Lecture 27 Gibbs Free Energy and Phase Diagrams
advertisement

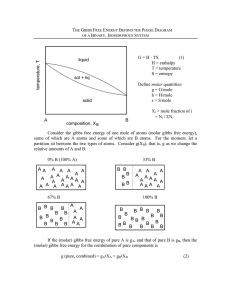
MIT 3.00 Fall 2002 181 c W.C Carter ° Lecture 27 Gibbs Free Energy and Phase Diagrams Last Time Interpretation of Gibbs Phase Rule Understanding Single Component Phase Diagrams Clausius-Clapeyron Equation Addition of a Soluble Species Consider the addition of a soluble species into the liquid phase (and suppose it is not very soluble at all in the solid phase) then Question: Will the Gibbs free energy of the liquid phase increase or decrease as it dissolves a soluble species? This is illustrated in the following figure: MIT 3.00 Fall 2002 182 c W.C Carter ° — G (molar Gibbs free energy) P =constant — solid G ∆T (Freezing point depression) — ∆Gmixing — liquid G (pure) — liquid G (solution) Tmelting (solution) Tmelting (pure) T (Temperature) Figure 27-1: Illustration of the freezing point depression when the molar Gibbs free energy of the high temperature phase is reduced. Thus we see that a soluble species in the liquid leads to “freezing point depression.” This is the reason that roads get salted when they get icy—and the reason that old-timers used to add salt to ice-water when making ice cream.