Tetrahedron Letters,Vol.25,No.21,pp 2253-2254,1984 0040-4039/84 $3.00 + ... Printed in Great Britain
advertisement
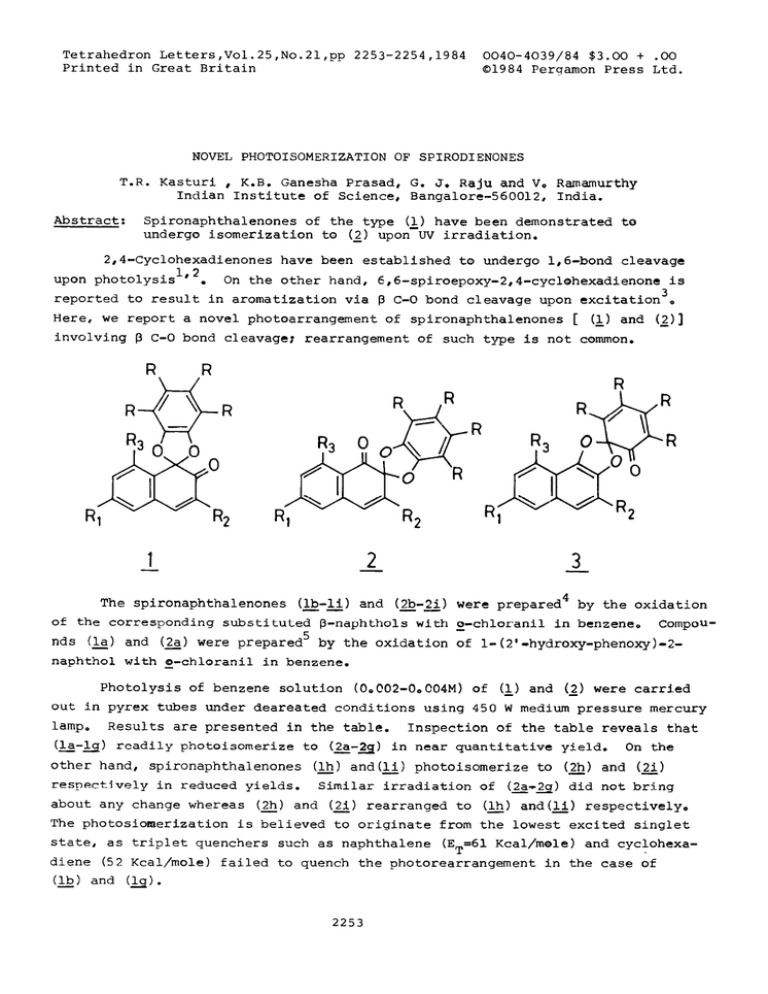
Tetrahedron Letters,Vol.25,No.21,pp 2253-2254,1984 Printed in Great Britain 0040-4039/84 $3.00 + .oo 01984 Perqamon Press Ltd. NOVEL PHOTOISOMERIZATION OF SPIRODIENONES T.R. Kasturi , K.B. Ganesha Prasad, G. J. Raju and Vo Ramamurthy Indian Institute of Science, Bangalore-560012, India. Abstract: Spironaphthalenones of the type cl_)have been demonstrated te undergo isomerization to (2) upon UV irradiation. 2,4-Cyclohexadienones have been established to undergo l,ó-bond cleavage upon photolysis 1,2 . On the other hand, 6,6-spiroepoxy-2,4-cyclehexadienone is reported to result in aromatization via B C-O bond cleavage upon excitation3* Here, we report a novel photoarrangement of spironaphthalenones [ (&) and <2)] involving B C-O bond cleavaget rearrangement of such type is not common. -1 -2 -3 The spironaphthalenones (Ib-li) and (2b-21) were prepared4 by the oxidation --of the corresponding substituted P-naphthols with o-chloranil in benzene, Compounds (la) and (2a) were prepared5 by the oxidation of l-(2'-hydroxy-phenoxyl-2naphthol with o-chloranil in benzene. Photolysis of benzene solution (OoC02-0o004M) of <&) and (2) were carried out in pyrex tubes under deareated conditions using 450 W medium pressure mercury lamp. (&-ti> Results are presented in the table. readily photoisomerize to (&-a) Inspection of the table reveals that in near quantitative yield. On the other hand, spironaphthalenones (lh) and(_) photoisomerize to (2) and (2) respectively in reduced yields. Similar irradiation of (2a+2q) did not bring - about any change whereas (2) and (21) rearranged to (lh) and(li) respectively, The photosiomerization is believed to originate from the lowest excited singlet state, as triplet quenchers such as naphthalene (ET=61 Kcal/mole) and cyclohexa- diene (52 Kcal/mole) failed to quench the photorearrangement in the case of ll&) and Cls>. 2253 2254 Table extent of isomerization (X1 Isomeric spirodienones (&)d R = R2= R3= R=H (2) (2) + (&) c-1 and (&): 100 0 (2) and (2b)r R:= R2= R3= H: R=Cl - 100 0 c-1 and (&): Rl= R2=H; R3=Me; R=Cl 100 0 (0) and (_)t Rl= R2=H: R3= pri; R=Cl 90 0 (5) and (2_e)rRl=R2= but: R3=H1 R=Cl 100 0 (12) and (22): Rl=R3=H; R2= but: R=Cl 90 0 (9) 95 0 (12) and (2h)t Rl= OMe: R2= R3= H; R=Cl - 25 75 c-1 75 25 and (_Z): Rl=CN: R2= R3= H: R=Cl and (2i)r Rl= Brt R2= R3= H: R=Cl - The photorearrangement could be visualized as occurring through the intennediacy of spirocyclohexadienone of the type (3) formed by initial 8 C-O cleavage. Further work, to have a deeper insight into its mechanism is under progress. Acknowledqement: We wish to thank Mr. V. Ramesh for helpful discussions. One of us (K.B.G.) wish to thank the Department of Atomic Energy for financia1 assistance. Referentes: 1) G. Quinkert, Anqew, Chem. Internat. edno, 2. 1072 (1972); H. Hart, D. A. Dickinson and W, Y, Li, Tetrahedronyett., 2253 (1975)t G. Quinkert, Pure. Appl. Chem ., -35 285 (1973); G. Quinkert, Fo Cech, Eo Kleiner and D. Rehm, Anqew. Chem. internat. edn,, 18 557 (1979). 2) T. Sala and M.V. Sargent, 3. Chem. Soco, Perk, Trans. II 870 (1981). 3) H.D. Becker, T, Bremholt and E, Adler, Tetrahedron Lett. 4205 (1972). 4) T. R. Kasturi, T. Arunachalam and G, Subramanyam, J. Chem. SOcc (C) 1257 (1970); ToR. Rasturi and R. Sivaramakrishnan, Indian. J. Chem. 16B 668 (1978). 5) T. R. Kasturi, K. B. Ganesha Prasad and B, Rajasekhar, Indian Jo Chem., 21B 813 (1981)o (Received in UK 29 February 1984)




