 1. Swain (ed.).
Assoc. Press.
pg 167-186.
Gerhold, H.D., and R.E. McDermott, E.J. Schreiner and J.A.
Winieski, eds.
1966.
Breeding Pest Resistant Trees.
Pergamon Press, London, 5O5p.
Gordon, J.C. and P. Larson.
1968.
Seasonal course of photosynthesis, respiration and distribution of Cl4 in young Pinus resinosa trees as related to wood formation.
Plant Physiol. 43:1617-1624.
Greenbank, D.0.
1963.
Hàst species and the spruce budworm.
In: Dynamics of Epidemic Spruce Budworm Populations,
R.F. Morris (ed.).
Memoirs of the Ent. Soc. of Can. No.
31. pg 219-223.
Helms, J.A.
1964.
Apparent photosynthesis of Douglas-fir in relation to silvicultural treatment.
For. Sci. 10:432-442.
Hermann, R.K.
1977.
Growth and production of tree roots: a review.
In: The Below Ground Ecosystem: A Synthesis of
Plant-Associated Processes, J.K. Marshall (ed.).
Range
Sci. Dep. Sci. Ser. No. 26.
Colorado State Univ., Fort
Collins.
pg 7-28.
Heron, R.J.
1965.
The role of chemotactic stimuli in the feeding behavior of spruce budworm larvae on white spruce
Can. J. of Zool.
43:247-269.
Herout, V.
1970.
Some their iSoprenoids.
relations between plants, insects and
Prog. in Phytochem.
2:143-202.
Hull, C.H. and N.H. Nie.
and Facilities for
1981.
SPSS UPDATE 7-9; New Procedures
Release 7-9.
McGraw-Hill Co., New York.
402p.
Kogan, M.
1975.
Plant resistance in pest management.
In:
Introduction to Insect Pest Management, R.Metcalf and W.
Luckmann (eds.).
John Wiley and Sons, New York.
pg
103-146.
Krueger, K.W. and W.K. Ferrell.
1965.
Comparative photosynthetic and respiratory responses to temperature and light by Pseudotsuga menziesii var. menziesii and var. glauca seedlings.
Ecol.
4b:794-801.
Langenheim, J.H., S.P. Arrhenius and J.C. Nascimento.
1981.
Relationship of. light intensity to leaf resin composition and yield in the tropical leguminous genera Hymenaea and
Copaifera.
Biochem. Syst. Ecol.
9:27-37.
32
Lavender, D.P., G.B.Sweet, J.B. Zaerr and R.K.Hermann.
1973.
Spring shoot growth in Douglas-fir may be initiated by gibberellins exported from the roots.
Sci.
182:838-839.
Lyon, R.L., C.E. Richmond, J.L. Robertson and B.A. Lucas.
1972.
Rearing diapause and diapause-free western spruce budworm
(Choristoneura occidentalis) (Lepidoptera: Tortricidae) on an artificial diet.
Can. Ent. 104:417-426.
Maxwell, F.G. and P.R. Jennings.
1980.
Breeding Plants
Resistant to Insects.
John Wiley and Sons, New York. 683p.
Mooney, H.A. and C. Chu.
1974.
Seasonal carbon allocation in
Heteromeles arbutifolia, a California evergreen shrub.
OecoIogia I4:295-3Ob.
Perry, D.A. and G.B. Pitman.
1983.
Genetic and environmental influences in host resistance to herbivory: Douglas-fir and the western spruce budworm.
Z. ang. Ent. (in press).
Rhoades, D.F.
1979.
Evolution of plant chemical defenses against herbivores.
In: Herbivores: Their Interaction with Secondary Plant Metabolites, G. Rosenthal and D.H
Janzen (eds.).
pg 3-54.
Rhoades, D.F. and R.G. Cates.
1976.
Toward a general theory of plant antiherbivore chemistry.
In: Biochemical
Interactions between Plants and Insects, Wallace and
Mansell (eds.).
Vol 10:168-213.
Robertson, J.L.
1979.
Rearing the Western Spruce Budworm.
CANUSA pub., USDA-Forest Service, Wash. D.C.
l8p.
Shiroya, T., .G.R. Lister, V. Slankis, G. Krotkov and C.D.
Nelson.
1966. Seasonal changes in respiration, photosynthesis, and translocation of C14 labelled products of photosynthesis in young Pinus strobus L. plants.
Ann. Bot. 30:81-91.
Steel, R.G. and J.H. Torrie.
1980.
Principles and Procedures of Statistics.
McGraw-Hill, New York.
633 p.
Thomas, A.W.
1976.
Effects of temperature on emergence of second i-nstar spruce budworm larvae.
Can. For. Serv.
Inform. Rep., Maritimes For. Res. Centre.
M-X-60.
8p.
Tingey, W.M. and S.R. Singh.
1980.
Environmental factors influencing the magnitude and expression of resistance.
In: Breeding Plants Resistant to Insects, F. Maxwell and
P. Jennings (eds).
John Wiley and Sons, New York.
pg
87-113.
33
Webb, W.L.
1975.
The distribution of photoassimilated carbon and the growth of Douglas-fir seedlings.
Can. J. For.
Res.
5:68-73.
Webb, W.L.
1977a.
Rates of current photosynthate accumulation in roots of Douglas-fir seedlings: seasonal variation.
In: The Below Ground Ecosystem: a Synthesis of Plant-
Associated Processes, J.K. Marshall (ed.).
Range Sci.
Dep. Sci. Ser. No. 26.
Colorado State University,
Fort Collins.
pg 149-152.
Webb, W.L.
1977b.
Seasonal allocation of photoassimilated carbon in Douglas-fir seedlings.
Plant Physiol.
60:320-322.
Woodman, J.N.
1979.
Variation of net photosynthesis within the crown of a large forest-grown conifer.
Photosynthetica
5:50-54.
Wright, L.C., A.A. Berryman and S. Gurusiddaiah.
1979.
Host resistance to the fir engraver beetle, Scolytus ventralis
(Coleoptera: Scolytidae).
4.
Effect of defoliation on wound monoterpene and inner bark carbohydrate concentrations.
Can. Ent.
111:1255-1262.
APPENDIX
LITERATURE REVIEW
The western spruce budworm (Choristoneura occidentalis) was established as a new distinct species in 1967 by Freeman, separating it from the eastern counterpart C. fumiferana
Clemens known since 1947 (Freeman, 1967).
This herbivore is a major defoliator of western coniferous forests, feeding primarily on Douglas-fir (Pseudotsuga menziesii var. glauca
(Beissn.)Franco), the true firs (Abies spp.) and Engelmann spruce (Picea engelmannii Parry) (Carolin and Honing, 1972).
In the western United States, defoliation doubled in 1982 infesting over 3.6 million hectares(ha) of forested land, and in eastern Oregon alone, visible defoliation increased fron
127,000 ha in 1981 to 606,000 ha in 1982 (Kucera and Taylor,
1983).
Epidemic populations seriously reduce the leaf area within a forest stand, causing losses in radial and height growth increments, top kill, stem deformity and a general weakening of the physiological state of the tree (Thomson,
1979; Furniss and Carolin, 1977; Shepherd etal., 1977).
The latter effect is known to predispose forests to attacks by other herbivores (i.e. bark beetles) and pathogens (i.e. root rots) (Wright etal., 1979; Mattson and Addy, 1975;
Schoenewei ss, 1975).
Spruce budworms lay eggs in late suniiier on host needles which hatch in about ten days.
First instar larvae disperse, spin hibernacula in bark crevices, moult to the 2nd-instar and
35
36 enter diapause (Furniss and Carolin, 1977).
Diapause emergence occurs after low winter temperatures satisfy the cold requirement, followed by a sufficient number of degree-days in the spring where solar radiation warms both air and needle temperatures (Thomas, 1976; Cameron etal., 1968; Rose and
Blais, 1954).
Larval emergence is normally synchronized with bud expansion if spring temperatures warm the soil and air microclimates gradually.
Tree phenology can then determine a species susceptibility to budworm damage, where a delayed budflush relative to larval emergence in spring, will significantly reduce mining and defoliation of the current years foliage (Edit and MacGillivray, 1972; Edit and Cameron,
1971; Edit and Little, 1970; Greenbank, 1963).
In Douglas-fir, the initiation of budflush has been shown to depend on both soil and air temperatures, where a delay in bud break occurs if soil temperatures remain low, even when the surrounding air is warm and favorable for growth (Lavender et al., 1973; Cleary and Waring, 1968).
It is highly probable then, that if the spring season exhibits unusually early periods of warm sunny days, raising ambient air temperatures before soils can heat up, larvae will be cued to emerge before buds begin their flush.
This asynchrony was observed by
Beckwith and Burnell (1982) and Edit and Little (1970) who found a large proportion of the post diapause 2nd-instar larvae mining old needles after a warm period in mid-April.
Since soils were still cold, buds remained dormant and inactive,
37 forcing the larvae to find another food source.
They also suggest that larvae may be dispersing to alternate hosts experiencing a more advanced stage of bud development.
Mortality rates are expected to increase when the host-insect phenologies are divergent, due to poor suitability of mature foliage, inadequate nutritional levels in the imature alternate host foliage, increased exposure time to predators while searching, and abiotic climatic factors endangering the dispersing larvae.
Foliar Defenses
Although plants have evolved numerous mechanisms of defense to reduce the severity of defoliation by herbivores, the most diverse and widespread strategy involves the allelochemic effects of certain secondary metabolites (Rhoades and Cates, 1976; Whittaker and Feeny, 1971).
Allelochemics are chemicals produced by one species which affect the survival, growth, vigor, behavior and/or population biology of another species (Reese, 1979; Whittaker and Feeny, 1971).
Defensive chemicals are normally divided into two large catagori es: quantitative digestibility-reducers or qualitative metabolic toxins (Cates and Rhoades, 1977; Feeny, 1976; Rhoades and Gates, 1976; Feeny, 1975).
Quantitative defenses can be either protein or carbohydrate complexing agents and include primarily condensed and hydrolyzable tannins (Rhoades, 1979;
Swain, 1979; Feeny, 1976).
Tannins reduce the nutritional availability of soluble proteins and polysaccharides, and are
38 capable of lowering digestive enzyme activity within the herbivore gut (Feeny, 1969; Goldstein and Swain, 1965; Pridham,
1963).
This is accomplished by forming covalent or phenolic hydroxyl-hydrogen bonds with either leaf or enzyme proteins, preventing breakdown into dietary nitrogen (i.e. amino acids) and carbon skeletons needed for conversion into insect tissues
(Swain, 1979; Feeny, 1970; Feeny, 1969).
Since tannins are normally sequestered in cellular vacuoles, when an insect attempts to chew on the leaf surface, cells are crushed thereby destroying compartmentalizatf on.
Tannins are released as the foliage is being ingested and bonds to any available protein
(plant or insect origin), and eventually causing starvation, death and/or a severe reduction in insect fitness (Feeny, 1970).
Qualitative defenses, however, contain a more diverse array of chemicals including monoterpenes, acetates, alcohols, phenols, alkaloids, cyanogenic glycosides and others (Cates and
Alexander, 1982; Rhoades and Cates, 1976).
These chemicals are toxins, attacking metabolic processes, disrupting insect physiology and often killing the non-specialist herbivore
(Feeny, 1976; Feeny, 1975).
Conifers are known to contain both tannins and toxins in a multifaceted defense strategy within both foliage and phloem resin ducts.
Seasonal changes in the accumulated levels of defense have been reported for many species.
Probably the most well known study was done by Feeny (1970) who observed steadily increasing levels of tannins in Quercus leaves from bud flush to maturity
39
This directly corresponded to a decreasing palatability of the foliage to the winter moth (Operophtera brumata), who have a synchronized life cycle with the time of early spring bud flush.
After mid-summer, when leaves are mature, larval vigor severely declines with a subsequent increase in larval mortality.
Numerous investigations involving spruce budworms indicate the same pattern.
Perry and Pitman (1983) reported current years foliage of Douglas-fir seedlings caused little mortality to larvae feeding on needles collected through the end of summer.
One year old foliage, however, was much less suitable for budworm growth and development revealing toxicity levels of varying degrees.
Studies where phenologies of coniferous budworm hosts are being observed, show that larval fitness and survival are significantly reduced when forced to feed on old foliage due to delayed bud break (Beckwith and Burnell, 1982;
Edit and MacGivillray, 1972; Edit and Cameron, 1971; Edit and
Little, 1970; Greenbank, 1963).
Heron (1965) found mature white spruce needles to have less total sugars and proline, but more glucosidic toxins.
These needles were less acceptable in preference tests compared to other tissues.
The seasonality of foliar defenses as maturity approaches can determine insect phenologies, leading to feeding behaviors coincident with their preferred hosts bud flush.
This in turn, may have the potential for genetic selection of trees with a
40 postponed budflush.
The relative level of toxicity or palatability of the mature foliage can influence population dynamics of the budworm, and knowledge of how these levels can be manipulated may affect susceptibility of forest stands.
Costs of Allelochemic Production
The evolution of allelochemic agents must depend on a balance between natural selection and the metabolic costs of synthesis (Whittaker and Feeny, 1971).
In addition, the overall energy budget of an individual plant will determine to what extent these resources are allocated to foliar defense.
Polyphenolic tannins and carbon-rich terpenoids require a substantial amount of energy to produce along their biosynthetic pathways (Chew and Rodman, 1979; Rhoades, 1979; Wright et al.,
1979).
These defensive compounds are derived from carbon precursors and energy needed in primary metabolism.
For example, acetyl Co-A, glycolytic intermediates, ATP and
NADPH2 are used in biosynthesis, and thus priorities must exist at any given time to relieve the competition (Wright et al., 1979; Harborne, 1977; Mooney and Chu, 1974).
Wright et al.calculated 90 molecules of ATP must be sacrifised or diverted from other physiological areas inorder to synthesize one molecule of monoterpene.
They include all glucose and energy lost or used from the start of the Krebs cycle to the end of the mevalonic acid pathway.
Tannins have heavier molecular weights than toxins, typically containing 60 carbon atoms (i.e. tetrameric
41 procyanidin) (Swain, 1979) which could require two to six times more energy to make than other metabolites.
In addition to production costs, there are sequestration costs necessary to avoid autotoxicity, which include vacuole construction and other components to maintain an energy gradient for membrane transport within and between vacuoles(Fowden and Lea, 1979; McKey, 1974).
Given that overall costs are high, how is a plant able to allocate the available resources at any one time.
The amount of photosynthates accumulating in the resource pool must play a major role, and the subsequent changes in this pooi due to environmental inadequacies should directly affect a plants foliar defensive level.
Importance of Photosynthesis
Douglas-fir is classed intermediate to intolerant in its ability to withstand shaded conditions (Baker, 1950).
This species coninonly grows under high light intensities in its native habitat (sun species), thus will have a high capacity for photosynthesis at a saturating light intensity (Kramer and
Kozlowski, 1979; Boardman, 1977; Rabinowich, 1951).
The light compensation point where net photosynthesis begins, is consequently higher for these sun species, requiring more light to activate the photosynthetic system (Bohning and Burnside,
1956).
It is well documented that sun species increase their photosynthetic rates as radiation intensity is elevated (see numerous refs. in Boardman, 1977).
Studies directed
specifically at Douglas-fir show the same general pattern
(Brix, 1967; Krueger and Ferrell, 1965; Krueger, 1963).
The increase in photosynthetic activity will enlarge the pool of accumulating photoassimilates within plant tissues.
This should enhance the production of secondary substances due to the abundance of carbon and energy biosubstrates.
As a corollary, any reduction in light intensity resulting from partial shade, will lower photosynthetic activity and restrict the pool of available photoassimilates for allelochemic production.
Defenses will then suffer, increasing susceptability to insect attack.
Agricultural crop testing of resistant varieties under shaded conditions invariably resulted in lower resistance to both pathogen and insect attack (Fluck, 1963; Yarwood, 1959;
Loomis etal., 1957; Virtanen etal., 1957; Gaumann, 1950;
Bawden and Roberts, 1948).
Read (1968) also found lower disease resistance in pines under shaded conditions.
Langenheimetal., (1981) tested the effects of shade on resin quantity in tropical legume trees and found significant reductions in yield with decreasing light intensity.
They also found the same pattern when comparing leaves from the shaded lower crown, with leaves from the crown extending above the forest canopy in full sun.
Firmage (1981) tested the effects of low light conditions on a weedy herbaceous annual and found total resin oil per milligram leaf tissue was significantly reduced.
The lower yields of resins in low light environments
42
43 support the findings of Croteau etal. (1972) who found monoterpene synthesis occurs at energy-poor sites.
In addition, terpene synthesis accelerated when photosynthates, such as sucrose, were abundant.
Burbott and Loomis (1969) reported that monoterpene degradation and turnover occurs in peppermint, and when photosynthates are limited, turnover increases thereby releasing carbon and energy into primary metabolic pathways.
Although most investigations were conducted on herbaceous annuals, the overall mechanism and course of events in photosynthesis can be expected to occur in coniferous trees classified as "sun species".
Within forests, low light ibtensity is comon, resulting from unmanaged densely stocked stands.
Tree susceptability to insect attack may then be higher in these stands, due to lower levels of secondary chemical production and/or increased catabolic turnover of existing terpenoid constituents.
Carbon Allocation and Seasonal Patterns
Competitive demands for energy and carbon can not be met simultaneously throughout a plant, and it appears that a priority system exists at any given time of the year (Chung and
Barnes, 1980; Mooney and Chu, 1974; Loach and Little, 1973).
The source-sink concept developed by Wareing and Patrick (1975), describes the flux of materials from production centers
(sources) to the utilization centers (sinks) within a plant and season.
Depending on the overall abundance of photosynthates,
a proportion of available resources is diverted to the physiological organ establishing the greatest sink (Gordon and
Larson, 1968; Webb, 1977; Krueger and Trappe, 1967).
Spring shoot and needle expansion are the strongest sinks at that time of the year (Webb, 1977), where defenses and storage take low priority (Chung and Barnes, 1980; Mooney and
Chu, 1974).
During the development period, carbon is utilized primarily for synthesis of proteins and complex sugars (Mooney,
1972) during construction of new tissues.
When growth slows and differentiation processes predominate, the bulk of carbon and energy are diverted to the synthesis of essential oils, lignins for cell wall thickening and reproductive structures
(Loomis, 1932).
This is evident in the higher proportion of photosynthates allocated to phenolic compounds (protection) during the nongrowing season (Chung and Barnes, 1980; Mooney and Chu, 1974).
If resources are limiting at any point in time, due to a source constraint (i.e. low light, reducing photosynthetic activity), the relative amount normally allocated to a biochemical fraction during that season must also decline.
The overall result of low light intensity, then should be a reduction in seedling resistance due to a lower amount of finite resources available for defense (McLaughlin and
Schriner, 1980).
44
BIBLIOGRAPHY
Baker, F.S.
1950.
Principles of Silviculture.
McGraw-Hill,
New York.
399p.
Bawden, F.C. and F.M. Roberts.
1948.
Photosynthesis and the predisposition of plants to infection with certain viruses.
Ann. Appl. Biol.
35:418-428.
Beckwith, R.C.,, and D.G. Burnell.
1982.
Spring larval dispersal of the western spruce budworm (Lepidoptera:
Tortricidae) in north-central Washington.
Envirn. Ento.
11:828-832.
Boardman, N.K. 1977.
Comparative photosynthesis of sun and shade plants.
Ann. Rev. Plant Physiol. 28:355-377.
B3hning, R.H. and C.A. Burnside.
1956.
The effect of light intensity on rate of apparent photosynthesis in leaves of sun and shade plants.
Am. J. Bot. 43:557-561.
Brix, H.
1967.
An analysis of dry matter production of
Douglas-fir seedlings in relation to temperature and light intensity.
Can. J. Bot. 45:2063-2072.
Burbott, A.J. and W.D. Loomis.
1969.
Evidence of metabolic turnover in peppermint.
Plant Physiol. 44:173-179.
Cameron, D.G., G.A. McDougall and C.W. Bennett.
1968.
Relation of spruce budworm development and balsam fir shoot growth to heat units.
J. Econ. Ento.
61:857-858.
Carolin, V.M. and F.W. Honing.
1972.
Western Spruce Budworm.
USDA Forest Pest Leaflet, 53.
8p.
Cates, R.G. and H. Alexander.
1982.
Host resistance and susceptibility.
In: Bark Beetles of North American
Conifers; Mitton and Sturgeon (eds.).
Univ. Texas
Press.
527p.
Cates, R.G. and D. Rhoades.
1977.
Patterns in the production of antiherbivore chemical defenses in plant comunities.
Biochem. Sys. Ecol. 5:185-193.
Chew, F.S. and J.E. Rodman.
1979.
Plant resources for chemical defense. In: Herbivores: Their Interaction with
Secondary Plant Metabolites, G. Rosenthal and D.H. Janzen
(eds.).
pg 271-307.
45
46
Chung, H. and R.L. Barnes.
1980.
Photosynthate allocation in
Pinus taeda, II.
Seasonal aspects of photosynthate allocation to different biochemical fractions in shoots.
Can. J. For. Res.
10:338-347.
Cleary, B.D. and R.H. Waring.
1969.
Temperature: collection of data and its analysis for the interpretation of plant growth and distribution.
Can. J. Bot. 47:167-173.
Croteau, R.,' A.J. Burbott and W.D. Loomis.
1972.
Biosynthesis of mono- and sesqui-terpenes in peppermint.
Phytochem.
11:2937-2948.
Edit, D.C. and C.H. Little.
1970.
Insect control through host-induced asynchrony: a progress report.
J. Econ.
Ento. 63:1966-1968.
Edit, D.C. and M.D. Cameron.
1971.
Delayed budbreak and spruce budworm survival.
Bi-Monthly Res. Notes.
27:28-29.
Edit, D.C. and H.G. MacGillivray.
1972.
Resistance of seven fir species to spruce budworm and other insects.
Bi-Monthly Res. Notes.
28:2-3.
Feeny, P.
1969.
Inhibitory effect of oak leaf tannins on the hydrolysis of proteins by trypsin.
Phytochem. 8:2119-2126.
Feeny, P.
1970.
Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars.
Ecol. 51:565-581.
Feeny, P.
1975.
Biochemical coevolution between plants and their insect herbivores.
In: Coevolution of animals and
Plants; L. Gilbert and P. Raven (eds.).
Univ. Texas
Press.
pg 1-19.
Feeny, P.
1976.
Plant apparency and chemical defense.
In:
Biochemical Interaction Between Plants and Insects; J.
Wallace and R. Mansell (eds.).
Rec. Adv. Phytochem.
10:1 -40.
Firmage, D.H.
1981.
Environmental influences on the monoterpene variation in Hedeoma drummondii.
Biochem.
System. and Ecol.
9:53-58.
Flack, H.
1963.
Intrinsic and extrinsic factors affecting the production of secondary plant products.
In: Chemical
Plant Taxonomy, 1. Swain (ed.).
Assoc. Press.
pg 167-186.
47
Fowden, L. and P.J. Lea.
1979.
Mechanism of plant avoidance of autotoxicity by secondary metabolites, especially by nonprotein amino acids.
In: Herbivores: Their
Interaction with Secondary Plant Metabolites; G. Rosenthal and D. Janzen (eds.).
pg 135-160.
Freeman, T.N.
1967.
On coniferophagous species of
Choristoneura (Lepidoptera: Tortricidae) in North
Mierica.
I.
Some new forms of Choristoneura allied to
C. fumiferana.
Can. Ent.
99:449-456.
Furniss, R. and V.M. Carolin.
1977.
Western Forest Insects.
USDA-Forest Service Misc. Pub. 1339.
654p.
Gaumann, E.
1950.
Principles of Plant Infection.
W.B.
Brierley (transl.) London: Crosby Lockwood.
543p.
Goldstein, J.L. and 1. Swain.
1965.
The inhibition of enzymes by tannins.
Phytochem. 4:185-192.
Gordon, J.C. and P. Larson.
1968.
Seasonal course of photosynthesis, respiration and distribution of C14 in young Pinus resinosa trees as related to wood formation.
Plant Physiol. 43:1617-1624.
Harborne, J.B.
1982.
Introduction to Ecological Biochemistry.
Academic Press, London.
278p.
Heron, R.J.
1965.
The role of chemotactic stimuli in the feeding behavior of spruce budworm larvae on white spruce.
Can. J. of Zool.
43:247-269.
Kramer, P.J. and 1.1. Kozlowski.
1979.
Physiology of Woody
Plants.
Academic Press, New York.
811p.
Krueger, K.W.
1963.
Comparative photosynthesis and respiration rates of Douglas-fir seedlings from Vancouver
Island and Montana under various conditions of light and temperature.
PhD. Thesis, Oregon State Univ.
Corvallis,
Or.
80p.
Krueger, K.W. and W.K. Ferrell.
1965.
Comparative photosynthetic and respiratory responses to temperature and light by Pseudotsuga menziesii var. menziesii and var.
glauca seedlings.
Ecol.
46:794-801.
Krueger, K.W. and J.M. Trappe.
1967.
Food reserves and seasonal growth of Douglas-fir seedlings.
For. Sci.
13:192-202.
Kucera, D.R. and R.G. Taylor.
1983.
Spruce budworms situation in North America 1982.
Env. Canada, Misc. Pub. 1436.
22p.
48
Langenheim, J.H., S.P. Arrhenius and J.C. Nascimento.
1981.
Relationship of light intensity to leaf resin composition and yield in the tropical leguminous genera Hymenaea and
Copaifera.
Biochem. Syst. Ecol.
9:27-37.
Lavender, D.P., G.B.Sweet, J.B. Zaerr and R.K.Hermann.
1973.
Spring shoot growth in Douglas-fir may be initiated by gibberellins exported from the roots.
Sci.
182:838-839.
Loomis, W.E.
1932.
Growth-differentiation balance vs.
carbohydrate-nitrogen ratio.
Am. Soc. Hort. Sci.
29:240-245.
Loomis, R.S., S.D. Beck and J.F. Stauffer.
1957.
The European corn borer, Pyrausta nubilalis(Hubn.) and its principal host plant.
V.
A chemical study of host plant resistance.
Plant Physiol.
32:379-385.
Mattson, W.J. and N.D. Addy.
1975.
Phytophagous insects as regulators of forest primary production.
Sci. 190:515-522.
McKey, D.
1974.
Adaptive patterns in alkaloid physiology.
Nat.
108: 305-320.
Am
McLaughlin, S.B. and D.S. Shriner.
1980.
Allocation of resources to defense and repair.
Plant Disease, Vol.
5:407-431.
Mooney, H.A.
1972.
The carbon balance of plants.
Ann. Rev.
Ecol. System.
3:315-346.
Mooney, H.A. and C. Chu.
1974.
Seasonal carbon allocation in
Heteromeles arbutifolia, a California evergreen shrub.
Oecologia 14:295-306.
Perry, D.A. and G.B. Pitman.
1983.
Genetic and environmental influences in host resistance to herbivory: Douglas-fir and the western spruce budworm. Z. ang. Ent. (in press).
Pridham, J.B.
1963.
Enzyme chemistry of phenolic compounds.
Perg. Press, New York.
l42p.
Rabinowitch, E.I.
1951.
Photosynthesis and related processes.
Vol. 2: part 1.
New York: Interscience.
12O8p.
Read, D.J.
1968.
Some aspects of the relationship between shade and fungal pathogenicity in an epidemic disease of pines.
New Phytol.
67:39-48.
49
Rhoades, D.F.
1979.
Evolution of plant chemical defenses against herbivores.
In: Herbivores: Their Interaction with Secondary Plant Metabolites, G. Rosenthal and D.H
Janzen (eds.).
pg 3-54.
Rhoades, D.F. and R.G. Gates.
1976.
Toward a general theory of plant antiherbivore chemistry.
In: Biochemical
Interactions between Plants and Insects, Wallace and
Mansell (eds.).
Vol 10:168-213.
Reese. J.G.
1979.
Interactions of allelochemics with nutrients in herbivore food.
In: Herbivores: Their Interaction with Secondary Plant Metabolites.
G. Rosenthal and D.
Janzen(eds.).
pg. 309-330.
Rose, A.H. and J.R. Blais.
1954.
A relation between April and
May temperatures and spruce budworm larval emergence.
Can.
Ent.
86:174-177.
Schoeneweiss, D.F.
1975.
Predisposition, stress and plant disease.
Ann. Review Phytopath.
13:193-211.
Shepherd, R., J. Harris, A. VanSickle, L. Fiddick and L.
McMullen.
1977.
Status of western spruce budworm on
Douglas-fir in British Columbia.
Can. For. Serv. Rep.,
Pac.For. Res. Centre.
5p.
Swain, 1.
1979.
Tannins and lignins.
In: Herbivores: Their
Interactions with Secondary Plant Metabolites.
G.Rosenthal
and D. Janzen(eds.).
pg. 657-682.
Thomson, A.J.
1979.
Evaluation of key biological relationships of western budworm and its host trees.
Can.
For. Serv., Victoria, B.C.
BC-X-l86.
l9p.
Virtanen, A.I., A. Aura and T Ettala.
1957.
Formation of benzoxazolinone in rye seedlings.
Svom. Kern.
B30:240-245.
Wareing, P.F. and J. Patrick.
1975.
Source-sink relationships and the partition of assimilates in the plant.
In:
Photosynthesis and productivity in different environments.
J. Cooper(ed.).
IBP, Vol.
3:481-499.
Cambridge Univ. Press.
Webb, W.L.
1977.
Seasonal allocation of photoassimilated carbon in Douglas-fir seedlings.
Plant Physiol.
60:320-322.
Whittaker, R.H. and P.P. Feeny.
1971.
Allelochemics: Chemical interactions between species.
Sci.
171:757-770.
Wright, L.C., AA. Berryman and S. Gurusiddaiah.
1979.
Host resistance to the fir engraver beetle, Scolytus ventralis
(Coleoptera: Scolytidae).
4.
Effect of defoliation on wound monoterpene and inner bark carbohydrate concentrations.
Can. Ent.
111:1255-1262.
Yarwood, C.E.
1959.
Predisposition.
In: Plant Pathology.
J. Horsfall and A. Diamond(eds.).
Vol. 1:521-562.
50