Session #18: Homework Solutions
advertisement

Session #18: Homework Solutions
Problem #1
(a) In a diffractometer experiment a specimen of thorium (Th) is irradiated with
tungsten (W) L α radiation. Calculate the angle, θ , of the 4th reflection.
(b) Suppose that the experiment described in part (a) is repeated but this time the
incident beam consists of neutrons instead of x-rays. What must the neutron velocity
be in order to produce reflections at the same angles as those produced by x-rays in
part (a)?
Solution
(a) ν =
1
5
=
(74 − 7.4)2 R → λ = 1.476 × 10-10 m
36
λ
Th is FCC with a value of Vmolar = 19.9 cm3
∴
4
a3
=
NA
Vmolar
1/3
⎛ 4 × 19.9 ⎞
→ a= ⎜
⎟
⎝ 6.02 × 1023 ⎠
λ = 2d sinθ ; d =
= 5.095 × 10-8 cm
a
2
h + k2 + l2
4th reflection in FCC: 111; 200; 220; 311 → h2 + k2 + l2 = 11
λθ =
2a sinθ
h2 + k2 + l2
⎛ λ h2 + k2 + l2
→ = sin-1 ⎜
⎜
2a
⎝
⎞
⎛
⎞
⎟ = sin-1 ⎜ 1.476 11 ⎟ = 28.71o
⎜
⎟
⎟
⎝ 2 × 5.095 ⎠
⎠
(b) λneutrons = λ x-rays
λneutrons =
h
h
h
6.6 × 10−34
=
, ∴ v=
=
= 2.68 × 103 m / s
p
mv
mλ 1.675 × 10−27 × 1.476 × 10−10
Problem #2
A Debye-Scherrer powder diffraction experiment using incident copper (Cu) Kα
radiation gave the following set of reflections expressed as 2θ : 38.40°; 44.50°;
64.85°; 77.90°; 81.85°; 98.40°; 111.20°.
(a) Determine the crystal structure.
(b) Calculate the lattice constant, a.
(c) Assume that the crystal is a pure metal and on the basis of the hard-sphere
approximation calculate the atomic radius.
(d) Calculate the density of this element which has an atomic weight of 66.6 g/mol.
Solution
Follow the procedure suggested in lecture:
(1) Start with 2θ values and generate a set of sin2 θ values.
(2) Normalize the sin2 θ values by generating sin2 θn /sin2 θ1 .
(3) Clear fractions from the “normalized” column.
(4) Speculate on the hkl values that would seem as h2+k2+l2 to generate the sequence
of the “clear fractions” column.
(5) Compute for each θ the value of sin2 θ /(h2+k2+l2) on the basis of the assumed hkl
values. If each entry in this column is identical, then the entire process is validated.
(a) For the data set in question, it is evident from the hkl column that the crystal
structure is FCC (see table below).
(b)
λ2
4a2
=
λCu
Kα
sin2 θ
h2 + k2 + l2
= 0.0358,
= 1.5418 Å, ∴ a =
(c) In FCC,
2a = 4r, ∴ r =
1.5418
(4 × 0.0358)1/2
= 4.07 Å
2
× 4.07 Å = 1.44 Å
4
(d) Here we’ll use atomic mass and atomic volume.
ρ=
6.02 × 1023
m 4 atoms NA atoms
× (4.07 × 10−8 cm)3 = 10.15 cm3
;
∴ Vmolar =
=
3
4
V
V
a
molar
∴ ρ=
66.6 g/mol
10.15 cm3 / mol
= 6.56 g/cm3
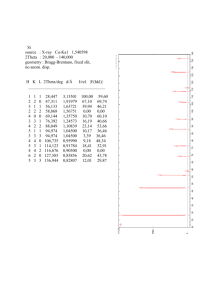
Data Reduction of Debye-Scherrer Experiment:
2
sin2
normalized
38.40
44.50
64.85
77.90
81.85
98.40
111.20
0.108
0.143
0.288
0.395
0.429
0.573
0.681
1.00
1.32
2.67
3.66
3.97
5.31
6.31
clear
fractions
3
4
8
11
12
16
19
(hkl)?
sin 2 h + k2 + l2
111
200
220
311
222
400
331
0.0360
0.0358
0.0359
0.0358
0.0358
0.0358
0.0358
2
Problem #3
The following diffractometer data (expressed as 2θ ) were generated from a
specimen irradiated with silver (Ag) Kα radiation: 14.10°; 19.98°; 24.57°; 28.41°;
31.85°; 34.98°; 37.89°; 40.61°.
(a) Determine the crystal structure.
(b) Calculate the lattice constant, a.
(c) Assume that the crystal is a pure metal and on the basis of the hard-sphere
approximation calculate the atomic radius.
(d) At what angle θ would we find the first reflection if, instead of Kα radiation, we used
silver L α radiation to illuminate the specimen?
Solution
We follow the same approach as described in the answer to Problem 2.
(a) See table below. It is evident that the crystal structure is BCC. Look at the hkl
column.
(b)
λ2
2
4a
=
sin2 θ
2
2
2
h +k +l
(c) In BCC,
= 7.53 × 10−3 , λ Ag
Kα
3a = 4r ∴ r =
(d) λ = 2 dhkl sin θ , dhkl =
λL given by: ν = λ-1 =
α
= 0.574 Å ∴ a =
0.574
4 × 7.53 × 10-3
= 3.31 Å
3
× 3.31 Å =1.43 Å
4
a
a
⎛ λ ⎞
=
∴ θ = sin-1 ⎜
⎟
2
⎝ 2a ⎠
h2 + k2 + l2
5 R(Z
36
- 7.4)2 =
5
36
× 1.1 × 107 (47 - 7.4)2 = 2.40 × 109 m-1
⎛ 4.17 ⎞
o
→ λ = 4.17 Å ∴ θ = sin-1 ⎜
⎟ = 63.0
⎝ 2 × 3.31 ⎠
Data Reduction of Diffractometer Experiment: incident x-ray AgK
2
sin2
14.10
19.98
24.54
28.41
31.85
34.98
37.89
40.61
0.0151
0.0301
0.0452
0.0602
0.0753
0.0903
0.1054
0.1204
normalize
d
1.00
1.99
2.99
3.99
4.99
5.98
6.98
7.97
clear
fractions
try again
1
2
3
4
5
6
7
8
2
4
6
8
10
12
14
16
α
→ λ =0.574 Å
hkl
110
200
211
220
310
222
321
400
103
sin h 2 + k 2 + l2
2
7.550
7.525
7.533
7.525
7.530
7.525
7.529
7.525
Problem #4
What is the maximum wavelength ( λ ) of radiation capable of second order
diffraction in platinum (Pt)?
Solution
The longest wavelength capable of 1st order diffraction in Pt can be identified on the
basis of the Bragg equation: λ = 2d sin θ . λmax will diffract on planes with maximum
interplanar spacing (in compliance with the selection rules): {111} at the maximum
value θ (90°). We determine the lattice constant a for Pt, and from it obtain d{111}.
Pt is FCC with a value of atomic volume or Vmolar = 9.1 cm3/mole.
Vmolar =
NA 3
a ; a=
4
3
9.1 × 10−6 × 4
= 3.92 × 10−10 m
NA
If we now look at 2nd order diffraction, we find 2 λ = 2d{111} sin90°
a
∴ λmax = d{111} =
3
=
3.92 × 10−10
3
= 2.26 × 10−10 m
Problem #5
What acceleration potential V must be applied to electrons to cause electron
diffraction on {220} planes of gold (Au) at θ = 5°?
Solution
We first determine the wavelength of particle waves ( λp ) required for diffraction and
then the voltage to be applied to the electrons:
λ = 2d{220} sin θ = 2
aAu =
λ=
3
4 × 10.2 × 10−6
6.02 × 1023
2 × 4.08 × 10-10
8
eV =
λP =
h
2meV
8
sin5°
= 4.08 × 10−10 m
sin5° =
mv2
, ∴ v=
2
h
=
mv
a
4.08 × 10−10
2
× 0.087 = 0.25 × 10−10 m = λp
2eV
m
, ∴ V=
h2
2λ2me
= 2415 V
Problem #6
How can diffraction on {110} planes of palladium (Pd) be used to isolate Kα
radiation from the “white” spectrum of x-rays emitted by an x-ray tube with a copper
(Cu) target? Rationalize your answer and provide an appropriate schematic drawing.
Solution
{110} planes of Pd cannot be used to isolate K α radiation from the x-rays emitted
by a tube with a Cu target. Pd has FCC structure and any reflection on {110} planes
are destructively interfered with by corresponding {220} planes, composed of
“center” atoms.
X
O
X
d(220)
O
O
X
O
X
X
O
X
X
O
X
X
X
X
X
X
O = corner atoms
X = center atoms
X
O
X
d(110)
O
X
O
d{220} =
1
1
d
→ Δx for {220} reflections = Δx for {110} reflections
2 {110}
2
MIT OpenCourseWare
http://ocw.mit.edu
3.091SC Introduction to Solid State Chemistry
Fall 2009
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.