Membrane Osmometry: Polymer Molecular Weight
advertisement
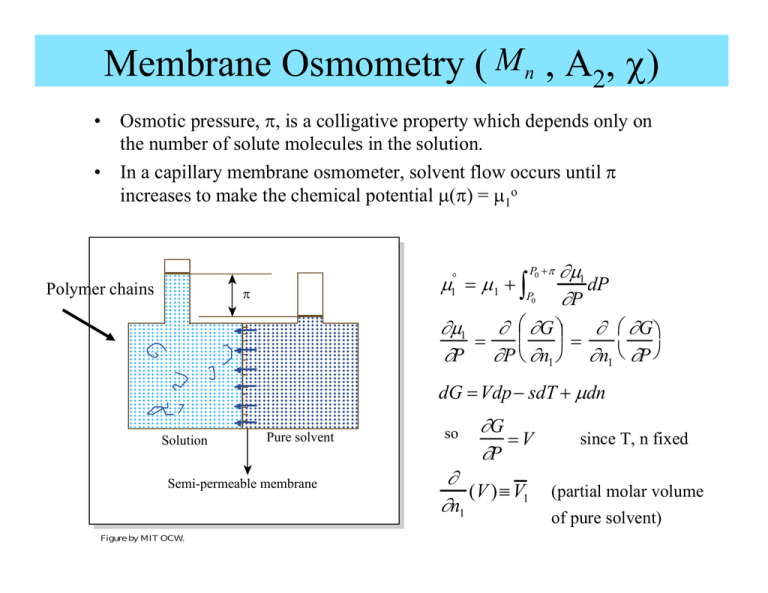
Membrane Osmometry ( M n , A2, χ) • • Osmotic pressure, π, is a colligative property which depends only on the number of solute molecules in the solution. In a capillary membrane osmometer, solvent flow occurs until π increases to make the chemical potential μ(π) = μ1o Polymer chains ∂μ1 dP ∂P ∂μ1 ∂ ⎜⎛ ∂G ⎞⎟ ∂ ⎛ ∂G ⎞ = = ∂P ∂P ⎝ ∂n1 ⎠ ∂n1 ⎝ ∂P ⎠ P0 + π μ = μ1 + ∫P ° 1 p 0 dG = Vdp − sdT + μdn Solution Pure solvent Semi-permeable membrane Figure by MIT OCW. so ∂G =V ∂P ∂ (V )≡ V1 ∂n1 since T, n fixed (partial molar volume of pure solvent) Membrane Osmometry cont’d P0 +π μ = μ1 + ∫P ° 1 0 V1 dP = μ1 +πV1 μ1 − μ1o = − πV1 Recall F-H expression for a dilute solution (φ2 small): ⎡ −φ 2 2⎤ μ1 − μ = RT⎢ +( χ −1/ 2) φ2 ⎥⎦ ⎣ x2 ° 1 Rearranging: ⎡ ⎛ φ2 ⎞ ⎛ 1 ⎛⎜ φ 22 ⎞ ⎤ ⎞ ⎟ + −χ π = RT ⎢ ⎜ ⎠ ⎝ V 1 ⎠ ⎥⎦ ⎣ ⎝ V1 x 2 ⎠ ⎝ 2 Since solution is dilute: recall φ2 ≅ V ≅ n1V1 so V1 ≅ V1 n2 x 2 n1 2 ⎡⎛ n2 ⎞ ⎛ 1 n ⎛ 2⎤ 2⎞ ⎞ π = RT ⎢⎝ ⎠ +⎝ − χ ⎠ ⎝ ⎠ V1x 2 ⎥ V 2 ⎣ V ⎦ The osmotic pressure is a power series ⎛n ⎞ in ⎜⎝ 2 ⎟⎠ i.e., π depends V on the number of molecules of solute per unit volume of the solution. Osmotic Pressure cont’d • • For a polydisperse polymer solution, n2 = Σ ni where ni is the number of moles i of polymer of molecular weight Mi in the solution. The number average molecular weight of the polymer is simply ∑ ni Mi m Mn = = ∑ ni n2 The concentration in grams/cm3 of the polymer in the solution is just Hence the osmotic pressure can be written ⎡ c m n2 Mn ⎛n c2 = = or ⎝ 2 ⎞⎠ = 2 V V V Mn • ⎞ V1 x 22 ⎤ 1 ⎛1 = RT⎢ +⎜ − χ⎟ 2 c2 ⎥ ⎝ ⎠ c2 Mn ⎦ ⎣ Mn 2 π The “reduced osmotic pressure” when plotted as a function of the solute (i.e. polymer) concentration will give a straight line with: Key Information: π c2 RT Intercept: Mn Slope: Three types of behavior: x x x χ < 1/2 (good solvent) x 2 1 2 2 n ⎛1 ⎞V x χ − ⎜ ⎟ ⎝2 ⎠M x x x x x x x x x x χ = 1/2 (θ condition) χ > 1/2 (poor solvent) c2 Osmometry cont’d ⎡ 1 ⎛1 ⎞ V1 x 22 ⎤ = RT⎢ +⎜ − χ⎟ 2 c2 ⎥ ⎝ ⎠ c2 Mn ⎦ ⎣ Mn 2 • At the θ condition, 2nd term disappears (recall χ = 1/2) and solution is ideal 2 π πV ≅ n RT • The slope of the reduced osmotic pressure yields a measure of the polymer segment-solvent F-H interaction parameter, ie we have a way to experimentally determine χ. • For gases, the pressure is often developed as a function of 2 3 concentration P = RT ( A1c+ A2c + A3c +...) • We can therefore identify the virial coefficients A1 and A2 as: A1 = 1 Mn ⎛1 ⎞ ⎜ − χ⎟ ⎝2 ⎠ A2 = 2 ρ 2V1 Comments about Osmometry 1. 2. We assumed mean field conditions - local environment is similar everywhere. We employed a dilute solution approximation with φ2<<1 . The requirements force the experimental conditions to be chosen such that: (1) System is “thermodynamically concentrated” c2 > c2* (2) System is “mathematically dilute” φ2 << 1 c2* is the so called overlap concentration; the polymer concentration at which the coils just begin to touch and hence the solution is reasonably uniform in composition (i.e. mean field situation, no large regions of pure solvent) c2* ≡ concentration at overlap ≅ c2 ≈ c * 2 M /NA mass of polymer molecule = 3 volume of coil ⎛ r 2 1/ 2 ⎞ ⎟ ⎜ ⎠ ⎝ c2 > c2* Schematic Polymer-Solvent Structure at Various c2 C Overlap Concentration Semi-Concentrated Solution Figure by MIT OCW. SEC/GPC (Size Exclusion Chromatography)/(Gel Permeation Chromatography) • SEC allows measurement of the entire molar mass distribution enabling all molecular weight averages to be computed for comparison with other techniques. Preparative GPC uses 25-50 mg of a polymer per run and allows one to fractionate polydisperse samples. • Apparatus: Set of columns (2-6) containing solvent swollen crosslinked PS beads. The beads are selected to have pore sizes in the range of 102 –105Å. A run consists of injecting 0.05 ml of a dilute solution (requires only ~0.1 mg of polymer !), then allowing the solution to transverse the columns and monitoring the eluting polymer molecules using various detectors. In order to “see” the molecules, one needs sufficient “contrast” between the solvent and solute (polymer). Three types of detectors are commonly used: – Ultra violet absorption – Infrared absorption – Refractive index changes • : : : UV Detector IR Detector RI Detector The solvent is chosen to be a good solvent for the polymer, to have a different refractive index from the polymer and to not have light absorption in the range for which the polymer is absorptive. Schematic of SEC Separation Process Swollen PS Xlinked Bead GPC Column Porous Interior of PS Bead Porous Gel Greatly magnified Magnified Cross linked PS beads in well packed column Diffusing chain PS chain network Pore Polymer molecules outside beads : 102 A - 105 A Figure by MIT OCW. Principles behind SEC involves… • Competition between 2 types of entropy 1. 2. • The interplay of both types of entropies results in substantially different residence times in the column for the different polymer molar masses. 1. 2. 3. • ΔSmix favorable entropy of mixing of polymer and solvent inside pores. ΔSconf unfavorable loss of conformation entropy of large size polymers (high MW) when entering smaller pores in the gel (column packed with swollen Xlinked PS beads). Mixing entropy gain drives smaller chains to enter and diffuse throughout entire gel Intermediate size chains access a portion (the larger pores) of the pore volume in the gel Conformational entropy loss prevents larger chains from entering the gel GPC is not an absolute method so it is necessary to calibrate the MW vs. elution volume curve using known narrow fraction samples of the same polymer in the same solvent at the same temperature. Normally researchers employ PS in THF at 23°C for PS-based calibration. Thus samples are referred to as eg “60,000 g/mol on a PS-basis,” meaning that the particular sample exited the column at an elution volume corresponding to a 60,000 g/mol PS sample going through the same column using THF at 23°C (a good solvent) as the carrier medium. A Simple GPC Chromatograph solvent carrier reservoir polymer solution of mixed Mi inject (typical flowrate ~ 1 cm3/min) GPC column(s): 4-6 columns in series packed column of 10μm diam. crosslinked PS gel beads pore size ~ 102Å – 105Å hν Concentration UV, IR, or RI signal Detector Preparative GPC (collect fractions of eluted solution) For a Polymer with Narrow Weight Distribution UV, IR or R.I. signal high MW low MW Elution volume → time → SEC cont’d • The volume eluted Ve consists of 2 parts: External to the Beads: “Void” Volume Vo (outside volume) Pore Volume Vi (inside volume) Internal to the Beads: • Depending on the size of the molecule passing through the column all or only a portion of the total volume is visited/explored (called pore permeation) by the diffusing molecules. pure solvent eluted volume Ves = Vo + Vi polymer solution eluted volume Vep = Vo + ViKse • where Kse is the size exclusion equilibrium constant. K se (x 2 ) ≡ c (x ) polymer concentration inside bead = 2i 2 polymer concentration outside bead c 2o (x 2 ) Kse = 1 for x1 = 1 solvent, i.e. no exclusion Kse = f(x2) < 1 for x2 > x1 Kse → 0, ie total exclusion from pores for x2→∞ ΔGpp = Go – Gi change in Gibbs free energy for pore permeation by polymer • Solvent and column material are chosen such ΔHpp of the polymer is = 0 Δ Gpp = -TΔSpp = -RT ln Kse ΔSpp is the change in entropy for pore permeation. This depends mainly on the relative sizes of the polymer chain and the pore. solving K = eΔS/R se SEC cont’d Furthermore, we can approximate: ⎡⎛ pore surface area ⎞ ⎤ ΔS pp = − R⎢⎜ ⎟ (diameter of chain)⎥ ⎣⎝ volume of pore ⎠ ⎦ here Vep Vep = Vo + ViKse Vep = Vo + Vi e −⎛⎜ As r 2 ⎝ 1/ 2 ⎞ ⎟ ⎠ is the elution volume of the polymer and As is the pore S/V ratio As depends on the pore/column and <r2>1/2 depends on solvent, T and the MW of that polymer chain entering the pore GPC Calibration Elution Volume vs. MW PS Calibration PS PI PB PVCH useful range of separation V0 + Vi log MW → last out Vep V0 first out Chain size (Molecular Weight) THF solvent Elution volume → Detector Sensitivity/Selectivity: An Example Polydispersity Index (PDI) GPC result 22.0 Response 17.6 PDI = 13.2 Mw Mn 2 Column GPC 8.8 PDI = 1.12, apparently a nice sample… 4.4 0.0 10.0 11.2 12.4 13.6 14.8 16.0 17.2 18.4 19.6 20.8 22.0 Retention Volume (ml) 6 Column GPC (high resolution) PDI >> 1.12!! [The Truth hurts!!] 38 39 40 41 42 43 44 45 46 47 Elution Volume (ml) Figure by MIT OCW. Note very different elution volume values Scattering of Radiation: Dilute Polymer Solution • M w , A2(χ) and <Rg2> (Zimm plot) • SALS (small angle light scattering) SAXS (small angle X-ray scattering) SANS (small angle neutron scattering) I0 r I0 Figure by MIT OCW. q= 4π λ no sin θ 2 Scattering Intensity from a Solution of Polymer Chains in Solvent • Geometry of apparatus: sample detector distance r, scattering angle θ • Optical constants and material properties: λ, n(or α), dn/dc2 • Thermodynamics of polymer-solvent system: c2, M w and A2(chi) Basic Scattering Equation 1 ⎡ 1 Kc2 ⎤ 2 .... = + + A c 2 2 ⎥⎦ ΔR(θ ) P(θ ) ⎢⎣ M w We will derive this. Note the nice set of variables…that we would like to be able to determine K = optical constants c2 = polymer concentration P(θ ) = particle scattering factor, known for various particle geometries ⎞ ⎛1 ⎜ −χ⎟ 2 A2 = ⎝ 2 ⎠ = 2 nd virial coefficient ρ 2 V1 ΔR(θ ) = excess Raleigh ratio = Rsolution − Rsolvent Light X-ray Neutron ~ excess scattering intensity Scattering arises from… (Δα)2 polarizability fluctuations (Δρ)2 electron density variations (Δb)2 neutron scattering length variation Scattering I: from Density Fluctuations A dilute gas in vacuum • Consider small particles: – (situation:~ point scatterers) dp << λradiation Particles dp Incident radiation λ • Scattered Intensity at scattering angle θ to a detector r away from sample: I 8π (1+ cos θ ) α = polarizability of molecule Iθ = α I0 = incident beam intensity λr • For N particles in total volume V (assume dilute, so no coherent 4 2 0 2 4 2 scattering) N Iθ = Iθ V ' ⎛N⎞ ε = 1 + 4 π⎜ ⎟ α ⎝V⎠ ε = dielectric constant ε = n2, ε(ω) = frequency dependent Fundamental relationship: index of refraction polarizability N n = 1+ 4π α V Can approximate n gas dn ≅ 1+ c dc dn = refractive index increment dc c = conc. of particles per unit volume 2 ⎛ dn ⎞ ⎛ dn ⎞ n = ⎜1 + c ⎟ ≅ 1 + 2⎜ ⎟ c + . . . ⎝ dc ⎠ ⎝ dc ⎠ 1 (dn / dc) c Solving gives α= 2 π (N /V ) the polarizability 2 gas So by analogy for a polymer-solvent solution: dn n ≅ n0 + c2 dc 2 n 2 ≅ n 02 + 2 n 0 dn c2 dc 2 Rayleigh and Molecular Weight of Gases 2 2 π 1+ cos θ ) ⎛ (dn / dc) c ⎞ 8 ⎛ ⎞ N ( ' Iθ = ⎜ ⎟ ⎜ ⎟ ⎝V ⎠ λ4 r 2 ⎝ 2π (N /V ) ⎠ simplifying 2 2 2 2 π 1+ cos θ I ⎛ ⎞ dn ( ) 2 Iθ' = 0 4 2 ⎜ ⎟c λ r (N /V ) ⎝ dc ⎠ 2 and since N c = V M / N AV This expression contains several parameters dependent on scattering geometry, so we define Iθ' R= I 0 (1+ cos 2 θ )/r 2 Rayleigh Ratio, R as 2 which equals ⎛ dn ⎞ 2π 2 ⎜ ⎟ M c ⎝ dc ⎠ R= λ4 N AV Or just Where K is a lumped optical constant ⎛ dn ⎞ 2π 2 ⎜ ⎟ ⎝ dc ⎠ K≡ λ4 N AV 2 R = K•M•c Note, for polymer-solvent solution: ⎛ ⎞ 2 dn 2 2π n 0 ⎜ ⎟ dc ⎝ 2⎠ K≡ λ4 N AV Rayleigh measured the molecular weight of gas molecules using light scattering! 2


