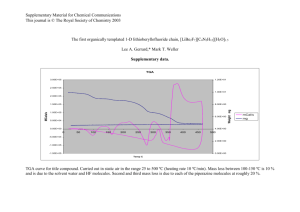
Evaluation of the microbial adhesion to hydrocarbon assay applied to... bacterium
advertisement

Evaluation of the microbial adhesion to hydrocarbon assay applied to a hydrocarbon degrading bacterium by Lawrence Otto Schmidt A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Environmental Engineering Montana State University © Copyright by Lawrence Otto Schmidt (1995) Abstract: The outer surface of microbial cells contain a variety of chemical compounds which may be involved in the attachment of cells to surfaces. Hydrophobic/hydrophilic interactions play a large role in attachment, leading to the development of the concept of cell surface hydrophobic!ty as a measure of the tendency of a cell to attach to a surface. One of the most popular tests of this tendency is the Microbial Adhesion to Hydrocarbons (MATH) test. In a typical assay, a cell suspension is contacted with a small volume of hydrocarbon, and the removal of cells by the hydrocarbon is determined. The goal of this project was to evaluate the effects of test conditions and bacterial growth conditions on the results and precision of the MATH test using a single species of hydrocarbon degrading bacteria. Cells were grown under either carbon limited (C:N = 5:1) or nitrogen limited (C:N = 15:1) conditions, and MATH contact assays were performed at various agitation intensities, with various hydrocarbon volumes, and for various mixing times. In addition, the results of the assay were evaluated using light absorbance and using viable plate counts to determine cell removal due to the hydrocarbon. Appropriate negative controls were also run in the absence of hydrocarbon to account for wall effects in the test vessels. The results of the study were analyzed according to traditional methods, using percent removal and rate of removal, and compared to the literature. Additional statistical methods were used to evaluate the variance in results and to determine how much of the variance could be attributed to experimental test factor values versus how much was due to inherent variability in the assay. In general, reproducibility was found to be poor, owing perhaps to the strong tendency of the test organism to form clumps in aqueous solution. Multiple linear regression models and analysis of variance (ANOVA) produced p-values for the factors that often showed statistical significance, but the actual effects of the test conditions were found to be negligible from a practical standpoint. Only the agitation rate was found to be significant according to all test methods: at low agitation rates, significantly lower removals from aqueous phase were observed than at high rates. - 'I "I EVALUATION OF THE MICROBIAL ADHESION TO HYDROCARBON ASSAY APPLIED TO A HYDROCARBON DEGRADING BACTERIUM by Lawrence Otto Schmidt A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Environmental Engineering MONTANA STATE UNIVERSITY Bozeman, Montana April 1995 © COPYRIGHT by Lawrence Otto Schmidt 1995 All Rights Reserved U%ni & A .5 4 7 APPROVAL of a thesis submitted by Lawrence Otto Schmidt This thesis has been read by each member of the thesis committee and has been found to be satisfactory regarding content, English usage, format, citations, bibliographic style and consistency, and is ready for submission to the College of Graduate Studies. A^;/Zi m s Datey Chairperson, Graduate Committee Approved for the Major Department Z / Date Head, Major Department Approved for the College of Graduate Studies Date Graduate Dean "/ Ul STATEMENT OF PERMISSION TO USE . In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Montana State University, I agree that the Library shall make it available to borrowers under rules of the Library. If I have indicated my intention to copyright this thesis by including a Copyright notice page, copying is allowable only for scholarly purposes, consistent with “fair use” as prescribed in the U.S. Copyright Law. Requests for permission for extended quotation from or reproduction of this thesis in whole or in parts may be granted only by the copyright holder. Date iv TABLE OF CONTENTS PAGE LIST O F TA B LES ........................................................................................... vii LIST O F FIG U R ES ......................................................................................... viii A B ST R A C T ix ......................................................................................................... IN T R O D U C T IO N .............................................................................................. Surface Characteristics of Cells ................................... ......................... M A TH te st ............................................................................................... Consistency of the MATH test ............................................... Physical Factors ......................................................................... Physiological Factors ............................................................... Importance of the MATH test . ................................................. Goal of R esearch ................................................................................. H ypotheses .............................................................................................. I I 3 3 4 5 5 6 6 B A C K G R O U N D ................................................................................................. Cell Surface H ydrophobicity .......... ........... ....................................... Previous R esearch .............................................................................. . Measurement of Cell Surface H ydrophobicity ..................................... H ydrophobicity A ssays ..................... ....... ......................................... BATH and MATH Assays ......................... ........................... Contact A ngle M easurem ent .................................................. Hydrophobic Interaction Chromatography ............................. Salt A ggregation T est .............................................................. Tw o Phase P artition ............................... ................................ Binding of Molecular Probes ........ ........................................... A dhesion to H ydrophobic Surfaces ....................................... D irection o f Spreading ............................................................ Im age A nalysis ......................................................................... Physiological Effects on Cell Surface H ydrophobicity....................... ' G row th Phase ............................................................................. G row th T em perature ................................................................ Cell G eneration ......................................................................... Cell Type and Preparation ...................................................... Physical Factors Effecting the MATH test ........................................ C ontinued R esearch .............................................................................. Im portance of Research ........................................................... C om parable T ests .................................................;................... 8 8 11 12 13 13 14 14 15 15 16 16 16 17 18 18 19 19 19 20 21 21 22 V TABLE OF CONTENTS (continued) M E T H O D S .......................................................... P relim inary W ork .................................................................................. Iso la tio n ....................................................................................... S u b stra te ...................................................................................... C:N ratio calculations ................................................. N utrient M edium ...................................................................... Experim ental Design ............................................................................... M A TH A ssay ............................................................................ C:N R atio ............. Experim ental Conditions ........................................... A gitation R ate ............................ H ydrocarbon V olum e .............................................................. H ydrocarbon Screening ......................................................... Tim e of Mixing ........................................................................... E xperim ental Procedure ..................................................................... In o cu latio n ................................................................................ W ashing Procedure ............................................................... R esuspension o f Cells ........................................................... A ddition to T est Tubes ......................................................... A g ita tio n ....................................... Cell Enum eration Techniques ............................................... Cell E num eration ................................................................... A bsorbance M easurem ents..................................................... 24 24 24 25 26 26 27 27 27 28 28 29 29 30 30 30 31 32 32 33 33 34 34 R E SU L T S ............................. T est T ube O bservations ........................... P ercent Rem oval D ata .......... Results from Statistical A n aly sis........................................................... R egression A nalysis ............................................................... 36 36 42 48 48 D IS C U S S IO N .................................................................................................... A nalysis o f D ata ............. P ercent Rem oval ...................... K inetic A pproach ................................................................... G raphical A nalysis ................................................................ S tatistical A nalysis .............................................................. R egression ................................................................................ D ata Inclusion ........................................................... S ignificant Factors .................................................... p V alues .......................................... Significant Coefficients ............... V ariation Between Experim ents .......................................... Means and Standard E rror ................................... 50 50 50 51 57 59 59 60 61 61 63 64 65 vi TABLE OF CONTENTS (continued) C O N C L U S IO N ................................................................................................. 70 R E FE R E N C E S ................................................................................................. 71 A P P E N D IC E S .................................................................................................. A ppendix A .............................................................................................. Experim ental Results ............................................................ Appendix B ............................................................................................ Im age A nalysis Research ....................................................... j 75 76 77 95 96 LIST OF TABLES Table 1. 2. 3 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. C om position o f nutrient m edia ............................................................ Settings for Agitaiton rate, hydrocarbon volume and mixing tim e ......... Absorbance and Percent Removal for Three H ydrocarbons................. Composition of buffer solution .................................................................. Exam ple of a Typical Experim ent ....................................................... Absorbance Responses for Cells Cultured at a C:N ratio of 5 :1 ............ Cell Count Responses for Cells Cultured at a C:N ratio of 1 5 :1 ........... Absorbance Responses for Cells Cultured at a C:N ratio of 5 :1 ............ Cell Count Responses for Cells Cultured at a O N ratio of 1 5 :1 ........... Absorbance and Cell Count Data for Cells Cultured at a C:N ratio of 5:1 for Experiments with Interference........................ ......... Constant Terms and Coefficients for Responses ............................... Percent absorbance data showing the change from the lowest settings to the highest settings for absorbance and cell counts............. T he p values for six responses ......................................................... Constant terms and coefficients for responses ................. .................. Values for Interexperimental and Intraexperimental Error.................... Page 27 28 29 32 33 43 44 45 46 47 49 51 62 64 65 viii LIST OF HGURES Figure Page 1. Cells Within the Circle are at the Hydrocarbon-water Interface.................... 2. Test tube containing unmixed hydrocarbon and cell suspension 3. Control test tube after agitation. (Agitation at a setting of 60 for 40 sec.)...... 4. T est tube agitated w ith carbon ................................................................. 5. Control test tube. (Agitated at a setting of 60 for 20 s e c .) ........................ 6. Control test tube. (Agitated at a setting of 140 for 20 sec.) ..:................. 7. Test tube containing .2 ml of hydrocarbon. (Agitated at a setting of 60 for 20 seconds) ................................................................................................... 8. Test tube containing .2 ml of hydrocarbon. (Agitated at a setting of 140 fo r 20 seco n d s)............................................................................................... 9. Test tube containing I ml of hydrocarbon. (Agitated at a setting of 60 for 20 seconds) ................................................................................................... 10. Plots used to determine the removal rate (k) and removal coefficient (K) for cells grown in a culture containing a 5:1 C:N ratio............................. 11. Plots used to determine the removal rate (k) and removal coefficient (K) for cells raised with a 15:1 C:N ratio ..................................................... 12. Effects of agitation setting, hydrocarbon volume and time of mixing on the percent removal (ideal) for isolate growth at a 15:1 C:N ratio for a b so rb an ce ...................................................................................................... 13. Percent removal (ideal) at two mixing times with variation of agitation rate and hydrocarbon volume ................................................................... 14. Initial cell counts for cultures raised at 5:1 and 15:1 C:N ratio including the cell counts for interference d a t a ............................................................. 15. Responses In (Ao/A) and In(CoZC) vs hydrocarbon volume including the standard error o f the averages ............................................................ 16. Responses In(AoZA) and In(CoZC) vs agitation setting including the standard error of the averages ................................................................... 17. Responses in (AoZA) and In(CoZC) vs time of mixing including the standard error of the averages ................................................................... 37 38 38 39 39 40 40 41 41 52 54 57 59 61 67 68 69 IX ABSTRACT The outer surface of microbial cells contain a variety of chemical compounds which may be involved in the attachment of cells to surfaces. Hydrophobic/hydrophilic interactions play a large role in attachment, leading to the development of the concept of cell surface hydrophobicity as a measure of the tendency of a cell to attach to a surface. One of the most popular tests of this tendency is the Microbial Adhesion to Hydrocarbons (MATH) test. In a typical assay, a cell suspension is contacted with a small volume of hydrocarbon, and the removal of cells by the hydrocarbon is determined. The goal of this project was to evaluate the effects of test conditions and bacterial growth conditions on the results and precision of the MATH test using a single species of hydrocarbon degrading bacteria. Cells were grown under either carbon limited (C:N = 5 :1) or nitrogen limited (C:N = 15:1) conditions, and MATH contact assays were performed at various agitation intensities, with various hydrocarbon volumes, and for various mixing times. In addition, the results of the assay were evaluated using light absorbance and using viable plate counts to determine cell removal due to the hydrocarbon. Appropriate negative controls were also run in the absence of hydrocarbon to account for wall effects in the test vessels. The results of the study were analyzed according to traditional methods, using percent removal and rate of removal, and compared to the literature. Additional statistical methods were used to evaluate the variance in results and to determine how much of the variance could be attributed to experimental test factor values versus how much was due to inherent variability in the assay. In general, reproducibility was found to be poor, owing perhaps to the strong tendency of the test organism to form clumps in aqueous solution. Multiple linear regression models and analysis of variance (ANOVA) produced p-values for the factors that often showed statistical significance, but the actual effects of the test conditions were found to be negligible from a practical standpoint. Only the agitation rate was found to be significant according to all test methods: at low agitation rates, significantly lower removals from aqueous phase were observed than at high rates. I INTRODUCTION Measurements of cell surface hydrophobicity are made by a variety of methods. The MATH (microbial adhesion to hydrocarbon) test is potentially the most useful of the techniques. The MATH test is simple, fast and reasonably reproducible. The main drawback with the method is its lack of quantification. The MATH test needs to be tested for reliability and further the factors which influence the test the most should be determined. Surface Characteristics of Cells The biochemical composition of the outer surfaces of microorganisms strongly affects the nature and extent of their interaction with and attachment to substrata. Once a cell contacts a surface, a variety of processes maybe involved in microbial attachment (Kjellberg 1984). Structure-mediated processes can be initiated by extracellular polymers which project from the cell surface. These extracellular polymers attach to surfaces by two mechanisms: specific and nonspecific binding. Specific binding requires a complementary site on the binding surface to the extracellular polymer of the bacteria. Nonspecific binding involves macromolecules or extracellular polymers that interact with the surface of the substratum or macromolecules present on the surface (Group Report 1984). A number of possible nonspecific binding processes can occur including: ionic, dipolar, hydrogen bonding, and hydrophobic/hydrophilic surface interactions. Cell surface hydrophobicity describes cells with hydrophobic surface characteristics. The hydrophobic nature of these cells promotes attachment to substrates which may provide access to essential nutrients. Attachment of bacteria at the hydrocarbon- 2 water interface is a good example of a hydrophobic interaction. These bacteria are able to colonize surfaces that are unavailable to other cells, thus providing a competitive advantage in nutrient availability. The hydrocarbon-water interface is a unique environment which may offer protection for the bacteria from predators (Marshall 1976). These bacteria need to be able to detach from oil once all the long-chain n-alkanes they grow on have been utilized. Acinobactercalcoaceticus RAG -1 releases encapsulated emulsans which cause the bacteria to desorb from the hydrocarbon. The emulsan forms a hydrophilic polymeric film around the depleted oil droplet that hydrophobic bacteria cannot attach to (Rosenberg and Kaplan 1987). Hydrophobic organisms have been found in a variety of environments. In the medical field, studies on adhesion of bacteria to host tissues indicate that hydrophobicity is an important factor for the infection process. (Enzer and Douglas 1992). Another example in medicine is the persistence of bacteria which are able to adhere to plastics used in medical devices (Rosenberg and Doyle 1992). In the petroleum industry methods are needed for quick and cost effective removal of insoluble hydrocarbons. Bacteria that are able to adhere to the hydrocarbon-water interface may play an integral part in development of these methods (Goswami and Singh 1990). Chemical surfactants are used extensively in industry, agriculture and medicine. Surface-active compounds that aid in emulsification of the oil phase into the aqueous phase increase the interfacial area available for microbial contact (Atlas 1984,672); Advantages of natural surfactants over manufactured surfactants are their ease of biodegradation and their selectivity for a specific surface. Emulsan, for instance, is specific for a mixture of aliphatic and aromatic hydrocarbons. The use of Acinobaetercalcoaceticus RAG-1 to produce emulsan and other extracellular polysaccharides by fermentation processes is another area of research incorporating bacteria with hydrophobic surfaces (Shabtai and Wang 1989). 3 MATHTest Many researchers are working with bacteria that have hydrophobic character and would like to be able to compare the cell surface hydrophobicity of their organism to others. A quantitative measure of hydrophobicity should be able to predict the tendency of bacteria to attach to a hydrophobic surface. The MATH (microbial adhesion to hydrocarbon) test has been used extensively to screen bacteria for hydrophobic character by measuring the tendency of organisms to attach to a hydrophobic surface. This test was first developed in 1980 by Rosenberg as a possible quick and easy method to determine the hydrophobic character of cells. The test has developed to the point where some researchers believe that it is a quantitative measure of cell surface hydrophobicity (Lichtenberg et al. 1985). In a typical experiment, test tubes or cuvettes containing a cell suspension and hydrocarbon are agitated at a fixed rate for a given time period. The agitation of the solution allows the microorganisms to come into contact with the hydrocarbon. The phases are then allowed to separate, thfe lower aqueous phase is separated from the upper hydrocarbon phase and absorbance measurements are made on the aqueous phase. Removal of cells from the aqueous phase (as measured by change in absorbance) is then used as a measure of hydrophobicity. Consistency of the MATH Test In order for the MATH test to be useful, results and comparisons throughout a study must not be affected significantly by the results of error inherent in the MATH test. Correlations between the MATH test and other methods for determining hydrophobicity have been observed. A linear correlation was observed for Pasteurellamultiocida when 4 percent adhesion to hydrocarbon was compared to percent retained on octyl-Sepharose (Darnell et al. 1987). The result of another method comparison was a direct correlation between cell-surface hydrophobic!ty and adhesion to epithelial cells (Ener and Douglas 1986). Epithelial cells make up the epithelium, the cellular tissue covering surfaces, forming glands and lining most of the cavities in the body. Evaluation of the hydrophobicity of the following organisms, Enterobacteraerogenes, Klebsiella oxytoca, Saccharomyces carsbergensis and Kluyveromyces fragilis varied widely depending on the measurement technique (Moses and Rouxhet 1987). The MATH test proved to be the most inconsistent of those techniques. The MATH test does not always yield internally consistent results. For example. Sweet et al. (1987) state, “current methods of assessing bacterial hydrophobicity as a function of adherence to liquid hydrocarbons (especially hexadecane) do not always yield reproducible results.” Sweet etal. determined in their study that p-xylene should be used instead of hexadecane. Factors which affect the internal consistency of the MATH test are both physical and physiological. Physical Factors Physical factors which affect the MATH test include agitation rate, hydrocarbon volume, time of mixing and test tube size. The hydrocarbon volume will affect the surface area available for attachment, as will the agitation rate. More vigorous agitation may also increase the transport of cells to the interface. Time may also affect the results since the longer the cell suspension is in motion the more likely it is that a microbe will come into contact with the hydrocarbon. If the MATH test is to be internally consistent, the effects of these factors on response should be minimized. 5 Physiological Factors Physiological factors affect the growth of cells, subsequently causing changes in the chemistry of the cell surface. The carbon to nitrogen ratio is one such factor. A C:N ratio of 7.7:1 has been determined to support balanced growth and product synthesis (emulsan production) fox Acinetobactercalcoaceticus RAG-1 (Shabtai and Wang 1989). Carbon limited media will typically limit the ability of organisms to produce extracellular, materials particularly exopolysaccharide. Conversely, a high C:N ratio should produce extracellular materials which could lower the hydrophobic!ty of the cells. Other nutrient conditions that affect the MATH test are phosphate limitation, inorganic ions and dissolved organic matter. Culture conditions such as temperature, pH, and ionic strength can also affect the results of the MATH test. Last, generation variation between cultures may affect the surface properties and thus the cell surface hydrophobicity of cells. Importance of the MATH test For the MATH test to be useful, results and comparisons throughout a study must not be affected significantly by errors inherent in the MATH test. As the number of comparisons between data from the MATH test increases, it is important to recognize the limitations of this test. This study will evaluate the effects of mixing time, agitation intensity, hydrocarbon volume and growth conditions on the performance and precision of the MATH test. 6 Goal of Research The goal of this project is to evaluate the effects of test conditions and bacterial growth conditions oh the results and precision of the MATH test. The specific objectives are: 1) Evaluate the reproducibility of the MATH test with a single hydrocarbon­ degrading bacterial species. 2) Evaluate the effects of mixing intensity, mixing time and hydrocarbon volume on the test 3) Evaluate the effect of C:N ratio during growth of bacteria on the results of the MATH test. The following null hypotheses were established as a basis for statistical evaluation of the experimental results. Each of these hypotheses were tested using viable cell counts and light absorbance as measures of the cell concentration in the suspension. Hypotheses I) For a given set of experimental and bacterial growth conditions, the MATH test yields reproducible results. 2) The time of mixing in the assay does not significantly affect the results. 7 3) The mixing intensity does not significantly affect the results. 4) The hydrocarbon volume does not significantly affect the results. 5) The C:N ratio during growth of the bacteria does not significantly affect the results. 6) Cells are not removed from suspension in the test when hydrocarbon is not present. 8 BACKGROUND Cell Surface Hydrophobicitv The surface of many microbes can have a hydrophobic nature. This phenomenon promotes interactions such as partitioning of bacteria at liquiddiquid and liquid: air interfaces and adhesion of bacteria either to host tissue or to nonwettable solid surfaces (Marshall 1976). This property allows some bacteria to attach to hydrocarbons and use it as substrate, increasing cellular mass and population at the hydrocarbon: water interface (Rosenberg et al. 1982). Many other examples of microbial adhesion to hydrophobic surfaces exist, including adhesion to contact lenses, elemental sulfur, mineral particles, biomaterials and others thus emphasizing the importance of this phenomenon (Rosenberg and Doyle 1990). The term hydrophobicity is misleading, as “hydrophobicity” literally translates as “water aversion” and therefore contradicts the ability of many microbial cells to disperse readily in water. Dispersion in aqueous solutions allows microbes to come into contact with the desired surface, whether hydrophobic or hydrophilic in nature. Many hydrocarbon­ degrading organisms have specifically adapted to surviving and interacting with the insoluble substrates they use as their carbon source. The nature of bacterial attachment to hydrophobic surfaces or interfaces is a subject that has proved to be a source of dispute between physical chemists and microbiologists. At the Dahlem Konferenzen in 1984 a group of scientists made up of microbiologists and physical chemists formed a consensus on a number of definitions relating to mechanisms of attachment. The group agreed upon two basic mechanisms for bacterial attachment to hydrophobic surfaces: non-specific and specific binding (Marshall 1984). Non-specific binding involves electrostatic or hydrophobic interactions, while specific binding involves 9 complementary sites on the bacterial surface and the colonizable surface with high affinities for each other (Sweet et al. 1987). The complexity of defining and understanding the nature of microbial adhesion to surfaces demands further research. Microbial adhesion to hydrocarbons at the oil-water interface is a special case of microbial attachment to hydrophobic surfaces. In nature, microbial growth on waterinsoluble hydrocarbons requires direct contact between bacterial cells and the oil phase. The first step in hydrocarbon degradation typically involves oxygenase an intracellular enzyme. With hydrocarbons being very nonpolar, and thus insoluble in water, it follows that the microbe must come into direct contact with the nonaqueous phase. Microorganisms cannot grow inside the nonaqueous phase as they require water as well as soluble nitrogen and phosphorus. As a result, hydrocarbon-degrading bacteria grow at the hydrocarbon-water interface. There are four processes involved in biodegradation of hydrocarbons: adhesion, growth, desorption and surface renewal. Adhesion is the process by which the bacteria become attached to the water-petroleum interface. Hydrocarbon-degrading bacteria are able to adhere to the hydrocarbon through hydrophobic interactions. Thin fimbriae which extrude from the cell membrane allow hydrocarbon-degrading bacteria to adhere to the hydrocarbon. Three observations support this. First, mutants that lack thin fimbriae fail to adhere to the hydrocarbons, and grow poorly on the hydrocarbon substrates. Secondly, subjecting cells with thin fimbriae to high shear causes loss of cell surface hydrophobicity and the ability to adhere to hydrocarbons. Finally, revertants of nonadhering species regain the ability to adhere to hydrophobic surfaces (Rosenberg and Kaplan 1987). Following adhesion/adsorption, growth occurs at the hydrocarbon-water interface. In this stage there are strong intercellular interactions, as supported by the observation that desorption of cells from oil yields cell clumps. It is important to note that in initial growth, cells multiply at an exponential rate until the surface is covered. When the surface cell 10 population reaches a monolayer, the surface area becomes the limiting factor, and the biomass increases at an arithmetic rate. This indicates why the amount of hydrocarbon surface area to which microbes can attach limits degradation of hydrocarbons. There are two factors involved in extending the exponential growth phase: hydrocarbon emulsification, and the transfer to a new substrate. Hydrocarbon emulsification increases the surface area available for cell growth. When a 2 mm oil droplet is broken down to droplets of 10 jm \ diameter, a typical value for bacterial-induced emulsions, the surface area increases by 200 times. The interfacial tension between the bacterial-coated hydrocarbons and water is lower than between pure hydrocarbon and water. It is thought that emulsifying agents utilized by bacteria play a role in oil degradation at the microscopic level. Bacterial cells produce extracellular emulsans (polysaccharidecontaining emulsifiers) which adhere to hydrophobic compounds. These encapsulated emulsans are present on cell surface. When the hydrocarbon droplets useful substrates have been utilized the encapsulated emulsan is released. The released emulsan forms a polymeric film which plays a key role in desorption and surface renewal. The depleted oil droplet is covered by a film which now has hydrophilic character restricting attachment of hyrophobic bacteria (Rosenberg and Kaplan 1987). During the growth phase of the bacteria cells at the hydrocarbon-water interface, the emulsan is tightly bound to the cell. The emulsan capsule is released from the cell surface when starvation conditions occur. Then emulsan adsorbs avidly to the oil droplet, thereby displacing the cells to the aqueous phase. These “used” droplets are covered with a very stable monomolecular film of emulsan that prevents bacteria from reattaching to the depleted droplet (Inouye 1985). The fatty-acid ends are oriented towards the hydrophobic organic phase while the polar hydroxyl and carboxyl groups are faced towards the aqueous phase. The released capsule-deficient bacteria are free to attach to fresh substrate. 11 Previous Research The earliest reference to cell surface hydrophobicity was in Mudd and Mudd’s classic paper in 1924 in which experiments of bacterial attachment at the oil-water interface were studied. This research opened up a new field of research in microbial interaction with surfaces and interfaces. The study attempted to understand the physical-chemical factors involved in penetration of bacteria through epithelia cells of the animal body by first investigating the mechanisms of transport of a simpler system. Mudd and Mudd investigated the kinetic mechanism of bacterial transport at the interface between two imiscible fluids and postulated that the mechanism was dependent primarily upon the interfacial surface tension forces at the interface. Marshall and Cruickshank studied cell surface hydrophobicity and the orientation of certain bacteria (1972). Photographs portray Flexibacter CW7 cells at the hydrocarbon-water interface oriented perpendicular to the hydrocarbon surface (Marshall 1976). Another focus of the research was microbial oxidation of oil products, at first for development as a potential protein source, and later as a means of cleaning up after oil spills. One such study measured the interfacial area of hydrocarbon and its relationships to specific growth rates in yeast fermenters. This study (Wang and Ochoa 1972) determined that the specific growth rate is directly related to the specific hydrocarbon interfacial area. The MATH assay was developed in 1980 and proposed bacterial adhesion to hydrocarbon as a simple, general method for measuring cell surface hydrophobicity. The method was based on the percentage of adherent cells to various liquid hydrocarbons subjected to agitation for brief periods. It was also discovered that hydrocarbon degraders were not the only bacteria with the ability to adhere to the bulk hydrocarbon (Rosenberg, Gutnick, and Rosenberg 1980). During this time many different hydrophobic interactions 12 were studied, including adhesion to mineral surfaces, fish surfaces, oral tissue and to inert surfaces such as polystyrene (Rosenberg and Doyle1990). Research in the late 1980s and early 1990s continues to look at the adhesion of bacteria to different surfaces and the further development of quantitative measurement methods. During this time the MATH assay has been redesigned a number of times in an attempt to increase the reproducibility of the test results. A method that has proved to be successful in capturing the kinetics of surface colonization in dynamic systems is image analysis. Using the image analysis system Escher determined that sorption-related processes are a function of the bulk cell concentration and interface dynamics (Escher 1986). In 1990, Mueller looked at the adsorption process and the effects of different substrata on the sticking efficiencies of Pseudomonas aeruginosa and Pseudomonas fluorescens bacteria. The importance of the MATH test in determining cell surface hydrophobicities of mutant and nonmutant strains of Acinetobactercalcoaceticus RAG-1 and the role of emulsan in surface renewal cannot be understated. Through the use of MATH, Rosenberg was able to determine the roles played by the extracellar thin fimbreae and encapsulated emulsan. From this work a novel method for comparing cell surface hydrophobicities was produced and has proved to be among the most useful methods to date. Measurement of Cell Surface Hydrophobicitv Historically, measurements of cell surface hydrophobicity have been used to compare the relative affinities of bacteria for different surfaces or to compare the influence of different experimental conditions such as nutrient, media or growth phase effects. Some of these methods can be very time-consuming and labor-intensive, prohibiting their usefulness in many laboratory situations. The development of the MATH assay was a 13 breakthrough in regard to the relative ease of collecting data indicating a certain affinity of the bacteria toward the hydrocarbon-water interface. Unfortunately, this assay does not always agree with some of the other methods. Even when comparing different experiments using the MATH assay, the results are often hard to interpret. One such example is the case of A. viscous cells that adhere to hexadecane only after vortexing and not when mixed with gentle agitation (Rosenberg 1991). Rosenberg’s research in developing a simple test for cell surface hydrophobicity has undergone many alterations since he first published the method in 1980. Hydrophobicity Assays BATH and MATH Assays In his first paper Rosenberg described a simple method for determining cell surface hydrophobicity using BATH (bacterial attachment to hydrocarbon). This was later changed to MATH (microbial attachment to hydrocarbon) in part because of extensive use of the assay in studies of eucaryotic organisms (Rosenberg and Doyle 1990). The MATH assay utilizes the fact that many hydrophobic microbes suspended in buffer media, when agitated, will adhere to hydrocarbon and form emulsions at the hydrocarbon-water interface. Microbes in a suspension of buffer solution are placed in a test tube to which the hydrocarbon (hexadecane, octane or xylene) is added in varying amounts. The test tubes are vortexed for 120 seconds, after which the mixture is allowed to separate and the absorbance of the aqueous layer is measured. The absorbance is plotted against the hydrocarbon volume, thus revealing the affinity the bacteria have towards the hydrocarbon as a function of hydrocarbon volume (Rosenberg etal. 1980). Lichtenberg etal. (1985) proposed a kinetic approach for the MATH assay. In this procedure varying amounts of 14 hydrocarbon are added to the cell suspensions and are agitated for fixed consecutive time periods. Following each agitation the phases are allowed to separate and the absorbance of the aqueous layer is measured. By plotting the logarithm of the percentage of cells remaining in the aqueous phase as a function of time, the results yielded a linear relationship. The authors determined, “the slope of the curve is a quantitative expression of the affinity of the cells tested for the water: oil interface, and denote it as the removal coefficient (K) of the cells from the bulk aqueous phase by the hydrocarbon” (Lichtenberg etal. 1985). The test was further developed with the use of polystyrene cuvettes as the experimental vessel instead of test tubes (Sharon etal. 1986). It is noteworthy that in a scientific citation in 1988, a paper in which E. Rosenberg is a co-author, the use of the kinetic method is not employed (Pines etal. 1988). Contact Angle Measurement Contact angle measurement (CAM) is a standard method for studying hydrophobic surfaces. The method measures the surface free-energy of solid surfaces. CAM was first used in measuring the hydrophobic surface properties of bacteria in 1972 (van Oss and Gillman). In order to measure the contact area the surface needs to be homogeneous, flat and dry. Therefore, conditions for using CAM on microbial studies requires that the cells be flat and dry. Microbial mats on agar plates is one method used for these studies. This method could prove useful in its own way, but it takes special equipment and technical experience to measure the contact angles. Hydrophobic Interaction Chromatography Hydrophobic interaction chromatography (HIC) measures microbial adsorption to octyl-Sepharose or phenyl-Sepharose beads (Rosenberg and Doylel990). A column 15 packed with an aqueous suspension of beads bearing either octyl or phenyl groups is treated with suspension of cells. As the cells pass through the column some of them stick to the beads. The percentage adhering is determined from the loss of turbidity or radioactivity in the elute compared to the initial level. Controls filled with untreated beads have had cells adhere to them . Another problem is that some researchers have used salting-out agents to increase the amount of adherence to the beads (Rosenberg and Doylel990). The nature of suspension solution often plays a role in the results in this test method. The method has potential if all tests are performed under the same conditions. Salt Aggregation T est The salt aggregation test (SAT) is an extremely easy technique for studying the aggregate behavior of cells (Rozgonyi etal. 1985). The cells are challenged with increasing amounts of salting-out agents (ammonium sulfate), and as the salt concentration increases, cells start to clump together. Measurements are compared by observing at what concentration clumping began. This method will not work with bacteria that clump readily without added salt and also may be dependent on initial cell concentrations. Two Phase Partition Two phase partition (TPP) measures the distribution of cells between two immiscible fluids (Rosenberg and Doyle 1990). Usually one contains dextran and the other polyethylene glycol. The bacteria are added to the system and thoroughly mixed. After the mixture separates, samples are taken from each phase and the amount of cells in each phase is determined. In some cases cells may bind at the interface, causing problems for recovery and interpretation of the results. The test is also sensitive to changes in concentration, batches and molecular weight distribution of the two polymers. 16 Binding of Molecular Probes This method uses hydrophobic molecular probes that bind at the outermost surface of cells to determine hydrophobicity. Kjellberg, Lagaercrantz, and Larson studied binding of radiolabled dodecenoic acid to bacterial cells to quantitatively determine the cell surface hydrophobicity (1980). Molecular probes often will aggregate without the addition of polar or charged groups, which can interfere with the results. Another potential problem is the internalization of some of the probes, which may cause cell damage (Rosenberg and Doyle 1990). Adhesion to Hydrophobic Surfaces Microbial adhesion to hydrophobic surfaces can be another very useful method. Study of bacterial adhesion to hydrophobic surfaces such as polystyrene employs a variety of techniques (Rosenberg and Doyle 1990). Some examples are measurements of radiolabeled dodecanoic acid to bacterial cells and microspheres of polystyrene in a aqueous suspension directly observed via microscope or dynamic observations using image analysis. One advantage to this method is the ability to use contact angle measurements to determine the hydrophobicity of the surface. One advantage of polystyrene is the transparency of the material, which allows direct observation of the surface with conventional microscopy. Direction of Spreading Direction of spreading (DOS) was developed to observe differences between the hydrophobicity of individual cells and the surfaces of bacterial colonies (Sar 1986). The direction in which a drop of water spreads when placed at the border between the bacterial 17 lawn and another surface determines the nature of the bacterial lawn surface. The border surfaces used to make the measurements are: agar/bacteria, glass/bacteria, polystyrene/ bacteria. The droplet tends to move away from the border of the microbial lawn towards the material surface if the microbial lawn is more hydrophobic. Therefore, the more hydrophobic the bacteria is the more the water droplet will move towards the hydrophobic material. Sar found that the method, when tested against the MATH assay, will sometimes gives higher values for cell surface hydrophobicity. Image Analysis Image analysis enables dynamic systems to be studied. Bacterial adhesion to stationary surfaces can be studied by passing cell solutions through a flow cell and measuring directly the rates of attachment, detachment and growth on the surface. This method allows direct observation of the overall processes in a dynamic system through the use of microscopy and video techniques. The adhesion of S. sanguis 12 to FEP (fluoroethylenepropylene) and glass surfaces in real time using image analysis is described by Busscher et al. (1987). The adhesion kinetics of S. sanguis 12 during the first four to six hours of exposure to both substrata under various conditions yielded the initial rate of adhesion and the number of cells adhering in a stationary state. Escher in 1986 and Mueller in 1990 also used image analysis to determine the kinetics of bacterial attachment to surfaces. Escher’s goal was to determine the influences of independent parameters such as the fluid dynamics, biomass concentration in the bulk fluid, surface characteristics and cell physiology on early colonization of surfaces. Mueller’s goal was to determine the effects of different surfaces, environmental conditions, and microbial species on early bacterial colonization in continuous flow systems. Mueller used the MATH assay to determine the hydrophobicity of the bacteria in his study, but did not determine the hydrophobic character 18 of the surfaces tested. Comparisons between the hydrophobic surface character and the cell surface hydrophobicity may have helped to determine if a correlation between the two methods could be found. Physiological Effects on Cell Surface Hydrophobicity Growth Phase Microbial populations in batch culture follow a typical growth cycle with three distinct phases: lag, exponential, and stationary. Lag phase is a time period just after innoculation of the media during which little or no growth occurs. During the exponential phase cells grow and replicate exponentially as long as nutrients are available in the culture media. Once the microbes have utilized most of the available nutrients they enter the stationary phase and growth of the cells slows down. Cells in an environment in which nutrients are limited begin to slow down the synthesis of cellular components. Stationary phase is not static. During this time, for example, E. coli cells will continue to produce DNA after protein synthesis has been reduced (Neidhardt etal. 1990). Physiological differences between cells harvested from different phases of growth have also affected the results of the MATH assay. Lichtenberg et al. (1985). report that S. pyogenes cells harvested during the logarithmic growth phase had a removal coefficient (K) several orders of magnitude lower then those harvested during stationary growth. Rosenberg reported similar results in 1980 when he compared the lag phase and stationary phase hydrophobicities of S. marcescens cells. For this reason, care has to be taken that the time of harvesting is constant, so that physiological characteristics of the cells do not change substantially between experiments. 19 Growth T emperature Cultures incubated at different temperatures do not always have the same physical or chemical characteristics. These differences will often affect the MATH assay and other hydrophobicity tests. Sharon etal.1986 grew S. marcescens at 30°C and 35°C, and a greater removal coefficient (more hydrophobic) for the cells grown at 30°C was observed. The temperature of culture solutions needs to be kept constant between experiments for valid results. Cell Generation Isolation of microbial cells for experiments may require cells to be removed from the original batch culture onto a more ‘friendly’ medium such as agar plates. In some cases, cells grown and subsequently transferred to another medium may lose some of their original characteristics (Rosenberg and Doyle 1990). Cells taken from cultures and then suspended should be of the same generation for each experiment to keep errors due to loss of genetic material at a minimum. Cell Type and Preparation Microbial cells when washed may lose some of their hydrophobic character. This could result from a loss of surface components when the bacteria are rinsed, agitated and resuspended. Microbes exist in a number of states in nature, such as in biofilms, microbial mats and cells in suspension. The microbes in the biofilm will not have the same characteristics as microbes in suspension or in a microbial mat. Sar found that bacteria in microbial mats had higher DOS and contact angle measurements correlating to higher hydrophobicity than readings on suspended cells from the MATH test (1987). Washed 20 cells and unwashed cells were prepared, measurements of cell surface hydrophobic!ty by DOS and MATH methods were made. The results for both methods were similar except that the washed cells in some cases had lower values for the MATH test (Sar and Rosenberg 1987). The authors suspect that removal of the hydrophobic slime from the cells decreases the hydrophobicity values obtained by the MATH test. Physical Factors Effecting the MATH Test The MATH test can be influenced by factors that do not depend on the physiological characteristics of the microbial cell as affected by growth conditions. Some outside influences such as adsorption to water films, properties of the aqueous phase, presence of amphipathic contaminants, cell concentration and cell clumping can affect bacterial adhesion to the hydrocarbon-water interface. These factors can have pronounced effects on the results by either increasing or decreasing the MATH measurement. Adhesion to the sides of glass vessels or other containers will likely cause higher MATH readings. Cells normally in the bulk liquid which adhere to the agitating vessel wall lower the final absorbance reading in the MATH test. The cells on the wall have not interacted with the hydrocarbon and should not be included in the test. A control vessel, a container treated the same without hydrocarbon, can be used to subtract the amount of cells adhering to the sides of the mixing vessel after agitation. Bacteria which are extremely hydrophobic have a tendency to adhere to one another. This tendency makes it difficult to get a well-mixed suspension of cells and consistent readings for initial absorbance of the cell suspension. Cell concentration may also prove to influence microbial adherence to hydrocarbons, so that cell concentrations must be constant between single experiments. The ionic strength of the buffer solution affects the extent of adhesion of bacteria to hydrocarbons. Salting-out agents sometimes cause bacteria to aggregate. This causes more 21 of the bacteria to adhere to the hydrocarbon. Equipment used during the experiment must be clean and free of amphipathic contaminants. Glassware should be treated to remove all surfactants from the surface, otherwise interference with the MATH test will occur. Surfactants will interact with the cells in suspension and keep them from adhering to the hydrocarbon, reducing the hydrophobicity measurement. Even though there are a number of factors that influence the results, standard procedures and cleanliness can limit the effects on any one experiment. Continued Research Importance of Research Hydrophobicity of hydrocarbon degraders is not the only area where the MATH test and other methods are used. Cell surface properties of bacteria isolated from fish have been studied using MATH and DOS methods Sar and Rosenberg 1987). This research was used to determine the surface character of the bacteria found on fish scales. The researchers found that the some of the bacteria have hydrophobic characteristics though the values for DOS were often greater then those for MATH. It is thought that the hydrophobic surfaces of the bacteria may aid the fish in motility by reducing the frictional force of water against the fish. The development of mouthwash is another case where the MATH test has proved useful. In 1982 Weiss et al. found that a high proportion of oral bacteria isolated from extracted teeth and steel bands adhered to hexadecane. Other studies had earlier indicated that microbes which adhere to hydrocarbons will also behave similarly when treated with various nontoxic oils. Mouthwashes with an oil phase were tested against commercial 22 mouthwashes. The result was a significant reduction of oral microbial activity and bad breath when mouthwashes containing oil were used (Rosenberg 1991). The previous examples illustrate the importance of cell surface hydrophobicity and the need for different adhesion tests. Hydrophobicity tests are used to elucidate the surface properties of microbial cells to help determine the possible interactions between the cells and a surface. Knowledge of this sort is useful, especially when one considers the vast number of systems that have colonizable surfaces. Understanding the mechanisms of bacterial degradation of hydrocarbons at the interface would also give valuable information about how bacteria are able to utilize this hydrophobic carbon source. If the kinetics of bacterial attachment, desorption, growth and surface renewal can be quantified the information obtained could help researchers develop methods to maximize degradation of oil in natural or man-made systems. Comparable Tests One of the flaws of the MATH test is its inability to allow for comparison between different experiments. Many of the factors involved in reproducibility have less to do with the overall experimental method than with standardization of factors influencing the test. Another factor that may prove to influence the results of the MATH test are “user errors”. Some researchers may interpret a method differently then the author intended. For example, the method used to vortex the cells may have a pronounced effect on the results of the test. Even though there are better tests which allow comparisons between cell surface hydrophobicity, few of them are as fast and simple as MATH. To make comparisons between experiments from many laboratories using MATH is, at this time, rather inconclusive. There are cases where MATH was compared to other methods of determining cell surface hydrophobicity, the bacteria came from one generation 23 of bacteria which were scraped from agar plates (Dillon and Koohmaraie 1986). The ability to compare a variety of microbes from a simple test would allow investigators to screen bacteria with unknown surface properties for desired or undesired traits with some assurance that their results are valid. Such a method would make it easier to distinguish nonadhering hydrocarbon degraders from adhering hydrocarbon degraders. A MATH test that is quantifiable would also allow results to be easily compared so that influencing factors can be more easily studied. 24 METHODS The methods section is divided into four parts: preliminary work, experimental design, and experimental procedure. The preliminary work describes the isolation of the bacteria and background experiments and procedures. The next section describes experimental design followed by the experimental procedure portion which describes how each experiment was carried out. Preliminary Work Isolation The bacteria used for this research project were isolated from a contaminated soil sample using a modification of technique designed to isolate acetonitrile-utilizing bacteria (Chapatwala et al. 1990). These isolates were transferred to silica slant tubes into which the,hydrocarbon hexadecane was added as substrate (Funk and Krulwich 1964). The colonies on the slant tube that were able to degrade the hexadecane were further isolated to obtain single colonies. From the slant-tubes, colonies were selected for transfer to tryptone glucose extract agar plates. Transferred colonies were then streaked on the agar plates to isolate individual colonies once more. All of the slant tubes and agar plates were incubated at 35° C until enough growth occurred to allow for individual isolation of colonies. The colonies were transferred to 250 ml flasks containing PUM buffer nutrient media with hexadecane as the carbon source. These flasks were then placed on a shaker table that controlled the temperature and agitation rate for increased oxygen availability. The bacteria grew slowly, breaking down the hydrocarbon while creating a murky solution with a great 25 amount of bacterial clumping and emulsion production. From this procedure two isolates with the desired characteristics were cultivated for use in all experiments. The characteristics of the two isolates were similar, although one isolate was pink in color while the other was clear. Both bacterial isolates degraded hexadecane, produced emulsions and collected at the hydrocarbon-water interface. Only the pink colonies were used in the MATH experiment. The pink isolates were chosen for two reasons; they were easier to spot at the hydrocarbon-water interface and individual colonies were easier to discern from groups of colonies. The isolates grew slowly on hexadecane and the suspension remained viable for over a month after which it became necessary tosubculture. Substrate The substrate desired for growth of cells in batch cultures had to have certain characteristics in order for the desired results to be achieved. Using an insoluble substrate such as hexadecane was not practical because the amount of substrate available to the cells is not easily quantified. Further, residual hydrocarbon adherence to cell surfaces could interfere with the MATH test results. It was also required that the substrate contain no nitrogen in the molecular formula. The first choice was fatty acids, such as formate, nbutyrate, iso-butyrate, propionate or acetate. These substrates dissolve readily in aqueous solutions and are often intermediates in the metabolism of straight-chain alkanes such as hexadecane. The isolate was able to metabolize all of the fatty acids. However, the inability to easily sterilize fatty acids decreased their desirability as the substrate. Ethanol was tried as a substrate because it is simple in chemical make-up and resembles the end of a fatty acid or alkane chain. Also, the alcohol did not have to be sterilized before addition to the nutrient media. The isolates were able to use ethanol, although the growth rates were extremely slow. 26 C:N ratio calculations Calculations for relative amounts of carbon in the form of ethanol and nitrogen as ammonium chloride were based on an article by Sykes (1975). Growth yield (Ybioh ) and values for the carbon-to-nitrogen ratios for balanced growth were determined. The range varied from 6.26:1 to 4.94:1. The C:N ratio is important because it has been determined that the relative availability of carbon, nitrogen and phosphorus determines the cells reproduction and production of extracellular polymeric substances. When both carbon and nitrogen are plentiful, the cells grow and reproduce. As nitrogen is depleted, some cells will then switch to recycling of nitrogen. In this case carbon may be excreted from the cells as extracellular polymeric material (EPS). By picking a C:N ratio above that for balanced growth the cells should have enough carbon to grow readily. A C:N ratio of 15:1 is high enough above the minimum requirement that an excess of carbon will be available to the cells in solution. The physical and chemical surface properties should therefore be different for cells grown at a C:N ratio of 15:1 than for those grown at 5 :1. NutrientMedium The choice of medium in the growth experiments was determined from adjustments made to the media used to isolate hydrocarbon-degrading organisms. The components of the nutrient media passed through various changes until one that did not interfere with the absorbance experiments was found. Initial choices of media formed precipitates that were carried through the cell suspension preparation and contributed to the absorbance. Initial cell counts of these experiments were usually lower than those for experiments that did not have a precipitate left over from the nutrient medium. This interference was not noticed initially because the interference from the precipitate did not occur until low concentrations of cells in the solution occurred. When the precipitates were present, an initial drop in 27 absorbance was observed at low agitation rates and mixing times. When the higher agitation rates were used no further drop in absorbance was observed. At the point where the absorbance no longer changed cell numbers continued to decrease. The absorbance of the precipitate in the solution became a background that did not change with the continued removal of cells. For this reason a modified Buschnell-Haas broth was used for the culturing of the bacteria (Table I). Table I. Composition of nutrient media. Magnesium Sulfate Calcium Chloride ' Monopotassium Phosphate Dipotassium Phosphate Ferric Chloride (M gS04*7H20) (CaCl2^ H 2O) (KH2PO4) (K2HPO4) (FeCl3^riH2O) Ammonium Chloride for C:N 15:1 Ammonium Chloride for C:N 5:1 (NH4Cl) (NH4Cl) 0.2 g/1 0.02 g/1 1.0 g/1 1.0 g/1 0.625 ml of O.Olg/ml stock solution added to each 125 ml flask. 0.319 ml of 10 mg/ml stock solution added to each 125 ml flask. 0.955 ml of 10 mg/ml stock solution added to each 125 ml flask. Experimental Design M ATHAssay C:N Ratio The MATH test was used to determine if the carbon to nitrogen ratio (C:N) would affect the hydrophobicity of a hydrocarbon-degrading bacterium. C:N ratios of 5 :1 and 15:1 were used, representing carbon-limited and carbon-rich environments respectively. In the breakdown of hydrocarbons by bacteria in sea-water the limiting nutrient is nitrogen (Atlas 1984). For carbon-limited cells the C:N ratio is 5:1, although there.can be some variation (Characklis and Marshall 1990,145). The carbon-rich C:N 28 ratio at 15:1 is higher then the ratio (7.7:1) needed for maximum production of emulsan for Acinetobacter calcoaceticus RAG-1 (Shabtai and Wang 1989). Experimental Conditions Concurrent with testing the stated hypothesis on the effects of the C:N ratio, the experiments were designed to obtain statistical data on the accuracy and precision of the MATH test itself. The design of this part of the experiment involved three factors of the MATH test: agitation rate, hydrocarbon volume, and time of agitation (Table 2). Two different methods were used to obtain this data: absorbance measurements and viable cell counts. These methods were also compared to determine if there were significant differences in the results of the two cell enumeration techniques. Table 2. Settings for agitation rate, hydrocarbon volume and mixing time. AgitationRate 60 100 140 Hydrocarbon Volume in ml. 0.2 0.6 1.0 Tim eofM ixing in seconds. 10 20 40 AgitationRate Three agitation rates were used in the test. A Maxi Mix II (vortexer source) was used at the highest setting and the rates were controlled using a Powerstat type 116B (voltage regulator) on three settings; 60,100 and 140 (Rosenberg 1980). The rate of rotation was measured by strobe light for each of the settings. The rotation rate of the mixing plate and the fluid inside the test tubes for each agitation rate measured as rpm (rotations per minute) were: 1470 at 60, 2810 at 100, and 3100 at 140. 29 Hydrocarbon Volume The amount of cell suspension in the test tubes was held constant at 5 ml. Three different hydrocarbon amounts (0.2 ml, 0.6 ml and 1.0 ml) were used in the experiments In the initial MATH test, 1.2 ml of cell suspension was subjected to varying volumes of hexadecane, xylene and octane. The volume of hydrocarbon ranged from 0 to 0.2 ml. A later testing procedure used 5 ml of cell suspension to I ml of hydrocarbon. For the reported data, the larger volume technique was used because cell counts and absorbance measurements after agitation required the full 5 ml of cell suspension. Hydrocarbon Screening To determine which hydrocarbon would be used for the research the three hydrocarbons (hexadecane, xylene and octane) were tested. The experimental design compared the results of each hydrocarbon’s efficiency and variability for removal of bacterial cells from the media. Initial results indicated the three methods had similar percent removals and reproducibility (Table 3). The experimental error associated with the three methods seemed to be similar, and in some cases the experimental error was large. Table 3. Absorbance and Percent Removal for Three Hydrocarbons Hydrocarbon Xylene Octane Hexadecane I nit. Abs. .301 .302 .290 10 sec. .209 .213 .215 Time of Mixing 20 sec. 40 sec. .196 .192 .204 .198 .209 .204 80 sec. .191 .196 .191 % Removal 3 6 .5 % 3 5 .0 % 3 4 .1 % This finding was similar to results obtained by Sweet et al( 1987), where it was determined that residual hydrocarbon can adhere to bacteria and increase the refractive index of the 30 bacteria. The residual could be removed by aerating the solution for one minute causing desorption from the bacterial surface. The results using this method were less reliable for hexadecane than for xylene. One potential problem that can occur when using xylene as the hydrocarbon is lysis of the cells. This would cause lower absorbance measurements to be observed in the experiments. Sweet etal. determined that lysis of cells was not occurring in the presence of xylene (1987). By aerating a suspension of cells and xylene overnight, the xylene was removed and the original absorbance was recovered. Viable cell counts confirmed that the cells were not being lysed. This research followed the procedure of Sweet etal. by using xylene as the hydrocarbon and aerating the cell suspensions to remove xylene attached to the cells or dissolved in the water. Tim eofM ixing Researchers using the MATH test have used agitation times ranging between 5-120 seconds. To minimize the number of mixing periods the point where continued mixing no longer changed the absorbance was determined. After using three different hydrocarbons and mixing times running to 120 seconds, it was determined that no significant changes were taking place after 40 seconds. Table 3 also shows this trend, with absorbance values changing little after 40 seconds of mixing. Experimental Procedure Inoculation Numerous authors cite growth phase as affecting the MATH test. It was for this reason that all cells were harvested during stationary phase at the same interval after 31 inoculation of the culture. The soil isolate had a slow growth rate and did not reach the stationary phase until the seventh day of incubation. To determine when stationary phase was reached, 5 ml samples were taken twice a day from the culture for nine days. Stationary phase was reached when the absorbance measurements no longer increased. Stationary phase was also confirmed from cell count data. Once the stationary phase was determined, a harvest time was picked that would be constant in all the experiments. All cells for each experiment were therefore harvested between 190-194 hours, or about 8 days after inoculation. The culture flasks were agitated at 110 rpm on a New Brunswick Scientific water bath shaker table at 37° C. First generation cells, scraped from R2A agar plates, were suspended in 125 ml of Bacto Bushnell-Haas Broth nutrient medium (Table 3). For a 5:1 C:N, 0.955 ml of stock solution was added to the nutrient medium, while for a 15:1 C:N, 0.319 ml of solution was added. Filter sterilized ferric chloride (0.625 ml) was added separately because of its tendency to precipitate when autoclaved. 3 1 3 ]A of ethanol, the substrate, was added to the nutrient medium. After 8 days, the cell suspension was transferred to centrifuge bottles and placed in the centrifuge. Washing Procedure The cell suspension was added to centrifuge tubes and placed in a RC5C .Sorvall Instruments centrifuge set to run for 25 minutes at 12,000 xg. After centrifuging, the liquid was poured off and the pellet was washed with 50 ml of buffer solution (Table 4) which had been filtered through a .2 pim filter to remove any precipitate formed by the autoclaving process. The cells were vortexed to break up the pellet and placed in the centrifuge again. The same procedure was then performed twice more. 32 Table 4. Composition of buffer solution. Monopotassium Phosphate (KH2PO4) Dipotassium Phosphate_______ (K7HPO4) 22.2 g/1 7.26 g/1 Resuspension of Cells The cells were resuspended in sufficient phosphate buffer solution to produce the desired absorbance of 0.2 at 500 nm. This usually gave a total test volume of between 100150 ml. The initial cell counts were determined by plate counts. Addition to T est tubes Experiments were initially carried out in polystyrene cuvettes following the procedure of Sharon etal. (1986). The isolate tended to stick to the surface of the polystyrene cuvette after agitation. Controls that lacked the hydrocarbon had substantially lowerabsorbance values after agitation than the initial absorbance measurements. Therefore, the MATH tests were carried out in 16 * 150 mm Kimex test tubes even though the procedure was more tedious. To remove potentially interfering surfactants, test tubes were washed by hand and rinsed three times in distilled water, placed in a muffle furnace set at 500 °C for four hours. The test tubes were then capped and autoclaved for twenty minutes. This process kept the test tubes free of biological and chemical contaminants that could interfere with the experiments. Five milliliter portions of the cell suspension were divided among test tubes, generally using between 12 to 15 test tubes. Hydrocarbon was added to the cell suspension in three different volumes: 0.2 ml, 0.6 ml and 1.0 ml. Each portion of hydrocarbon would be in triplicate and three test tubes Would be free of hydrocarbon as controls. In some cases, when a greater volume of cell suspension was available, a larger experiment could be run. Table 5 shows a typical experimental array of test tubes with the 33 relative amounts of hydrocarbon, agitation rate and time of mixing. The test tubes were then placed at a constant temperature of 30° C for 20 minutes. T able 5. Example of a Typical Experiment Agitationrate HC volume .60 / 0.2 100 / 0.6 140 / 1.0 Control 10s V V V . V 20s V V V V 40s V V V V Agitation After the 20 minute equilibrium period one of the test tubes was placed on the mixer plate and agitated at the desired setting and time period. The test tubes were randomly selected to help average out any error in the transfer of cell suspension to the test tubes. The setting on the Powerstat would be changed only after all the tests for that particular agitation rate were finished. The experiment progressed until all samples and controls were mixed at their prescribed agitation rate for a given time period. Cell Enumeration Techniques Two methods were used to determine the concentration of cells in the suspension media; viable plate counts and absorbance measurements. Enumeration of viable cells using PC (plate Count) methods gives information on the number of cells able to reproduce and form colonies on agar plates. It is thought, however, that in some cases injured cells that are still performing some biological functions will not be able to grow on the agar plates (Yu, Pyle, and McFeters 1992). Absorbance measurements provide information on the numbers of particles without regard to viability or culturability. Further, absorbance 34 measurements are not as adversely affected by clumping as plate counts. Cell counts were initially to be determined using direct microscopic counts. Direct microscopic counts did not work however, due to the tendency of bacteria to clump when passed through the (0.2 jUm pore size) black polycarbonate membrane filter (Nuclepore). Cell Enumeration A modified drop plate method was used for the PC (plate count) cell enumeration (Miles and Misra 1938). The cell suspension was separated from the hydrocarbon layer by carefully pipeting the lower aqueous layer to clean test tubes. From the aqueous suspensions of some of the test tubes, I ml of supernatant was removed and transferred to a dilution tube containing 9 ml of autoclaved buffer and I /d of homogenization fluid and homogenized to disperse the cells (Camper etal 1985). Serial ten-fold dilutions of the culture were then performed. Petri dishes containing R2A agar were divided into three equal portions, one for each of the ten-fold dilutions (e.g., IO"4, IO"5, IO"5). Ten 10 ]A drops of a diluted cell suspension were placed in each section of the p e tri. The plates were incubated at 35° C for eight days before colonies became large enough to count. The cell counts were determined by counting the number of colonies at a dilution where no fewer than 10 and no more then 50 colonies were observed for each drop of sample at a particular dilution. The remaining cell suspension was set aside for absorbance measurements. Absorbance Measurements Absorbance was measured using a Milton Roy Spectrpnic 601 absorbance spectrophotometer at 500 nm using buffer media alone as the blank. An initial cell absorbance of approximately 0.200 was used. For absorbance measurements, the remaining cell suspension transferred from the original test tubes were aerated for one 35 minute (air flow 3 ml per second) to remove any excess hydrocarbon from the liquid and the small amounts that stuck to suspended cells. Absorbance measurements were then taken of all the test tubes containing the cell suspension, including controls. Measurements were made after transferring 2 ml of sample to a I cm square (4 ml) polystyrene cuvette which could be placed in the spectrophotometer. 36 RESULTS The results are divided into three sections; observations, percent removal measurements and statistical analysis. The first section includes photographs depicting test tubes used in the experiments. The second section describes different analyses used to describe and interpret the absorbance data. The statistical analysis section describes the general design of the experiment and the statistical results. Test Tube Observations Agitation at all settings and times produced an emulsion of xylene and water, which separated into layers after about 30 minutes of rest. The adhering cells collected at the hydrocarbon-water interface and could be observed as clumps causing deformation (Figure I). As shown in figure 2, some clumping of cells occurred even before agitation of the solution. Following agitation, the cell suspension in the control test tubes behaved differently than in the hydrocarbon-filled test tubes. In the controls, more of the suspended cells adhered to the glass surface in clumps than in the hydrocarbon-containing test tubes. Figure 3 shows a typical control test tube after agitation and Figure 4 shows a test tube with hydrocarbon agitated at the same rate and time period as the control. Figure 5 shows a control test tube which had been vortexed for 20 seconds with an agitation setting of 60, while Figure 6 depicts a control vortexed for 20 seconds at a setting of 140. Figure 7 depicts a sample with a hydrocarbon volume of 0.2 ml agitated at 60 for 20 seconds while figure 8 depicts a test tube containing the same volume, but agitated at 140 and Figure 9 depicts an agitation setting of 140. The emulsion at the interface can be observed in the photographs, as well as the differences in adherence to the surface of the glass in the 37 Figure I Cells within the white circle are at the hydrocarbon-water interface. 38 Figure 2 Figure 3 Test tube containing unmixed hydrocarbon and cell suspension. Control test tube after agitation. (Agitated at a setting o f 60 for 40 seconds.) 39 Figure 4 Figure 5 Test tube agitated with hydrocarbon. (I ml hydrocarbon with agitation setting of 60 and mixing time of 40 seconds.) Control test tube (Agitation setting o f 60 and mixing time o f 20 seconds.) 40 Figure 6 Figure 7 Control test tube. (Agitated at a setting of 140 for 20 seconds.) Test tube containing .2 ml of hydrocarbon. (Agitated at a setting of 60 for 20 seconds.) 41 Figure 8 Figure 9 Test tube containing .2 ml of hydrocarbon. (Agitated at a setting of 140 for 20 seconds.) Test tube containing I ml of hydrocarbon. (Agitated at a setting of 60 for 20 seconds.) 42 controls (Figures 3,5,6) and hydrocarbon (Figures 4,7-9) and at the different rates of agitation. Percent Removal Data Results from the MATH produced six types of data. First, the initial absorbance (Ao) and cell counts (Co) were determined for the cell suspension. After the agitation step was complete and the cell suspension was allowed to settle, absorbance (Ac) and cell counts (Ce) measurements for the control volumes were taken. The final absorbance (A) and cell counts (C) for the test tubes containing hydrocarbon were also m easured. Several combinations of these parameters were developed, and the results were analyzed arithmetically and statistically. For the arithmetic method the responses were, percent removal (ideal), percent removal (control) and percent removal (adjusted), defined as follows: 1) Percentremoval (control) = ((control-final)/control)* 100 2) Percent removal (ideal) = ((initial-final)/initial)* 100 3) Percent removal (adjusted) = ((control-final)/initial)* 100 where percent removal = percentage of cells removed from the suspension, initial = initial absorbance or cell count measurements, control = control absorbance or cell count measurements, and final = final absorbance or cell count measurements. The percent removal (control) was compared to the percent removal (ideal) to determine which method of analysis was a better fit. The last method, percent removal (adjusted), used all three the components from the MATH test for the response. The adjusted response was used to 43 measure the percent removal after accounting for the bacteria lost to the surface of the glass test tube as determined in the controls. Table 6. Absorbance Responses for Cells Cultured at a C:N Ratio of 5 :1. % Removal (control) 10 20 40 Mean 50.3 66.7 Std. Dev. 13.7 4.1 n 2 2 Mean 63.8 Std. Dev. n Mean 56.9 76.3 Std. Dev. 9.1 0.7 n 2 3 Mean 80.0 Std. Dev. 8.5 n 5 Mean 86.5 Std. Dev. n Mean 89.5 78.0 Std. Dev. 0.9 14.4 n 2 2 Mean 72.2 Std. Dev. 1.6 n 2 Mean 79.1 81.9 Std. Dev. 8.1 4.3 n 2 2 Absorbance 5 :1 60/0.2 a 60/0.6 60/1.0 100/0.6 100/1.0 140/0.2 140/0.6 140/1.0 % Removal (ideal) 10 20 40 79.7 84.7 85.7 7.7 6.6 5.3 4 4 4 78.9 81.1 83.9 % Removal (adjusted) 10 20 40 21.0 32.5 0.3 4.0 2 2 33.3 82.5 5.3 5 86.8 6.8 4 85.5 7.0 4 90.6 4.1 5 98.8 90.0 4.9 5 91.4 4.2 4 26.6 1.1 2 89.5 7.4 4 87.6 0.7 2 89.6 2.7 4 90.7 5.1 3 86.6 0.7 2 90.5 3.6 3 92.3 4.7 4 89.3 0.4 2 91.5 1.9 4 53.7 19.1 2 32.3 0.7 2 42.7 13.9 2 22.7 10.5 3 39.7 16.9 5 7.7 38.8 11.1 2 35.2 1.2 2 a agitation setting / hydrocarbon volume. b n=l for means with no standard deviation listed. Absorbance response data from cell suspensions cultured with a C:N ratio of 5 :1 are documented in Table 6. The responses for percent removal (ideal) and percent removal (control) behave in a similar manner. The response for percent removal (control) varies 44 from 50.3 % to 81.9 % compared with 79.7 % to 91.5 % for percent removal (ideal). The values for the percent removal (adjusted) show no real tendencies although the errors are about the same as the other methods. There is a general upward trend for the responses as the agitation rate, hydrocarbon volume and time of mixing increase. The results from the cell count data show a greater percent removal then the absorbance data at the low factor settings (Table 7). The response for the percent removal (ideal) at high hydrocarbon Table 7. Cell Count Responses for Cells Cultured at a C:N Ratio of 5:1. % Removal % Removal Cell counts 5 :1 (control) (ideal) 10 20 40 a 10 20 40 43.9 81.1 60/0.2 Mean 97.8 98.7 Std. Dev. 14.1 0.2 0.3 n 2 2 2 60/1.0 Mean 33.4 35.0 97.4 98.7 Std. Dev. 30.2 0.7 0.3 n 2 2 2 100/0.6 Mean 90.6 99.6 Std. Dev. 12.1 0.1 n 4 4 "W 8 W T T 99.3 140/0.2 Mean 99.8 Std. Dev. 0.2 0.0 n 2 2 140/0.6 Mean 99.4 Std. Dev. n 97.8 140/1.0 Mean 99.7 99.7 Std. Dev. 2.5 0.1 0.3 n 2 2 2 % Removal (adjusted) 10 20 40 7.4 5.1 2 0.8 7.2 5.4 2 8.5 5.9 4 6.6 11.2 25.0 22.6 2 a agitation setting / hydrocarbon volume. b n=l for means with no standard deviation listed. volumes, agitation rate and mixing times is almost 100 %. The responses for percent removal (ideal) and percent removal (control) are consistently greater using cell counts than those using absorbance A somewhat different trend is found in the data for cell suspensions 45 raised at a C:N ratio of 15:1 (Tables 8 and 9). In this case the percent removal (adjusted) responses are almost the same for both cell counts and absorbance. The percent removal (ideal) with responses are greater for the cell counts than for absorbance, as was seen with the results obtained for cells cultured at 5 :1. In general, comparing Tables 6 and 8 or Tables Table 8. Absorbance Responses for Cells Cultured at a C:N Ratio of 15:1. Absorbance 15:1 a 60/0.2 Mean Std. Dev. n 60/0.6 Mean Std. Dev. n 60/1.0 Mean Std. Dev. n 100/0.2 Mean Std. Dev. n 100/0.6 Mean Std. Dev. n 100/1.0 Mean Std. Dev. n 140/0.2 Mean Std. Dev. n 140/0.6 Mean Std. Dev. n 140/1.0 Mean Std. Dev. n % Removal (control) 10 20 40 47.7 45.0 62.4 14.4 13.0 6 5 68.63 60.6 19.0 5 83.95 68.5 3.6 2 73.91 68.4 8.4 5 80 75.3 80.2 10.7 6 71.03 81.2 77.3 8.5 4 80.8 1.1 2 91.21 79.5 4.5 6 81.19 76.2 8.8 5 76.6 3.9 2 78.7 7.5 6 % Removal (ideal) 10.0 20.0 40.0 69.2 73.1 80.9 8.3 7.8 6.0 7 7 7 81.8 83.3 83.9 % Removal (adjusted) 10.0 20.0 40.0 31.1 41.8 34.0 12.1 15.7 11.3 6 3 5 36.5 76.9 9.0 7 91.8 1.8 2 86.4 2.1 7 90.0 79.2 8.4 7 91.8 1.8 2 88.6 4.5 7 89.5 84.3 3.9 7 92.3 LI 2 89.2 3.0 7 88.4 43.7 18.3 6 36.2 40.9 11.0 3 26.8 36.1 9.2 6 27.7 40.6 3.6 2 40.4 7.0 7 25.8 35.3 1.0 2 87.7 4.0 7 91.3 2.5 2 88.0 3.9 7 89.8 3.5 7 93.2 3.6 2 89.8 2.2 7 91.2 2.6 7 90.3 0.2 2 91.7 2.8 7 42.1 10.3 5 37.7 10.4 3 44.1 33.4 4.5 7 42.7 39.4 10.2 5 42.2 8.8 2 33.9 9.2 7 a agitation setting / hydrocarbon volume. b n=l for means with no standard deviation listed. 46 7 and 9, percent removals (ideal and control) were similar between the two C:N ratios using both absorbance and cell counts. The results obtained from some of the experiments were not incorporated into the data set. A precipitate that was not observable by the naked eye formed in some of the cell suspensions. Since initial cell suspensions were diluted to an absorbance of 0.2, the precipitate’s contribution necessitated a corresponding decrease in cell concentration. The Table 9. Cell Count Responses for Cells Cultured at a C:N Ratio of 15:1. % Removal (control) 10 20 40 6 0 / 0 . 2 Mean -22.6 54.4 a S t d . Dev 4 6 . 4 31.0 n 4 2 6 0 / 0 . 6 Mean 14.4 S t d . Dev n 6 0 / 1 . 0 Mean 1 1 2.6 39.0 S t d . Dev 1 3 4 . 3 4 4.9 n 4 2 1 0 0 / 0 . 2 Mean 7 4.4 S t d . Dev n 1 0 0 / 0 . 6 Mean 89.3 S td . Dev. 3.7 n 5 I 0 0 / 1 . C Mean 86.5 S t d . Dev n 1 4 0 / 0 . 2 Mean 7 9.9 90.6 96.2 S t d . Dev 8.9 0.9 n 3 3 1 4 0 / 0 . 6 Mean 91.2 S td . Dev. n 1 4 0 / 1 . C Mean 8 8.8 97.6 98.8 S t d . Dev 4.3 0.4 n 3 4 % Removal (ideal) Cell c o u n t s 15:1 10 52.7 31.4 4 20 95.3 40 74.9 22.1 4 83.5 78.2 21.2 3 92.4 5.4 4 95.6 3.6 4 a agitation setting / hydrocarbon volume. b n =l for means with no standard deviation listed. 9 4.4 91.2 8.8 4 % Removal (adjusted) 10 20 40 6.0 31.1 17.5 3 9.6 4 4 2.8 3 8.2 37.7 4 49.4 48.1 3 97.7 6.7 94.9 2.8 6 98.8 51.4 40.6 6 7.7 9 6.8 2.1 2 95.7 9 8.4 0.9 4 99.2 0.3 3 99.5 0.4 4 47.3 3 1.6 3 44.4 4 0.3 3 8.7 3 44.7 5 1.2 33.0 3 4 7.8 33.0 36.1 4 47 effect of the particulate matter then, was to lower the initial number of cells in the solution for the given absorbance of 0.200. The effect of the precipitate carried through the entire experiment. The absorbance measurements would drop initially until they reached a critical point. This point was where the particulate matter masked the cells’ effect on the absorbance. For the 5 :1 cultures with precipitate present, the average absorbance response for percent removal (ideal) varied from about 60 % to 80 % (Table 10). The percent removal (ideal) for cell counts was typically 20 % (Table 10) higher since the cell counts were not affected by the absorbance. Since species of bacteria behave differently at low cell Table 10. Absorbance and Cell Count Data for Cells Cultured at a C:N Ratio of 5:1 for Experiments with Interference. C:N 5:1 interference a 60/0.2 Mean Std. Dev. n 60/0.6 Mean Std. Dev. n 60/1.0 Mean Std. Dev. n 100/0.1 Mean Std. Dev. n 140/0.: Mean Std. Dev. n 140/1.1 Mean Std. Dev. n absorbance % removal (ideal) 10.0 20.0 40.0 62.5 64.2 69.5 11.7 11.8 13.4 7.0 6.0 7.0 78.9 80.0 82.2 0.0 1.6 2.4 2.0 2.0 2.0 64.8 71.5 72.8 6.7 1.9 3.0 4.0 3.0 4.0 70.3 70.5 71.6 13.7 14.6 12.2 5.0 8.0 5.0 72.2 74.4 76.0 7.6 7.3 8.1 4.0 4.0 4.0 73.3 69.1 75.5 13.6 16.4 15.6 7.0 4.0 6.0 cell counts % removal (ideal) 10.0 20.0 40.0 83.2 91.7 10.3 5.8 6.0 5.0 88.6 97.4 11.0 2.0 95.2 96.9 5.2 3.8 3.0 3.0 95.8 96.5 4.2 7.0 9 8 .1 98.9 2.3 1.5 3.0 3.0 98.3 98.9 2.6 1.1 5 4.0 a agitation setting / hydrocarbon volume. b n=l for means with no standard deviation listed. 48 concentrations than they do for high cell concentrations with respect to bacterial attachment to hydrocarbon the data in Table 10 were not used in further analyses. Results From Statistical Analysis The experimental design was governed by the statistical methods used to analyze the data. The design required that data points from the three factors (time of mixing, agitation rate, hydrocarbon volume) form the comers and the center point of a cube. The data collected for the absorbance measurements included other points within the cube. A linear regression model suing the software Minitab, was used to determine coefficients for each of the factors. ANOVA (analysis of variance), was performed using the software SAS to determine p values for each factor and the interexperiment and the intraexperiment errors. Regression Analysis Six response variables were determined from the experimental absorbance (A, Ac and Ao) and cell count (C, Ce and Co) data. The six transformed variables are all logarithms of ratios, as shown in the natural first column of Table 11. These responses were used to develop multiple linear regression models using the experimental factors rpm, time, C:N ratio and hydrocarbon volume. For the absorbance data when hydrocarbon was present, for example, the equation takes the form: In (A0ZA) = B0 + B1(rpm) + B2 (time) + B3 (C:N) + B4 (HG volume) where B0 through B4 are regression coefficients determined through least square error minimization. For each response, p-values and correlation coefficients R 2 were also 49 determined. Values for the regression coefficients (6 values) are shown in Table 11. The above linear regression model was expanded to include products of the factors to look for Table 11. Constant terms and Coefficients for Responses. Response Constant rpm Time C:N HC volume InAo/A 1.38 0.70 0.243 -0.625 0.362 In Co/C 1.98 2.62 0.801 -1.711 0.44 InAc/A 0.003 1.36 0.094 -0.123 0.413 In Cc/C -0.073 2.12 -0.616 -0.701 0.446 InAo/Ac 1.2 -0.617 0.177 -0.560 0.039 In Co/Cc 3.50 -0.054 -0.105 -0.813 0.088 interactions and quadratic terms for each factor to allow for nonlinear behavior. Step-wise linear regression using Minitab was used to determine which of the factors, cross factors and squared terms were important to the model. Interaction plots were analyzed first to determine which factors were important to the model. The interaction plot of the four factors at a given response helped to determine if a factor was likely to affect another. The first regression included all of the terms. The p values for the regression were analyzed and insignificant terms (p > 0.05) were removed. The method proceeded until only factors and cross factors with significant p values remained. ANOVA was used in the determination of the p values while Minitab was used to determine the regression coefficients. 50 DISCUSSION The discussion is divided into three sections. The first section discusses the classical approach of comparing MATH test results by percent removal. This section continues with a discussion on removal rate and removal coefficients as quantitative measurements of cell surface hydrophobicity. Analysis of the percent removal data is also approached arithmetically using graphs and standard deviation methods. The last section discusses the statistical approaches used to analyze the data. Analysis of Data Percent Removal The percent removal approach to analyzing the MATH test allows quick comparisons of the results but these results are not definitive. The information gained is limited to comparing the percent removal of bacteria by the hydrocarbon under various conditions but with no standard measure for comparison. Table 12 illustrates the variation in the data set by representing the data at the lowest settings to the highest for all three experimental factors. The variation in percent removal ideal and control are illustrated. For both absorbance and cell counts the response for the percent removal ideal is greater to start with. The percent error is larger for the control than for the ideal analysis method for the absorbance data. The cell count data is similar but with some exceptions had a lower standard deviation. The cell count data also did not have as many data points so the standard deviation is probably higher than its true value. From this representation of the data the following observations can be stated: 51 1) Error decreases as the factor settings increase from (60, 10, 0.2)* to the highest settings of (140, 40, 1.0)*. 2) Percent removal (control) error values are larger than those for percent removal (ideal). 3) For percent removal (ideal), the values at the highest settings show greater than 90% removal of the suspended cells. 4) Standard deviations decrease as settings increase for all data types. This is due largely to the near-100 % removals. *Settings are agitation, hydrocarbon volume (ml). Table 12. Percent absorbance data showing the change from the lowest settings to the highest settings for absorbance and cell counts. 50.3 ± 1 3 .7 -8 1 .9 ± 0 .4 percent removal (control) 79.7 ± 7 .7 - 9 1 .5 ± 1.9 percent removal (ideal) 47.7 ± 14.4 - 78.7 ± 7.5 percent removal (control) 69.2± 8.3 -9 1 .7 ± 2 .8 43.9 ± NA - 97.8 ± 2.5 percent removal (ideal) percent removal (control) 97.8 ± 0.2 - 99.7 ± 0.3 percent removal (ideal) -22.6 ± 46.4 - 98.8 ± 0.4 percent removal (control) 52.7 ± 3 1 .4 - 9 9 .5 ± 0 .4 percent removal (ideal) 5:1 A b sorb an ce 15:1 5:1 C e ll c o u n t s 15:1 KineticApproach In the MATH test the removal of bacteria by hydrocarbon is known to be a function of time and hydrocarbon to water ratio. Lichtenberg et al. proposed a kinetic approach to 52 Removal Rate (k) for an absorbance setting of 140 y 4= -0.1457): + 1.14 1. 1 0 -2 1.05 - y = -0.1757X + 1.0 e 1.00 Linear (0.2) Linear (I) Linear (0.6) 0.8 5 - - ........ ^1..^.5 .^(.l.)L...L.). R; = 0.996 7 time (min.) Removal Coefficient (K) for Absorbance Data 5:1 at agitation settings of 60 and 140. 1.4 1.2 0 .8 ...— •-3 .8688x + 0.5826 2 = 0.5147 ■ ---------------- '■■■■ ...I y = i-]V6456x ^ 0 5 f4 5 :“ 0.4 ■ 0.2 ♦ « 60 140 -Linear (60) - Linear (140) 1Linear (60) ■ Figure 10. Plots used to determine the removal rate (k) and removal coefficient (K) for cells grown in a culture containing a 5 :1 C:N ratio. 53 adherence of bacteria to hydrocarbon in 1985. The removal rate (k) was determined by dividing initial absorbance by experimental absorbance. The log of the quotient, multiplied by 100, was plotted against the time of agitation. The slope obtained was labeled k. Tb determine the removal coefficient (K), removal rates for different volumes of hydrocarbon (0.2,0.6, 1.0 in ml) multiplied by a factor of 2.3 were plotted against the V hAZw. The slopes for removal coefficients obtained by Lichtenberg etal. (1985) were consistent with those of Sharon etal. (1986). The results for k in this study are similar to those determined by Lichtenberg and Sharon. Two values for removal rate (k) from the Lichtenbefg data are: slope of 0.94 min'1 for 20 ]A and 0.6 min"1for 10 ]A volumes of hydrocarbon. Removal rates were calculated for both C:N ratios for agitation settings of 60 and 140. The log of cells remaining was plotted against the time of agitation in minutes. Values for the slope (k) in figure 10 are .26 min'1, . 15 m in'1 and . 17 min"1 for .2 ml, .6 ml and 1.0 ml of hydrocarbon respectively, at an agitation rate of 140. Most of the results for k yield positive slopes with R2 values of .90 or better. One example, where the slope deviated from the norm, occurred for a C:N of 15:1 , 1.0 ml of hydrocarbon and an agitation setting of 140 (Figure 11). The slope was positive, so that the value for k was negative and the linear regression produced an R2 value of 0.23. For this case, the data obtained are not represented by the model and therefore k should not be used to calculate the removal coefficient (K). Lichtenberg calculated a K of 376 min"1 for S . pyogenes, while Sharon etal. cited values of 4.7 min "1 for Streptococcus pyogenes and 72 min"1 for Acinobacter calcoaceticus. These values are very different from those obtained from the absorbance data for the isolated hydrocarbon-degrader. For cells grown at 5 :1 with an agitation setting of 60, K was determined to be 3.8 min Both Lichtenberg e ta l and Sharon etal. used substantially lower hydrocarbon volumes, from 5 ^ l - 20 p \ of hydrocarbon to 3 ml of 54 Removal Rate (k) for absorbance data with an agitaion setting of 140. 1.1 y = -0.2657 x + 1.12 R2 = 0 I? 75 L 1 .05 0 1 0. 2 1 < 0.95 < ^ % 0.9 y —-0.311 8x + 1.125 R2 = 0.9861 & 0.6 4 I -Linear (I) Linear (0.6) "Linear (0.2) y = 0.1543x - 0.8£ R2 = 0.23 )4 O 0.85 A 0.8 0. 2 0.4 time 0.6 0.8 (min.) Removal Coefficient (K) for absorbance data at agitation settings of 60, 100 and 140. 1. 3- 100 140 .............Linear (100) '""""“ '"Linear (60) ------ -Linear (140) * A M 0.5 0.3 Figure 11. Plots used to determine the removal rate (k) and removal coefficient (K) for cells raised with a 15:1 C:N ratio. Values for K were negative for the agitation rates of 60 100 and 140. 55 water. The reason for the low values compared to the results of Lichtenberg etal. may be due to the hydrocarbon volumes chosen. A t low hydrocarbon-water ratios the response would be influenced by the effects of a limited surface area for the bacteria to adhere to. On the other hand, when a large volume of hydrocarbon is used, the effects of the hydrocarbon surface area should be minimal. If this is true, then statistical analysis of the data should indicate that hydrocarbon volume has little effect on the numbers of cells adhering to the interface. The experiments of Lichtenberg etal. and Sharon etal. used different vessels for agitation of the hydrocarbon-water suspensions. Lichtenberg etal. used the conventional test tube method, whereas Sharon etal. investigated the possibility of using polystyrene cuvettes for agitation and absorbance measurements. They were interested in using cuvettes because it would simplify the amount of time needed to run an experiment. Cuvettes did not work for this research because the bacteria adhered to the sides of the cuvette. Another problem was loss of hydrocarbon and water during the agitation step. The hydrocarbonwater mixture would leak out from under the cuvette cap invalidating the method. Another important factor of the MATH assay is agitation rate. Lichtenberg etal. repeated their experiments using a higher agitation rate which resulted in a similar removal coefficient. The removal coefficient was 414 min ^ for S. pyogenes when subjected to a higher agitation rate while maintaining all other parameters identical to previous experiments. They state that it is possible that the removal coefficient is independent of agitation rate (Lichtenberg 1985). This is not supported by results of this study. The effects of agitation rate on the removal coefficients for both C:N ratios does not support that conclusion. In Figure 10 it is clear that K values for the two agitation rates are not similar. In fact, they have opposite slopes. The same conclusion can be drawn from Figure 11. The slopes for the three agitation rates are not the same. The negative slopes and low 56 coefficients could be the result of saturation with respect to hydrocarbon. The removal coefficients at greater hydrocarbon-to-water ratios should all have similar slopes because surface area is no longer limited with respect to the amount needed for a monolayer of cell attachment. The slopes for the removal coefficients of Figure 11 range from -1.05 m in _1to 3.8 min If the error bars were included, the slopes may not be statistically significantly different. It is possible that the curve is actually flat for high hydrocarbon-to-water ratios. In the case of low volumes of hydrocarbon there is a significant change in the surface area available to the cells for increasing volumes of hydrocarbon. Removal coefficients for this part of the curve should be much steeper than those for high hydrocarbon volumes. The statistical analysis should indicate the relative importance of agitation rate and hydrocarbon volume for a given response. Clumping of the bacteria may possibly be affecting the results of the removal rate and removal coefficient analysis. The tendency of these bacteria to aggregate may somehow influence the removal of the bacteria. The affect of the clumping may cause increased cell removal that is dependent on concentration. If the removal of cells was exponential then most of the removal would take place in the first seconds of agitation. This would cause the slopes to change little with increased mixing times. In most cases, percent removals were greater then 70% after 10 seconds of mixing and did not increase by more the 20% with continued mixing (Tables 6-9). It also looks like the C:N ratio may affect this part of the experiment. For hydrocarbon volumes of 1.0 ml the change in absorbance (AR=% removal ideal at 40 sec - % removal ideal at 10 sec) over the change in time (AT=40-10 sec). This gives rates of .24, .17, .05 min"1 for C:N of 5:1 and .37, .35, .25 min"1 for C:N of 15:1. The rate of change for the 5 :1 data is greater than the rate of change for 15:1 although the variance in the data may not support this conclusion. 57 Graphical Analysis A graphical representation including all the factors allows a comparison of the relative importance of each factor on the response (Figure 12). The importance of all three 3D plot for absorbance of C:N 15:1 % Removal (id ea l) S s * S 9 Time in second s agit rate / HC Figure 12. A three dimensional graph showing the effects of agitation setting, hydrocarbon volume and time of mixing on the percent removal (ideal) for isolate grown at a C:N ratio of 15:1 for absorbance. of the factors is illustrated in Figure 12. The percent removal is lowest at low hydrocarbon volumes, agitation settings and mixing time. As the agitation rate and volume of hydrocarbon increase the percent removal (ideal) increases. The percent removal (ideal) also increases as the mixing time changes from 10 seconds to 40 seconds. The effect of time of mixing can be observed especially well for the hydrocarbon volume of 0.2 ml and 58 an agitation rate of 60. The other relationships are not nearly as clear. The graph shows how little percent removal (ideal) changes at the higher settings. Cell counts for percent removal (ideal) increase as the agitation rate and hydrocarbon volume increase. A graph for percent removal (ideal) at two mixing times for different agitation rates and hydrocarbon volumes illustrate how agitation rate and hydrocarbon volume may influence adhesion of bacteria to hydrocarbon (Figure 13). The error bars for this graph also show how error decreases with increased mixing time, agitation setting and hydrocarbon volume. The two combined factors will influence the percent adhesion to different degrees. Hydrocarbon volume and agitation setting are paired in this representation and cannot be distinguished from each other. To determine the relative influence each factor has on the experiment, a different method of analysis has to be used. The percent removal increases the most as the hydrocarbon volume changes from 0.2 to 1.0. This may not be a real trend, it is likely that the agitation rate is affecting the removal rate more then the change in the hydrocarbon volume. Also, the error bar for (60/.2) at 10 seconds is greater then the percent removal (ideal) for that point (Figure 13). This is also true for the mixing time of 40 seconds, indicating that the data collected for small hydrocarbon volumes and low agitation settings is not very consistent. The methods described so far were not designed for statistical analysis, although the experiment was designed with a particular statistical method in mind. 59 no Percent removal for two mixing times (10 and 40 sec.) for cell counts at a C:N of 15:1. 100 ■o 90 > e 80 E ft 70 L K 60 50 40 00# . . . . . . . . . . . . I §n.I . . . . IJIIj. . . . . . . . . . . . . h i_l- - - h M -i- - 1- - - 1- - - h i Iffl— 110 sec. I 40 sec. I------- , M 5 O kO O O 's. VD O '■Jj O O agitation S S s e t tin g /H C (N 5 O O Tt O Tt volum e Figure 13. Graphical representation for percent removal (ideal) at two mixing times with variation of agitation rate and hydrocarbon volume. Statistical Analysis The experiment was set up so the method of least squares (regression analysis) could be applied to the results. Four factors: agitation rate, hydrocarbon volume, time of mixing and C:N ratio influenced the responses, In (Ao/A), In (Co/C), In (Ac/A), In (Cc/C), In (Ao/Ac) and In (Co/Cc). ANOVA (analysis of variance) was used to determine the variation between experiments and the adjusted R2 and p values. Regression Step wise regressions for each of the factors, cross products and squared terms were used to determine factors and cross factors important to the model. The data were 60 initially split up into three different groups: interference, agitation setting and agitation rate (rpm) data. The regression results were used initially to determine whether rpm or agitation setting as an indicator of mixing intensity, gave a better fit. The method of least squares was used to determine if interference data should be included with the rest of the data set. DataInclusion First the regression data for agitation rate (rpm) and agitation settings were compared. Analysis of the results of the regression determined that using rpm as the factor for agitation rate produced a better fit than agitation settings. This is supported by the higher R2 value (44%) calculated using rpm compared to R2 =39% if agitation is used. Next, the response for the interference data were analyzed. The regression analysis for the data with interference determined that it should not be included in the main data set. The p values for time, HCrH2O, and C:N were all greater than 0.05 for the absorbance data compared to the significant p values determined for the experiments without interference. The R2 value of 9% indicated that very few of the points of the interference could be explained by the model. It is interesting that R2 for the cell counts in the interference data was significantly higher than for absorbance. Absorbance may be more easily influenced by changing conditions of the media and test tubes. Cell counts are not influenced by impurities which affect the absorbance. If impurities are present which do not affect the removal of cells, but cause skewed absorbance readings, the more likely it is that the regression will have outliers. Cell counts for interference would however, only be affected by the initial absorbance reading, which was set around 0.02 for all experiments. In the cases with interference the initial cell counts were lower then those in normal experiments (Figure 14). The error bars for interference for cell counts of 5 :1 indicate that the data for the inter­ ference should be included. As stated earlier, the statistical analysis did not support the inclusion of interference results in the data pool. Another interesting observation is the 61 difference between the initial cell counts for the two culture types. The overlap of the error bars indicates that the two data sets are not statistically different (Figure 14). Initial Cell Count Comparison 2.50E+07 ^ 2.00E+07 g 1.50E+07 S Ave. Init. cell counts B Ave. Init. cell counts (int.) w I 1.00E+07 o O E 5.00E+06 * o 0.00E+00 5:1 cu ltu re 1 5:1 co n d itio n s Figure 14. Initial cell counts for cultures raised at 5 :1 and 15:1 carbon to nitrogen ratios including the cell counts for interference data. Significant Factors Regression analysis was used to fit data to a model. To determine the relative importance of a factor to the model, the magnitude of the p value is compared to 0.05. For values of p > 0.05, there is a very small chance that the factor is important to the model (Table 13). The coefficients of the linear regression also indicate, indirectly, the relative importance of each term. p Values The first response In (Ao/A) has no values of p that are insignificant to the model. The factors time of mixing, agitation, hydrocarbon volume and C:N ratio are all 62 important to the model. For the response In (Co/C) the same is true. This is not the case of the response In (Ac/A) since the coefficients for the factors of hydrocarbon volume and C:N ratio are statistically significant (p > 0.05). This difference could be related to wall effects in the control test tubes. If C:N influences the amount of cells which stick to the glass the controls could have a different response to changes in C:N ratio. One explanation for the lack of correlation with hydrocarbon volume is that the control absorbance (Ac) were uneffected by hydrocarbon while the test values (A) were effected. This explanation is not substantiated by the corresponding cell count regression where p < 0.05 for both hydrocarbon and C:N. Table 13. The p values for the six responses. response time agitation HC vol. C:N Exp (CN) In (Ao/A) 0.0001 0.0001 0.0001 0.0001 0.0001 0.0001 0.0041 0.0001 0.0001 0.0001 Z 0.0001 0.0001 0.0001 Sg H * In (Co/C) In(AcZA) In Cc/C) In (Ao/Ac) In (Co/Cc) ooooi %k49&$ i e F 0.0001 0.0007 * " ” 0 ,46 } :m mm 0.0001 0.0001 0.0001 At different C:N ratios, the production of extracellular materials is likely to be different. The 5 :1 raised cells could be producing a chemical that affects the absorbance of the solution but not cell counts. This may account for the differences in significance of the p value between the cell counts and the absorbance model responses. The last cases to be examined are those of the initial over the control measurements. The p-values for the hydrocarbon should be large because no hydrocarbon is actually involved in the experiment. For the absorbance response only the time of mixing affects the response 63 significantly. This may be because as the mixing time increases, additional cell clumping takes place. Absorbance values would decrease, but cell counts - which were performed after dispersing the clumps - would not be. Agitation rate may cause more clumping as more cells will collide as the rate increases. The more interactions the more likely it becomes that the cells will stick together. C:N ratio may also play a role in clumping of cells. Significant Coefficients The relative importance of factors to the model can be compared to the magnitude of the coefficients for factors in the model. The mean value of a factor times its coefficient is one way of determining a coefficient’s effect on the model. The results in Table 14 were calculated from the regression coefficients and the average value for a given factor. For example, the agitation rate coefficient for In (Ao/A) is 0.0002877 and the average value for rpm is 2433. The adjusted value is equal to the product of the terms or 0.70. For the response In (Ao/A) all the p values are significant and all of the adjusted coefficients are important to this model, so no terms can be removed. For In (Co/C) all of the factors are important to the model. The next case, In (Ac/A), has high p values for the HC volume, and C:N ratio. The adjusted coefficient contradicts the p value data for hydrocarbon and C:N. Both of these factors have significant coefficients, but the p values are greater than 0.05. One possible interpretation is that these factors contribute extensively to the model responses, but that scatter in the data (leading to a low p value) produce low confidence in the result. On the other hand, the coefficient/factor mean product for time is only 0.094, while the corresponding p-value was 0.0001, indicating good fit, but a low net affect of the time as a factor in this response. The results are inconclusive; some factors may not differ significantly from zero (high p-value) and yet the regression coefficient 64 predicts a significant change in response for a change in that factor. For the response In (C dC ) all the coefficients are important. This agrees with the results from the p value Table 14. Constant terms and Coefficients for Responses. Response Constant rpm Time C:N HC volume In Ao/A 1.31 0.70 0.243 -0.625 0.362 In Co/C 1.98 2.62 0.801 -1.711 0.44 InAc/A 0.003 1.36 0.094 -0.123 0.413 In C dC -0.73 2.12 -0.616 -0.701 0.446 In Ao/Ac 1.2 -0.617 0.177 -0.560 0.039 In Co/Cc 3.50 -0.054 -0.105 -0.813 0.088 analysis. The HC coefficients for In Ao/Ac and In Co/Cc are negligible and should not influence the model. This is consistent with the previous finding from the p values. The agitation coefficient for In Co/Cc is small enough to neglect, but this is not supported by the p value. Variation Between Experiments The inter and intra experimental errors were determined using the statistical program SAS for analysis of variance. The values for R2 were determined for the ANOVA model based on each C:N ratio level as a separate experiment. Values for the relative interexperimental and intraexperimental error and the adjusted R2 values can be found in Table 15. The results indicate that the responses not including the controls (In Ao/A), In(CoZC) have a higher interexperiment error than intraexperiment error. The reverse is true in the responses which include the control data. The worst case, control cell counts, has a value for inter experimental error which is 26 times larger than the intra experimental error. One possible explanation for this result is generation effects or possibly the age of the culture used. 65 Table 15. Response In (Ao/A) In (Co/C) In (A d A ) In (CdC) In (Ao/Ac) In (Co/Cc) Values for Interexperimental and Intraexperimental Error. Inter exp. error 0.105 0.780 0.069 0.230 0.016 1.390 Intra exp. error 0.060 0.260 0.074 0.530 0.026 0.052 R2 0.78 0.91 0.79 0.88 0.69 0.92 The R2 values are significantly higher when the experiment-to-experiment variation is accounted for. Cell counts fit the model better than the absorbance measurements. Conversely, the values for inter-experiment error are of the same order of magnitude as the coefficient/factor products in Table 14. Thus, one could conclude that the effects of the test and growth factors are no more significant than variations between experiments. Means and Standard Error Determination of the local and global variance and means with respect to test factors was carried out using the following procedure: I) The data were first divided into two groups; one for each C:N ratio. 2) The data were then sorted according to a single test factor ( e.g., agitation setting) and then according to experiment (each batch of cells). 3) Local means and variances were then determined within each experiment without regard to the other factor settings (for example, the local mean and variance with respect to agitation setting 60 in experiment I were determined without further segregation into time and hydrocarbon volume). 4) A group mean and variance were then computed for all the experiments at each C:N ratio and test factor value. 5) At each factor value, a mean and variance was computed for the two C:N ratios combined. The result was a mean response at that factor setting and a variance for all of the data at that factor setting. 6) Steps 2 through 5 of the above procedure were repeated for each of the other two test factors. 66 The results of this analysis are shown in the following figures. The first two graphs show the results for the responses In (Ao/A) and In (Co/C) vs. hydrocarbon volume (Figure 15). The graph shows that the average responses do not change much with increasing hydrocarbon volumes. The error associated with the responses indicates that the variation between experiments is greater than variation induced by changing hydrocarbon volumes. Results of this analysis on the other four responses (which include the controls) give essentially the same overall picture. In figure 16, the trends of the responses with respect to agitation are more significant. The error bars do not overlap between the middle and low settings but the high setting overlaps the middle setting. This indicates that at the lowest setting, the effects of the error are smaller than the effect of agitation setting. The agitation setting graphs would be similar to the agitation rate graphs (using rpm) except for more overlap for the high agitation rates (2800 and 3100). The last series is the response to time of mixing (Figure 17) . The error bars again overlap the means of the average responses. There does seem to be a slight trend toward increasing removal with longer mixing, but with the large overlap and small changes in the response it can probably be safely assumed that the time of mixing does not significantly change the results. 67 Average In (Ao/A) Response vs. Hydrocarbon Volume values for standard error of the averages 1.2 I 1.1 ? 1 <| 0.9 ^ < > ................... ^ j f < I ♦In Ao/A I I _ _ _ _ _ I_ _ _ _ _ 0.8 0.7 0.6 0 0.2 0.4 0.6 0.8 I 1.2 hydrocarbon volume (ml) Average In (Co/C) response vs Hydrocarbon Volume values for standard error of the averages 6 1.8 ♦In Co/C hydrocarbon volume (ml) Figure 15. Graphs of the responses In (Ao/A) and In (Co/C) vs. hydrocarbon volume including the standard error of the averages. 68 Average In (Co/C) Response vs Agitation Setting values for standard error of the averages 9 a 2 2-1 2 _Ib_ - jI 1.8 O U w I6 I4 - Aln Co/C 3k I2 I 4t) 63 80 100 120 140 160 agitation setting Average In (Ao/A) Response vs Agitation Setting values for standard error of the averages 19 _. ............. I I 4► I . i I0 w id I 09 8 - 4I 07 - 06 05 4<3 6(3 80 100 agitation setting 120 140 160 ♦InAo/A Figure 16. Graphs o f the responses In (Ao/A) and In (Co/C) vs. agitation setting including the standard error of the averages. 69 Average In (Co/C) Response vs Mixing Time values for standard error of the averages 2.4 2.2 ■............. ............. ............ ............ .............. 2 U ^ lB U ............ F#-I1 11 6 - ...... ..... ^► 4► ............ 4 >•............ ♦In Co/C I4 I2 I 5 K) U ---------- 1---------2( 25 3( tinie of mixing (se c) 3/5 4() 4i Average In (Ao/A) Response vs Mixing Time values for the standard error of the averages ♦In Ao/A time of mixing (sec) Figure 17. Graphs of the responses In (Ao/A) and In (Co/C) vs. agitation setting including the standard error of the averages. 70 CONCLUSION Evaluation of the MATH test for reproducibility proved to be a complicated task. The results were evaluated using two statistical approaches. ANOVA was used to determine the interexperimental and intraexperimental errors. A regression analysis was used to compare the significance of each of the test and growth factors on the response. The results from these analyses, when compiled with the results from the conventional methods (percent removal, removal rate and removal coefficients) were enough to draw some conclusions. The test is not reproducible for the results obtained using the (ideal) data. The interexperimental error was two times greater than the intraexperimental error. The effects of the factors varied and in some cases there was a statistical significance but not a practical significance. For the given hypotheses in the introduction the following statements can be made: 1) The MATH test does not yield reproducible results for the given set of test conditions and growth conditions for the bacteria used in this study. 2) The time of mixing will affect the results statistically although it is not of practical significance. 3) Mixing intensity has a practical and statistical significance to the results. 4) The hydrocarbon volume is neither statistically significant or practically significant for the hydrocarbon volumes used in this research. 5) The C: N ratio is not a significant factor in the removal of cells from solution. 6) Cells are removed when agitated without the presence of hydrocarbon. 71 REFERENCES Brown, W.A., Cooper, D.G. (1992) Hydrocarbon Degradation by Acinetobacter caJcoaceticus RAG-1 Using the Self-cycling Fermentation Technique. Biotechnology and Bioengineering, 40, 797-805. Busscher, H.J., Sjollema, J., van der Mei, H.C. (1990) Relative Importance of Surface Free Energy as Measure of Hydrophobicity in Bacterial Adhesion to Solid Surfaces, in Microbial C ellSurfaceHydropkobieity, Doyle, R.J. and Rosenberg, M., Eds., ASM Publications, Washington, D C., pp 335. Camper, A.K., LeChevallier, M.W., Broadway, S.C., McFeters, G.A. (1985) Evaluation of procedures to desorb bacteria from granular activated carbon. Journalof MircrobiologicalMethods, 3, 187-198. Chapatwala, K.D., Newaz, M.S., Richardson, J.D., Walfram J.H. (1990) Isolation and Characterization of Acetenitrile Utilizing Bacteria. Journal o f Industrial Microbiology. Courtney, H.S., Hasty, D.L., Ofek, I. (1990) Hydrophobicity of Group A Streptococci and Its Relationship to Adhesion of Streptococci to Host Cells, in Microbial CellSurface Hydrophobicity, Doyle, R.J. and Rosenberg, M., Eds., ASM Publications, Washington, D.C., pp 361. Duncan-Hewitt, W.C. (1990) Nature of the Hydrophobic Effect. inM icrobial CellSurface Hydrophobicity, Doyle, R.J. and Rosenberg, M., Eds., ASM Publications, Washington, D.C., pp 39. Ener, B., Douglas, L.J. (1992) Correlation between cell-surface hydrophobicity of Candidaalbicans and adhesion to buccal epithelial cells. FEMSMicrobiology Letters, 99, Escher, A. R. (1986) Colonization of a Smooth Surface by Pseudomonas aeruginosa: Image Analysis Methods. PH.D. Thesis, Montana State University. Fattom, A., Shilo, M. (1985) Production of emulcyan by Phormidium J-l: its activity and function. FEMS Microbiology Ecology, 31, 3-9. Funk, H.B., Krulwich T.A. (1964) Preparation of Clear Silica Gels than Can Be Streaked. J Bacteriology, 88, 1200-1201. Goswami, PI, Singh, H. (1991) Different Modes of Hydrocargon Uptake by Two Psuedomonas Species. Biotechnology and Bioengineering, 37, 1-11. Grcmp Report (1984) Mechanisms of Adhesion. In Microbial Adhesion and Aggregation, Inouye, M. (1987) Bacterial OuterM embranesAsM odelSystems. John Wiley & Sons, 72 Kjelleberg, S. (1984) Adhesion to Inanimate Surfaces. InM icrobialAdhesionand Aggregation, Ed K.C. Marshall, 51-70. Klotz, S. ( 1990) Role of Hydrophobic Interactions in Microbial Adhesion to Plastics Used in Medical Devices. In Microbial Cell SurfaceHydrophobicity, Doyle, R.J. and Rosenberg, M., Eds., ASM Publications, Washington, D.C., 107. Lachica, R.V. (1990) Significance of Hydrophobicity in the Adhesiveness of Pathogenic Gram Negative Bacteria, in Microbial Cell Surface Hydrophobicity, Doyle, R.J. and Rosenberg, M., Eds., ASM Publications, Washington, D.C., 297. Lichtenberg, D., Rosenberg, M., Sharfman, N., Ofek, I. (1985) A Kinetic approach to bacterial adherence to hydrocarbon. JournalMicrobiologyMethods, 4, 141-146. Marshall K. C. (1976) Interfaces in Microbial Ecology. Harvard University Press, Cambridge MA, 11-21. Marshall, K. C., Cruickshank, R. H. (1973) Cell Surface Hydrophobicity and Orientation of Certain Bacteria at Interfaces. Arch. Mikrobiol., 91, 29-40. Mudd, S., Mudd, E. B. H. (1924 a) The penetration of bacteria through capillary spaces. IV. A kinetic mechanism in interfaces. J. Exp. Med. 40, 633-645. Mudd, S., Mudd, E. B. H. (1924 b) Certain interfacial tension relations and the behavior of bacteria in films. J. Exp. Med., 40, 647-660. Mueller, R. F. (1990) Characterization of Initial Events of Bacterial Colonization at SolidWater Interfaces Using Image Analysis. M.S. Thesis, Montana State University. Pines, O., Shoham, Y., et. al. (1988) Unmasking of surface components by removal of cell-associated emulsan from Acinobacter_ Sp. RA G-1. Appl. Microbiol. Biotechnol., 28, Prokok, A., Ludvik, M., Erickson, L.E. (1972) Growth Models of Cultures with Two Liquid Phases. VIII. Experimental Observations of Droplet Size and Interfacial Area. Biotechnology and Bioengineering, Vol XIV, 587-608. Rittman, B.E. ( 1989) Detachmentfrom Biofilms, in Structure and Function o f Biofilms, Characklis, W.G., and Wilderer, R A ., Eds., 49-58. Rosenberg, E., Legmann, R., et. al. (1992) Petroleum Bioremediation - a Multiphase Problem. Biodegradation, 3, 337-350. Rosenberg, E. (1992) The Hydrocarbon-Oxidizing Bacteria. In Balows, A. (Ed) The Prokaryotes (pp. 446-459) Springer-Verlag, Berlin. Rosenberg, E.,Shabtai, Y. (1987) In Bacterial Outer Membranes as Model Systems. Masayori Inouye, Ed. (John Wiley & Sons, New York), pp. 311-342. 73 Rosenberg, E , Kaplan, N., Pines, O., Rosenberg, M., Gutnick, D. (1983) Capsular Polysaccharides Interference with Adherence of Acinetobacter calcoaceticus to Hydrocarbon. FEMS Microbiology Letters , 17, 157-160. Rosenberg, M. (1991) Basic and Applied Aspects of Microbial Adhesion at the Hydrocarbon: Water Interface. CriticalReviewsinMicrobiology, 18, No. 2,159-173. Rosenberg, M., Doyle, R.J. (1990) Microbial Cell Surface Hydrophobicity: History, measurement, and significance. In Microbial Cell Surface Hydrophobicity, Doyle, R.J. and Rosenberg, M., Eds., ASM Publications, Washington, D.C., pp I. Rosenberg, M. (1984) Ammonium Sulphate Enhances Adherence of Escherechiacoli J-5 to Hydrocarbon and Polystyrene., FEM SMicrobiology Letters, 25, 41-45. Rosenberg, M., Bayerm, E., Delarea, J., Rosenberg, E. (1982) Role of Thin Fimbriae in Adherence and Growth of Acinetobactercalcoaceticus RAG-1 on Hexadecane. A p p l and EnvironmentalMicrobiology., 44, No. 4, 929-937. Rosenberg, M., Gutnick, D., Rosenberg, E. (1980) Adherence of Bacteria to Hydrocarbons: A Simple Method for Measuring Cell-Surface Hydrophobicity. FEMS Microbiology Letters, 9, 29-33. Sar, N. (1987) Direction of spreading(DOS): a Simple Method for Measuring the Hydrophobicity of Bacterial Lawns. J. Microbiology Methods, 6, 211-219. Sar, N., Rosenberg, E. (1987) Fish Skin Bacteria: Colonial and Cellular Hydrophobicity. Microbial Ecology, 13, 193-202. Shabtai, Y ., Wang, D. I. C. (1990) Production of Emulsan in a Fermentation Process Using Soybean Oil (SBO) in a Carbon-Nitrogen Coordinated Feed. Biotechnology and Bioengineering, 35, 753-765. Sharon, D., Bar-Ness, R., Rosenberg, M. (1986) Measurement of the Kinetics of Bacterml Adherence to Hexadecane in Polystyrene Cuvettes. FEMSMicrobiology Letters, Stolzenback, K.D. (1989) Particle Transport and Attachment, in Structure and Function o f Biofilms, Characklis, W.G., and Wilderer, R A ., Eds., pp 33-47. Sweet, S.P., MacFarlane, T.W., Samaranayake, L.P. (1987) Determination of the Cell Surface Hydrophobicity of Oral Bacteria Using a Modified Hydrocarbon Adherence Method. FEMS Microbiology Letters, 48, 159-163. Wadstrom, T. (1990) Hydrophobic Characteristics of Staphylococci: Role of Surface Structures and Role in Adhesion and Host Colonization, in Microbial CellSurface Hydrophobicity, Doyle, R.J. and Rosenberg, M., Eds., ASM Publications, Washington, D.C., pp 315. Wang, Sy-Dar, Wang, D. I. C. (1990) Mechanisms for Biopolymer Accumulation in Immobilized Acinetobacter calcoaceticus System. Biotechnology and Bioengineering, 36, 402-410. 74 Yu, F.P., Pyle, B.H., McFeters, G.A. (1993) A Direct Viable Count Method for the Enumeration of Attached Bacteria and Assessment of Biofilm Disinfection. Journal o f Microbiological Methods, 17, 167-180. APPENDICES 76 APPENDIX A EXPERIMENTAL RESULTS 77 Experiment I Date: 7/13/94 C:N ratio: 15:1 V Results Time in Seconds Agit rat HC vol. 10 20 60 0.2 0.071 0.055 60 I 0.029 0.029 100 0.6 0.02 0.011 140 0.2 0.017 0.013 140 I 0.017 0.017 plate I 2 3 4 5 6 7 8 9 10 11 40 60 0.032 0.032 0.03 0.012 0.015 0.013 0.013 0.014 Control Time in seconds Agitatic 10 20 40 60 0.127 100 0.08 140 0.07 Dil. fact. ToL CC Std. dev. 0.00E+00 0.000 1.00E+05 5.26E+06 0.148 1.00E+05 3.76E+06 0.107 1.00E+05 4.62E+06 0.069 1.00E+05 3.48E+06 0.261 1.00E+05 1.46E+06 0.092 1.00E+05 1.00E+06 0.339 1.00E+04 1.06E+05 0.398 1.00E+06 1.24E+07 0354 0.00E+00 0.000 1.00E+05 6.22E+06 0.194 Cell Count data agit. rt, Plate counts, where each count represents a one ]a\. sample, he vol, time 1 2 3 4 5 0 I Ink. abs. .181 2 .097 (0.5) 55 62 55 50 41 3 .048 (0.25) 39 32 42 35 40 4 60/1/10 47 48 50 42 44 5 60/1/40 40 21 41 42 30 6 100/.6/20 14 16 14 16 13 7 140/1/10 15 10 11 6 8 8 140/1/40 14 8 16 6 9 9 60/10 14 17 15 10 6 10 11 140/40 69 51 61 79 51 Experiment 2 Date: 7/14/94 C:N ratio: 5:1 Low initial abs. Agitatic 60 100 140 I 2 3 4 5 6 7 8 52.6 37.6 46.2 34.8 14.6 10 10.6 12.4 Time in Seconds 10 20 40 0.024 0.012 0.014 0.022 0.012 0.01 0 0 0 0.001 0.005 0.004 0 0 0 Time in seconds 10 20 0.095 0.083 0.063 Plate counts, where I 2 10 6 12 12 21 19 38 33 21 19 65 66 15 17 24 1 2 3 4 5 6 7 8 Dil. fact. 1.00E+06 1.00E+06 1.00E+05 1.00E+05 1.00E+05 1.00E+05 1.00E+04 1.00E+04 T o tC C Std. dev. 8.20E+06 0.304 8.00E+06 0.468 2.20E+06 0.227 3.34E+06 0.168 2.04E+06 0.285 6.52E+06 0.076 O.OOE+OO 0.000 2.04E+05 0.265 40 each count represents 3 4 5 5 10 10 6 6 4 30 17 23 39 32 25 30 17 15 70 68 57 18 7.765 4.037 3.194 9.094 1342 3391 4.219 4393 62.2 12.050 plate Agitatio HC volt 60 0.2 60 I 100 0.6 140 0.2 140 I Control agit. rate/hc Initial .165 .06 (0.5) .041 (0.25) 60/.2/10 60/1/40 100/. 6/20 140/.2/10 140/.2/40 Averag Std. dev 28 10 Averag' 8.2 8 22 33.4 20.4 65.2 20.4 Std. dev 2.490 3.742 5.000 5.595 5.814 4.970 5.413 78 Experiment 3 Results Date: 7/11/94 Agitatio HC voli 60 0.2 100 0.6 140 I C:N ratio: 5:1 Time in Seconds 10 20 40 0.045 0.024 0.023 0.022 0.016 0.022 0.026 0.015 0.019 Low initial abs. plate 80 0.024 0.022 0.021 160 0.026 0.02 0.022 Control Agitatic 60 Time in seconds 10 20 I 2 3 4 5 6 7 8 Dil. fact. 1.00E+06 1.00E+05 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 ToL CC Std. dev. 2.16E+07 0.862 9.60E+05 0.519 7.60E+05 0.498 1.36E+05 0.377 1.90E+05 0.368 1.92E+05 0.251 7.40E+04 0.365 3.80E+04 0.220 100 140 Cell Count data I 2 3 4 5 6 7 8 Plate counts, agit. rate/he ■ I initial abs .0. 47 Dil. (0.5) 0.0 6 60/.2/10 14 60/.2/160 19 100/.6/40 15 140/1/10 22 140/1/40 12 140/1/160 3 where each count represents 2 3 4 5 35 6 15 5 6 6 16 14 6 5 8 5 7 10 18 14 26 26 10 18 16 26 14 18 6 7 5 7 4 3 4 5 Experiment 4 Results Date 7/15/94 Agitatio HC voh 60 0.2 60 I 100 0.2 100 I 140 I C:N ratio: 15:1 V Time in Seconds 10 20 0.063 0.05 0.022 0.026 0.018 0.018 0.018 0.018 0.019 0.02 plate 40 0.033 0.025 0.016 0.022 0.022 Control Agitatic 60 100 140 Time in seconds 10 20 0.111 0.069 0.095 10 Averag Std. dev 21.6 18.623 9.6 4.980 7.6 3.782 13.6 5.128 19 7.000 19.2 4.817 7.4 2.702 3.8 0.837 40 0.074 0.057 1 2 3 4 5 6 7 8 9 10 11 12 Dil. fact. 1.00E+06 1.00E+05 1.00E+05 1.00E+04 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+05 1.00E+05 1.00E+05 ToL CC Std. dev. 7.60E+06 0.996 2.60E+06 0341 8.20E+05 0380 4.50E+05 0.236 1.08E+06 0.327 1.00E+05 0.430 1.74E+05 0.242 9.20E+04 0.142 4.02E+04 0.164 6.60E+05 0.173 6.80E+05 0.263 6.20E+05 0.629 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 Plate counts, where each count represents agit. rate/hc ■ I 2 3 4 5 IniL Abs. 0.1 6 4 21 4 3 abs. .130 dil. 19 18 22 34 37 abs. .072 dil. 6 5 8 13 9 abs. .042 dil. 33 36 47 50 59 60/.2/10 8 8 9 13 10 60/1/40 6 12 15 12 5 100/. 2/20 22 20 18 16 11 100/1/20 11 9 8 8 10 140/1/20 33 34 41 45 48 60/10 5 6 7 7 8 100/20 9 8 7 5 5 140/40 2 2 8 9 10 10 Averag 0 7.6 26 8.2 45 10.83 10 17.4 9.2 40.2 6.6 6.8 6.2 Std. dev 7.570 8.860 3.114 10.607 3.545 4.301 4.219 1.304 6.611 1.140 1.789 3.899 79 Experiment 5 Date: 7/16/95 C:N ratio 5:1 Low initial abs. Init abs. Aggit./I IOs 20s 40s Oil. 60/.2 0.033 0.033 0.03 1/2 -I 100/.2 0.024 0.02 0.013 1/4 i( 100/1 0.015 0.008 0.008 140/.2 0.019 0.011 0.01 Control Aggit. 1 10s 20s 40s 60 0.092 100 0.088 140 0.086 Experiment 6 Results Date: 7/18/94 Agitatio HC volt 60 0.6 100 0.6 140 0.2 140 0.6 140 I C:N ratio: 15:1 V Time in Seconds 10 20 0.035 0.032 0.024 0.017 0.018 0.016 0.02 0.018 0.016 0.018 plate 40 0.031 0.016 0.016 0.018 0.016 Control Agitatic 60 100 140 Time in seconds 10 20 0.102 0.097 0.087 40 0.085 I 2 3 4 5 6 7 8 9 10 11 12 Dil. fact. 1.00E+05 1.00E+05 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+04 1.00E+05 1.00E+05 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 agit. rate/hc' Init abs. 0.1! 0.75 0.5 0.25 60/.6/20 100/. 6/20 140/.2/20 140/.6/20 140/1/20 60/20 100/20 140/20 Plate counts, where each count represents a one u\. sample. I 2 3 4 5 6 7 21 18 22 26 28 20 32 12 12 20 16 25 9 17 9 13 19 11 17 19 6 50 46 55 33 54 63 38 38 42 36 37 23 44 44 14 12 6 15 17 21 11 5 22 12 10 6 11 10 10 6 13 10 13 9 10 25 27 32 24 28 27 30 38 41 39 45 57 59 54 11 15 11 9 8 I6 9 13 9 14 10 14 14 12 8 19 15 11 53 62 16 15 8 33 53 8 8 T o tC C Std. dev. 2.45E+06 0.209 1.59E+06 0.312 1.24E+06 0.401 4.87E+05 0.195 4.05E+05 0.240 1.27E+05 0382 1.13E+05 0.419 1.05E+05 0.239 2.84E+04 0.101 4.73E+05 0.163 9.50E+05 0.273 1.20E+06 0.219 0 0 0 0 0 0 9 28 12 5 39 42 9 10 14 28 43 7 16 10 Averag Std. dev 31 24.5 5.126 21 15.9 4.954 14 12.4 4.971 56 48.7 9.476 37 40.5 9.710 6 12.7 4.855 12 113 4.739 12 10.5 2.506 30 28.4 2.875 44 473 7.732 11 9.5 2.593 10 12 2625 80 Experiment 7 Date: 7/19/94 C:N ratio: 5:1 Results Agitatio 60 60 100 plate Time in Seconds HC volt 10 20 40 0.6 0.038 0.032 0.034 0.2 0.048 0.034 0.029 0.6 0.032 0.038 0.038 I 2 3 4 5 6 7 8 9 10 11 12 Interference Control Agitatic 60 100 140 Time in seconds 10 20 0.094 0.099 0.054 40 Dil. fact. 1.00E+05 1.00E+04 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+03 1.00E+03 1.00E+04 1.00E+04 1.00E+04 T o tC C Std. dev. 1.27E+06 0.324 4.46E+05 0.182 3.85E+06 0.314 2.43E+05 0.254 1.89E+05 0.077 1.10E+05 0.249 4.50E+04 0.093 4.54E+04 0.084 3.29E+04 0.083 3.00E+05 0.193 3.80E+05 0.181 2.38E+05 0345 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 agit. rate/hc' Inif abs. .181 abs. .112 dil abs. .044 dil 60/.6/10 60/.2/10 100/.6/20 100/. 6/40 140/.6/10 140/.6/20 60/20 100/20 140/20 Experiment 8 Date: 7/21/1994 C:N ratio: 15:1 V Plate counts, where I 2 12 17 43 39 37 38 16 23 22 18 12 8 42 42 43 50 32 36 35 29 43 33 23 24 each count represents a one /d. sample. 3 4 5 6 7 13 16 19 8 15 53 34 49 40 32 45 58 57 37 24 34 17 25 18 31 19 20 19 18 17 12 13 16 10 8 51 45 41 41 47 41 45 45 51 50 35 36 36 31 28 35 23 39 23 26 31 34 37 35 35 15 20 16 19 17 Results Agitatio 60 60 100 140 140 plate Time in Seconds HC volt 10 20 40 0.2 0.038 0.024 0.025 I 0.022 0.025 0.014 0.6 0.024 0.012 0.022 0.2 0.013 0.011 0.012 I 0.016 0.019 0.012 1 2 3 4 5 6 7 8 9 10 11 12 Control Agitatic 60 100 140 Time in seconds 10 20 0 11 0.078 0.102 40 8 12 51 26 28 19 12 52 40 32 28 54 39 9 8 55 38 29 18 8 44 44 32 26 43 33 10 7 50 25 22 45 31 36 35 32 Averag' 12.700 44.600 38.500 24.300 18.889 11.000 45.000 45.400 32.900 30.000 38.000 23.800 Std. dev 4.111 8.127 12.104 6.183 1.453 2.739 4.183 3.806 2.726 5.793 6.864 8.203 Dil. fact. T o tC C Std. dev. 1.00E+06 1.35E+07 0305 1.00E+05 1.39E+06 0.259 0.00E+00 0.000 1.00E+04 2.23E+05 0.238 1.00E+04 6.33E+05 0.125 1.00E+04 7.62E+05 0.102 1.00E+04 2.23E+05 0.146 1.00E+04 2.29E+05 0.133 1.00E+04 9.25E+04 0.257 1.00E+05 1.21E+06 0.281 1.00E+05 1.18E+06 0.211 1.00E+05 1.14E+06 0.284 0.075 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 Plate counts, agit. rate/hc ■ I Init dil. .2 14 0.75 11 abs. .094 dil. 1/2 abs. .048 dil. 17 60/.2/20 61 60/1/20 74 100/. 6/20 20 140/. 2/20 21 140/1/20 10 60/40 13 100/20 13 140/10 15 where each count represents 2 3 4 5 7 11 17 21 12 10 17 19 20 62 85 24 26 8 12 11 6 26 63 83 29 22 13 10 11 7 20 68 69 20 23 10 17 10 12 31 78 64 24 23 11 6 11 14 6 11 10 7 12 25 67 83 20 18 9 15 18 48 73 11 8 10 8 17 17 9 15 16 28 60 70 20 28 16 63 74 5 8 12 15 16 14 9 21 22 15 12 10 Averag' Std. dev 10 13.50 4.12 17 13.90 3.60 87 12.11 8 14 22.33 63.33 76.20 22.25 22.88 9.25 12 11.78 11.40 5.32 7.94 7.79 3.24 3.04 2.38 3.41 2.49 3.24 81 Experiment 9 7/26/94 Init abs. .188 Dil. Abs. 1/2 0.076 1/4 0.033 C:N 15:1 V Control Experiment 10 Date: 8/28/94 C:N ratio: 5:1 V Aggit. E 10s 60 100 0.081 140 AggiUl60/.2 60/1 100/.2 140/.6 10s 0.055 0.041 0.013 0.013 20s 0.091 40s 20s 0.031 0.031 0.013 0.008 0.065 0.091 Results Agitatio 60 140 140 140 40s 0.031 0.022 0.013 0.018 plate Time in Seconds HC volt 10 20 40 0.2 0.029 0.026 0.031 0.6 0.026 0.026 0.021 0.6 0.028 I 0.024 0.029 0.021 Control Agitatic 60 Time in seconds 10 20 100 140 0.11 40 0.102 I 2 3 4 5 6 7 8 9 10 11 12 Dil. fact. 1.00E+06 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+05 1.00E+05 1.00E+05 T ot CC Std. dev. 1.49E+07 0.303 2.08E+06 0.262 3.84E+05 0.101 3.50E+05 0.139 2.28E+05 0.193 0.00E+00 0.000 8.20E+04 0.249 5.50E+04 0324 7.60E+04 0.299 7.90E+05 0.295 1.50E+06 0.290 1.39E+06 0.291 0.09 Cell Count data 7 8 9 10 11 12 Plate counts, where each count represents a one pi. sample. agit. rate/hc 1 1 2 3 4 5 6 7 Init abs. .20 12 19 13 12 13 21 15 abs. .088 dil. 25 28 18 17 26 13 27 abs. .042 dil. 43 46 37 33 39 40 38 60/.2/10 26 34 37 31 38 33 41 60/.2/40 16 17 21 25 28 22 20 140/.6/10 140/.6/40 5 6 7 10 11 7 9 140/1/10 3 7 4 9 5 5 7 140/1/40 5 10 8 7 10 4 10 60/40 10 6 11 9 8 10 9 100/20 17 10 10 16 19 19 15 140/10 8 20 10 13 21 12 15 Experiment 11 9/3/94 Init abs. .197 AggiVP Dil. Abs 60/0.2 C:N 5:1 1/2 0.083 60/1.0 1/4 0.027100/0.6 V 140/0.2 140/1 10s 20s 40s 0.06 0.043 0.036 0.05 0.048 0.032 0.034 0.027 0.022 0.042 0.029 0.029 0.026 0.019 0.019 Control AggiL E 10s 20s 40s 60 0.101 100 0.1 140 0.09 8 21 21 37 34 26 8 9 8 19 34 41 24 10 Averag Std. dev 14.89 4.51 14 20.80 5.45 37 38.40 3.89 35.00 4.85 22.80 4.39 10 8 4 8 4 11 5 9 7 12 14 14 22 12 6 5 5 8.20 5.50 7.60 7.90 15.00 13.90 2.04 1.78 2.27 2.33 4.35 4.04 82 Experiment 12 Date: 9/8/94 C:N ratio: 5:1 Interference Results Time in Seconds 10 20 40 0.094 0.094 0.093 0.087 0.08 0.078 0.076 0.078 0.071 Agitatio HC volt 60 0.2 100 0.6 140 I Control Time in seconds Agitatio 10 20 40 60 0.127 100 0 11 140 0.121 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 agit. rate/hc' Init. abs. .19! abs. .094 dil. abs. .058 dil. 60/.2/10 60/.2/40 100/. 6/20 140/1/10 140/1/40 60/10 100/20 140/40 100/. 6/20 Experiment 13 Date: 9/10/94 C:N ratio: 5:1 V Plate counts, where I 2 11 10 24 31 15 27 29 30 54 53 14 16 6 4 7 5 19 37 32 21 25 22 13 12 Time in Seconds HC volt 10 20 40 0.2 0.028 0.014 0.013 0.6 0.026 0.016 0.014 I 0.014 0.012 0.012 Control Agitatic 60 100 140 I 2 3 4 5 6 7 8 9 10 11 12 Dil. fact. 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+03 1.00E+03 1.00E+03 1.00E+04 1.00E+04 1.00E+04 1.00E+03 each count represents a one /<1. sample. 3 4 5 6 7 11 13 12 7 12 25 31 29 23 26 18 22 20 21 16 30 29 29 36 28 44 58 45 44 55 16 10 10 13 13 4 4 4 4 8 5 5 5 7 6 18 15 31 35 35 20 13 27 16 17 25 24 24 23 22 13 11 15 15 18 Results Agitatio 60 10 140 plate Time in seconds 10 20 0.07 0.072 40 plate I 2 3 4 5 6 7 8 9 10 11 8 18 29 18 25 55 13 5 6 23 20 25 10 Dil. fact. 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+03 1.00E+04 1.00E+04 1.00E+05 T o tC C Std. dev. 1.21E+06 0.235 2.78E+05 0.138 1.97E+05 0.173 2.95E+05 0.104 4.94E+04 0.126 1.33E+04 0.170 5.11E+03 0.301 5.67E+03 0.153 2.76E+05 0.292 2.06E+05 0.279 2.33E+05 0.128 1.35E+04 0.219 9 14 25 19 40 16 7 5 31 24 27 18 10 Averag Std. dev 13 12.10 2.85 35 27.80 3.82 21 19.70 3.40 29.50 3.07 46 49.40 6.22 12 13.30 2.26 5.11 1.54 5.67 0.87 32 27.60 8.07 16 20.60 5.74 16 23.30 2.98 10 13.50 2.95 T o tC C Std. dev. 0.00E+0O 0.000 9.70E+05 0.296 2.78E+05 0.159 3.40E+05 0.157 2.13E+05 0.147 1.14E+05 0.212 8.44E+03 0.272 1.06E+04 0.393 3.26E+05 0.138 3.87E+05 0.131 1.04E+06 0.334 0.08 Cell Count data 1 2 3 4 5 6 7 8 9 10 11 Plate counts, where each count represents a one ]A. sample agit. rate/hc I 2 3 4 5 6 7 Init. abs. .198 abs. .098 dil. 13 7 6 15 10 11 8 abs. .033 dil. 30 24 26 21 33 26 26 60/.2/10 36 26 39 36 40 30 25 60/.2/40 18 16 19 21 23 21 22 100/ . 6/20 8 8 11 12 10 12 11 140/1/10 7 7 5 7 10 11 7 140/1/40 6 8 10 9 8 7 10 60/10 35 36 33 37 32 28 33 100/20 46 41 31 40 44 40 39 140/40 9 10 11 5 15 13 10 10 Averag Std. dev 11 29 35 27 16 11 16 23 33 7 7 35 39 22 13 11 13 36 41 16 9 34 24 13 19 32 8 9.70 27.78 34.00 21.30 11.40 8.44 10.60 32.56 38.70 10.40 2.87 4.41 5.33 3.13 2.41 2.30 4.17 4.50 5.08 3.47 83 Experiment 14 9/11/94 C :N 15:1 Interference Init abs. Aggit./! IOs 20s 40s DiL 60/0.2 0.048 0.044 0.036 1/2 (60/1.0 0.052 0.046 1/4 ( 100/0.6 0.049 0.044 0.033 140/1 0.044 0.036 0.036 Control Aggit. E IOs 20s 40s 60 0.132 100 0.095 140 0.092 Experiment 15 Date: 9/13/94 C:N ratio: 5:1 Results Agitatio 60 100 140 plate Time in Seconds HC volt 10 20 40 0.2 0.093 0.088 0.081 0.6 0.084 0.079 0.081 I 0.079 0.083 0.082 Interference Control Agitatic 60 100 140 Time in seconds 10 20 0.183 0.189 0.143 1 2 3 4 5 6 7 8 Dil. fact. 5.00E+04 1.00E+04 I .OOE+04 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+03 ToL CC Std. dev. 7.70E+05 0.144 4.39E+05 0.148 3.46E+05 0.133 2.46E+05 0.248 1.26E+05 0.160 6.70E+04 0.282 4.86E+04 0.126 1.95E+04 0.194 7 15 44 31 8 15 44 36 22 22 9 15 32 28 14 13 6 55 25 5 45 15 46 23 40 0.192 Cell Count data agit. rate/hc I abs. .190 1/2 abs .075 1/4 abs .027 60/.2/10 60/.2/40 100/.6/20 140/1/10 140/1/10 Plate counts, where each count represents I 2 3 4 5 16 20 15 15 11 40 41 40 48 50 29 36 36 39 34 26 29 33 32 20 11 14 13 11 14 5 6 6 8 7 43 41 54 51 47 20 23 18 13 17 6 17 56 42 23 14 8 44 20 10 10 11 10 Averag Std. dev 15 15.40 2.22 44 43.90 6.51 34.56 4.59 24.56 6.09 16 12.60 2.01 5 6.70 1.89 60 48.60 6.13 21 19.50 3.78 84 Experiment 16 Date: 9/14/94 C:N ratio: 5:1 Interference Results plate Time in Seconds Agitatio HC volt 10 20 40 60 I 0.06 0.054 0.05 100 0.6 0.054 0.055 0.05 140 0.2 0.053 0.053 0.052 140 I 0.054 Control Agitatic 60 100 140 Time in seconds 10 20 0.14 0.104 40 DiI. fact. T o t CC Std. dev. 1.00E+06 1.94E+07 0.152 1.00E+04 5.12E+05 0.123 I 2 3 4 5 6 7 8 9 10 11 12 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+03 1.00E+05 1.00E+05 1.00E+05 2.51E+05 1.80E+05 7.25E+04 1.21E+05 3.15E+04 1.80E+04 2.73E+06 1.44E+06 1.04E+06 0.105 0.185 0.293 0.215 0.153 0.296 0.111 0.240 0.271 0.108 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 Plate counts, where each count represents a one n\. sample. agit. rate/hc ■ I 2 3 4 5 6 7 I abs. .204 18 17 26 19 18 21 21 1/2 abs. 0.04 39 59 55 50 52 58 46 1/4 abs. .028 60/1/10 27 22 24 28 26 26 22 60/1/40 15 22 15 17 24 22 17 100/. 6/20 11 5 8 9 6 6 5 140/.2/10 16 12 11 12 13 16 13 140/.2/40 29 21 29 35 34 31 29 140/1/10 26 24 22 20 13 13 15 60/10 22 32 29 30 28 24 26 100/20 13 10 13 13 21 11 15 140/40 16 10 10 8 8 14 11 Experiment 17 Date: 9/15/94 C:N ratio: 5:1 V Results plate Time in Seconds Agitatio HC volt 10 20 40 60 I 0.041 0.023 100 0.6 0.014 140 0.2 0.015 0.011 Control Agitatic 60 100 140 Time in seconds 10 20 40 0.094 I 2 3 4 5 6 7 8 9 10 11 8 15 49 9 20 47 10 Averag' Std. dev 19 19.40 2.95 57 51.20 6.29 28 16 8 8 34 21 27 16 8 27 16 21 16 11 35 16 28 18 9 9 38 10 Oil. fact. 1.00E+06 1.00E+05 1.00E+05 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+05 1.00E+05 1.00E+05 25.10 18.00 7.25 12.10 31.50 18.00 27.33 14.44 10.44 2.64 3.33 2.12 2.60 4.81 5.33 3.04 3.47 2.83 T o t CC Std. dev. 2.63E+07 0.193 2.41 E+06 0.190 1.67E+06 0.281 8.00E+05 0.328 4.00E+05 0.190 1.38E+05 0.213 1.60E+05 0.265 3.95E+04 0.086 1.27E+06 0.285 1.53E+06 0.200 1.90E+06 0.211 0.144 0.152 Cell Count data agit. rate/hc I abs. 0.204 dll 1/2 dll 1/4 60/1/10 60/1/40 100/.6/20 140/.2/10 8 140/.2/40 9 60/40 10 100/20 11 140/10 Plate counts, where each count represents a one ]*\. sample. I 2 3 4 5 6 7 26 35 23 25 30 22 32 30 26 17 20 27 28 25 20 10 10 18 19 19 14 7 7 5 6 6 7 12 34 56 39 36 27 42 42 17 17 16 13 17 9 12 17 21 13 15 8 20 13 37 39 39 33 39 44 43 20 16 16 10 10 12 11 18 16 13 11 13 19 16 19 15 20 13 13 20 24 8 29 20 20 8 37 13 15 44 8 11 21 9 22 24 9 43 10 22 39 11 18 23 10 Averag' Std. dev 19 26.30 5.08 4.58 24.13 13 16.70 4.69 13 8.00 2.62 44 40.00 7.60 14 13.80 2.94 16 16.00 4.24 38 39.50 3.41 13 12.70 3.62 18 15.30 3.06 22 19.00 4.00 85 Experiment 18 Results Date: 9/16/94 plate Agitatio 60 100 140 C:N ratio: 5:1 Dil. fact. T o tC C SId. dev. I contaminated 2 1.00E+04 3.89E+05 0.074 3 1.00E+04 3.02E+05 0.176 4 1.00E+04 1.46E+05 0.165 5 1.00E+04 8.22E+04 0.190 6 contaminated 7 1.00E+03 6.80E+03 0.316 8 contaminated 9 1.00E+05 1.84E+06 0.251 10 1.00E+05 1.40E+06 0.208 11 contaminated Time in Seconds HC volt 10 20 40 0.2 0.04 0.025 0.6 0.012 I 0.011 0.013 Interference Control Agitatic 60 100 140 Time in seconds 10 20 0.17 0.191 40 I 2 3 4 5 6 7 8 9 10 0.195 Cell Count data I 2 3 4 5 6 7 8 9 10 agit. rate/hc' dil I abs .204 dil 1/2 abs .1 dil 1/4 abs .( 60/.2/10 60/.2/40 100/.6/20 140/1/10 140/1/40 60/10 100/20 Experiment 19 Date: 9/17/94 C:N ratio: 5:1 V Plate counts, where each count represents a one /d. sample. I 2 3 4 5 6 7 8 9 10 Averag Std. dev 35 35 16 6 38 31 19 8 41 22 12 8 39 27 12 9 34 30 13 9 40 25 14 i6 41 28 14 9 37 38 13 11 43 36 15 8 41 9 3 5 9 10 7 7 7 5 18 16 20 18 16 13 14 15 13 13 Z3 10 14 18 18 12 20 11 Results Agitatio 60 100 140 100 38.90 30.22 14.60 8.22 2.88 5.33 2.41 1.56 6 6.80 2.15 28 18.40 14.00 4.62 2.92 18 plate Time in Seconds HC volt 10 20 40 0.2 0.045 0.039 0.034 0.6 0.03 0.024 0.02 I 0.019 0.016 0.016 0.6 0.023 Control Agitatic 60 100 140 Time in seconds 10 20 40 0.084 Dil. fact. T o tC C Std. dev. 1.00E+06 7.70E+06 0.318 0.00E+00 0.000 1.00E+04 9.00E+04 0.319 1.00E+04 1.63E+05 0.188 1.00E+04 8.33E+04 0312 1.00E+03 2.01E+04 0.181 1.00E+03 1.48E+04 0.208 1.00E+03 8.60E+03 0.199 1.00E+03 2.66E+04 0.112 1.00E+05 9.30E+05 0.273 11 1.00E+05 1.06E+06 0.425 12 1.00E+05 3.17E+06 0.119 I 2 3 4 5 6 7 8 9 10 0.091 0.125 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 Plate counts, where each count represents a one /d. sample. agit. rate/hc I 2 3 4 5 6 7 init abs .202 9 5 5 5 12 10 9 abs. .090 dil 1/2 abs. .044 dil 12 10 12 10 13 5 8 60/.2/10 14 17 13 16 14 18 18 60/.2/40 11 11 12 7 7 8 5 100/. 6/20 22 26 16 19 22 15 18 140/1/10 15 18 14 12 9 18 16 140/1/40 10 10 8 7 8 8 8 100/.6/20 24 30 28 31 25 26 30 60/40 9 5 9 7 8 14 12 100/20 5 7 5 10 8 9 16 140/10 41 30 28 34 33 3I 30 a*3RR*S&**&*S&*S 8 6 9 7 5 17 5 22 19 6 26 11 16 29 8 13 9 17 14 9 23 9 15 32 10 Averag Std. dev 9 7.70 2.45 7 23 24 13 12 23 9 15 29 9.00 16.30 8.33 20.10 14.80 8.60 26.60 9.30 10.60 31.70 2.87 3.06 2.60 3.63 3.08 1.71 2.99 2.54 4.50 3.77 8 # # 86 Experiment 20 9/ 18/94 Init abs. .200 Dil. Abs. 1/2 0.063 1/4 0.041 AggiUl 60/0.2 100/0.6 140/0.2 IOs 20s 40s 0.043 0.046 0.04 0.026 0.029 0.03 0.026 0.02 0.017 C:N 15:1 Control V Aggit. I IOs 20s 40s 60 0.146 100 0.131 140 0.101 Experiment 21 Results Date: 9/19/94 Agitatio HC voli 60 I 100 0.6 140 0.2 C:N ratio: 5:1 V plate Time in Seconds 10 20 40 0.033 0.03 0.028 0.016 0.013 0.013 0.017 0.018 0.013 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+04 1.00E+04 1.00E+05 2.79E+05 2.13E+05 1.80E+05 7.40E+04 1.18E+05 2.97E+04 3.20E+05 3.20E+05 1.62E+06 6 21 7 10 8 14 9 12 10 Averag Std. dev 14.22 3.73 32 17 21 6 9 49 29 33 9 25 27 18 5 16 49 29 39 13 18 19 20 5 16 44 33 27 17 23 22 15 7 14 41 25 27 13 32 Control Time in seconds 10 20 0.09 100 0.088 140 Agitatic 60 Dil. fact. T o tC C Std. dev. 1.00E+06 1.42E+07 0.263 I 2 3 4 5 6 7 8 9 10 11 40 0.202 0.251 0.196 0.409 0.270 0.160 0.159 0.159 0312 0.11 Cell Count data 1 2 3 4 5 6 7 8 9 10 11 Plate counts, where each count represents i agit. rate/hc 1 I 2 3 4 5 init abs ..208 18 10 13 13 17 abs. 108dil 1/2 abs. .036 dil 36 27 30 33 23 60/1/10 21 21 32 19 14 60/1/40 16 22 12 23 15 100/.6/20 14 6 6 11 9 140/.2/10 9 8 14 13 11 140/.2/40 46 46 38 43 37 60/10 31 33 37 20 28 100/20 28 33 31 42 28 140/40 14 18 19 28 16 18 5 8 36 32 32 15 27.90 21.33 18.00 7.40 11.80 29.70 32.00 32.00 16.20 5.63 5.36 3.53 3.03 3.19 4.74 5.10 5.10 5.05 87 Experiment 22 Results Date: 9/22/94 Agitatio HC voh 60 I 60 I 140 0.2 C:N ratio: 5:1 Time in Seconds 10 20 0.027 0.018 0.025 0.02 0.01 0.009 plate 40 0.01 0.01 0.01 V Control Agitatk 60 100 140 Time in seconds 10 20 40 0.043 I 2 3 4 5 6 7 8 9 10 11 Dil. fact. 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+04 1.00E+05 T o t CC Std. dev. 0.00E+00 0.000 0.00E+00 0.000 1.78E+05 0.238 2.44E+05 0.177 2.15E+05 0333 2.53E+05 0.251 1.76E+05 0.235 1.07E+05 0.245 3.30E+04 0.189 2.39E+05 0.264 9.00E+05 0.210 0.09 Cell Count data I 2 3 4 5 6 7 8 9 10 11 Plate counts, where each count represents a one u\. sample. agit. rate/hc I 2 3 4 5 6 7 initabs. 0.199 dil I abs. .062 dil 1/2 abs. .024 dil 19 19 18 26 20 17 10 60/1/10 22 24 30 25 18 22 20 60/1/40 17 21 35 15 20 12 23 60/1/10 25 34 29 32 18 20 18 60/1/40 17 17 15 18 26 18 14 140/.2/10 7 8 12 10 10 15 12 140/.2/40 20 38 31 40 37 26 33 60/40 18 14 17 23 28 30 33 140/10 13 10 8 8 9 9 7 tax p crim cn t 23 C :N 5:1 Init abs. AggiVI 10s 20s 40s Dil. 60/1 0.066 0.06 0.058 1/2 (100/0.6 0.034 0.03 0.03 1/4 ( 140/0.2 0.04 0.033 0.03 140/1 0.025 0.015 0.017 Interference Control 10/4/94 Aggit. I IOs 20s 40s 60 0.075 100 0.129 140 0.132 8 9 19 32 26 18 22 11 38 30 7 13 27 30 28 11 8 36 24 8 10 Averag' Std. dev 17 24 16 31 18 14 31 22 11 17.80 24.40 21.50 25.30 17.60 10.70 33.00 23.90 900 4.24 4.33 7.17 6.34 4.14 2.63 6.24 6.31 I RQ 88 Experiment 24 Date: 10/7/94 C:N ratio: 15:1 V Results Time in Seconds Agitatio HC volt 10 20 60 0.2 0.07 0.068 60 I 0.056 0.063 100 0.6 0.029 0.026 140 0.2 0.033 0.023 140 I 0.03 0.016 40 0.028 0.029 0.022 0.02 0.017 Control Agitatic 60 100 140 Time in seconds 10 20 0.118 0.111 40 0.081 2 3 4 5 6 7 8 9 10 11 12 13 14 15 1.00E+05 1.00E+05 1.00E+05 1.00E+04 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+05 1.00E+05 1.00E+05 1.39E+06 1.10E+06 1.41E+06 5.11E+05 7.00E+05 4.14E+05 2.21E+05 2.56E+05 6.80E+04 1.30E+05 2.01E+04 1.07E+06 1.48E+06 1.80E+06 0.289 0.113 0.235 0.140 0.261 0.116 0.248 0.143 0.167 0.202 0.221 0.165 0.188 0.126 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 13 14 15 agit. rate/hc initabs. 0.19 dil 1/2 d ill/4 60/.2/10 60/.2/40 60/1/10 60/1/40 100/. 6/20 140/.2/10 140/. 2/40 140/1/10 140/1/40 60/10 100/20 140/40 Plate counts, where I 2 14 14 20 13 12 10 10 14 49 51 8 7 43 37 18 16 32 29 5 8 13 14 18 20 13 12 17 10 15 19 each count represents a one pi. sample. 3 4 5 6 7 11 11 15 15 16 17 11 12 7 12 12 11 9 10 10 9 11 15 16 17 62 59 45 54 36 8 6 6 7 8 39 42 39 34 50 30 21 26 21 24 27 26 24 27 27 7 7 6 6 7 11 18 14 14 13 21 27 24 17 14 11 10 13 10 9 15 12 14 14 18 20 21 17 14 19 8 13 18 13 13 51 7 41 16 23 7 14 19 8 16 20 9 10 15 12 17 51 10 41 18 21 6 11 15 12 13 18 10 Averag. Std. dev 7 12.60 2.80 13.89 4.01 11 11.00 1.25 19 14.10 3.31 53 51.10 7.17 3 7.00 1.83 48 41.40 4.79 31 22.10 5.49 20 25.60 3.66 9 6.80 1.14 8 13.00 2.62 26 20.10 4.43 9 10.70 1.77 19 14.80 2.78 17 18.00 2.26 89 Experiment 25 Date: 10/8/94 C:N ratio: 5:1 Interference Results plate Time in Seconds Agitatio HC volt 10 20 60 0.2 0.084 0.078 60 0.2 0.09 0.079 60 I 0.087 140 0.2 0.073 0.065 140 I 0.069 0.065 40 0.078 0.071 0.061 0.06 0.068 Control Agitatic 60 100 140 Time in seconds 10 20 0.118 0.124 40 0.105 0.148 I 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Dil. fact. ToLCC Std. dev. 1.00E+05 1.32E+06 0.257 0.00E+00 0.000 1.00E+04 2.20E+05 0.233 1.00E+04 2.09E+05 0.202 1.00E+04 1.29E+05 0.202 1.00E+04 1.60E+05 0.179 1.00E+04 1.33E+05 0.279 1.00E+04 1.43E+05 0.218 1.00E+04 1.00E+05 0.271 1.00E+04 6.10E+04 0.196 1.00E+03 3.79E+04 0.078 1.00E+03 1.70E+04 0.232 1.00E+03 1.69E+04 0.237 1.00E+04 1.97E+05 0.225 1.00E+05 1.74E+06 0.128 1.00E+04 3.94E+05 0.090 1.00E+05 1.37E+06 0.220 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Plate counts, where each count represents a one /d. sample. agit. rate/hc ■ I 2 3 4 5 6 7 init abs .204 14 15 19 13 11 7 10 dil 1/2 d ill/4 24 29 28 23 25 20 14 60/.2/10 27 17 24 18 18 23 28 60/.2/40 15 16 10 11 9 11 12 60/.2/10 14 14 18 20 20 13 12 60/.2/40 13 13 8 19 16 15 12 60/1/10 20 10 14 15 14 16 12 60/1/40 13 14 9 10 5 7 10 140/.2/10 7 8 6 6 6 5 7 140/. 2/40 39 32 37 35 42 37 41 140/1/10 15 25 19 16 16 21 14 140/1/40 17 20 17 22 10 23 16 60/10 23 17 14 20 13 25 21 60/40 20 17 13 17 17 17 21 140/10 39 41 43 35 44 43 39 140/40 18 16 16 16 13 14 8 8 16 9 12 10 Averag Std. dev 15 13.20 3.39 23 17 16 15 10 17 12 7 40 14 17 24 16 39 13 14 18 14 18 18 11 9 4 37 13 13 16 17 33 13 20 19 15 16 9 11 5 39 14 24 19 38 10 22.00 20.90 12.90 16.00 13.30 14.33 10.00 6.10 37.90 17.00 16.90 19.70 17.40 39.40 13.70 5.12 4.23 2.60 2.87 3.71 3.12 2.71 1.20 2.96 3.94 4.01 4.42 2.22 3.53 3.02 90 Experiment 26 Date: 10/11/94 C:N ratio: 5:1 Interference Results Agitatio 60 60 140 140 plate Time in Seconds HC volt 10 20 0.2 0.061 0.048 I 0.071 0.061 0.2 0.058 0.055 I 0.056 I 2 3 4 5 6 7 8 9 10 11 12 13 14 15 40 0.047 0.051 0.045 0.038 Control Agitatic 60 100 140 Time in seconds 10 20 0.098 0.101 40 0.091 Dil. fact. 1.00E+06 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+03 1.00E+03 1.00E+03 1.00E+04 1.00E+04 1.00E+04 1.00E+03 T ot CC Std. dev. 8.60E+06 0.221 9.40E+05 0.355 1.78E+05 0.187 1.68E+05 0.255 1.14E+05 0326 2.08E+05 0.193 7.70E+04 0.260 4.32E+04 0.133 1.86E+04 0.218 2.42E+04 0.188 1.61E+04 0.148 3.10E+05 0.156 3.66E+05 0.126 2.38E+05 0.163 4.77E+04 0.131 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Plate counts, where agit. rate/hc ■ I 2 init abs .201 12 8 abs .075 dll I 11 14 abs .042 dil I 17 17 60/.2/10 13 15 60/.2/40 12 7 60/1/10 20 19 60/1/40 11 5 140/.2/10 41 49 140/.2/40 23 26 140/1/10 24 28 140/1/40 13 14 60/10 28 26 100/20 36 38 140/40 29 28 100/. 6/20 40 41 each count represents a one ] 3 4 5 6 8 11 7 7 4 9 9 10 22 24 18 17 13 20 17 13 6 12 11 11 22 22 24 23 7 6 8 9 35 53 38 45 20 22 15 14 24 27 20 24 16 20 14 18 24 40 28 34 38 36 44 29 26 25 25 24 43 49 57 53 a \. sample. 7 8 11 18 15 8 18 9 43 14 31 19 35 40 24 54 8 6 14 12 15 14 13 10 38 18 28 14 33 32 21 40 9 9 6 15 21 18 28 6 41 17 16 17 33 20 49 10 Averag Std. dev 10 8.60 1.90 6 9.40 3.34 18 17.80 3.33 26 16.80 4.29 15 11.40 3.72 19 20.80 4.02 6 7.70 2.00 49 43.20 5.75 17 18.60 4.06 20 24.20 4.54 16 16.10 2.38 29 31.00 4.83 36.63 4.63 16 23.80 3.88 51 47.70 6.27 91 Experiment 27 Date: 10/16/94 C:N ratio: 15:1 Interference Results plate Time in Seconds Agitatio HC volt 10 20 60 0.2 0.085 60 I 0.076 100 0.6 0.061 140 0.2 0.065 140 I 0.06 40 0.075 0.074 0.064 0.062 Control Agitatic 60 100 140 Time in seconds 10 20 0.148 0.144 40 0.1 I 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Dil. fact. 1.00E+05 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+03 I.OOE+04 1.00E+03 1.00E+05 1.00E+05 1.00E+04 T o t CC Std. dev. 1.27E+06 0.292 7.70E+05 0.324 1.98E+05 0.136 3.10E+05 0.210 2.90E+05 0.156 2.18E+05 0.221 2.04E+05 0.154 5.12E+05 0.064 1.56E+05 0.199 5.19E+04 0.072 1.79E+05 0.245 2.02E+04 0.177 7.44E+05 0.214 1.34E+06 0.173 3.99E+05 0.152 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 13 14 15 agit. rate/hc • init abs .200 abs .086 dil I abs .038 dil I 60/.2/10 60/.2/40 60/1/10 60/1/40 100/. 6/20 140/.2/10 140/.2/40 140/1/10 140/1/40 60/10 100/20 140/40 Plate counts, where each count represents a one ]A. sample. I 2 3 4 5 6 7 8 16 18 9 11 14 7 4 8 6 9 7 8 13 18 18 20 23 18 23 24 40 26 21 36 34 29 41 25 30 29 29 38 28 26 28 21 27 23 29 16 18 25 19 19 16 18 25 23 48 52 56 57 47 49 50 10 16 14 12 19 19 19 48 52 57 57 47 49 50 13 14 12 21 18 18 24 17 21 16 14 23 22 24 9 9 5 8 7 7 9 15 14 11 14 10 16 11 48 30 38 43 42 45 41 8 14 5 20 27 27 16 18 53 14 53 25 20 8 14 45 9 15 8 18 29 23 19 22 50 16 56 17 25 5 17 36 10 Averag Std. dev 15 12.70 3.71 9 7.70 2.50 16 19.80 2.70 27 31.00 6.50 35 29.00 4.52 21 21.80 4.83 19 20.40 3.13 50 51.20 3.29 17 15.60 3.10 50 51.90 3.73 17 17.90 4.38 20 20.20 3.58 7.44 1.59 12 13.40 2.32 31 39.90 6.05 92 Experiment 28 Date: 10/18/94 C:N ratio: 15:1 V Results Time in Seconds Agitatio HC volt 10 20 40 60 0.2 0.083 0.07 0.063 60 I 0.078 0.062 0.04 100 0.6 0.032 0.037 0.02 140 0.2 0.03 0.035 0.016 140 I 0.035 0.027 0.01 100 0.6 0.038 Control Time in seconds Agitatic 10 20 40 60 0.118 0.118 100 0.105 140 0.08 0.08 plate Dil. fact. I 1.00E+05 2 1.00E+05 3 1.00E+05 4 1.00E+05 5 1.00E+05 6 1.00E+05 7 1.00E+04 8 1.00E+04 9 1.00E+04 10 1.00E+03 11 1.00E+04 12 1.00E+03 13 1.00E+04 14 1.00E+05 15 1.00E+05 16 1.00E+05 17 1.00E+05 18 1.00E+05 T o tC C Std. dev. 1.57E+06 0.235 8.80E+05 0.292 1.12E+06 0.275 1.07E+06 0.265 7.56E+05 0.188 7.20E+05 0.205 3.28E+05 0.223 1.30E+05 0.154 2.24E+05 0.128 4.02E+04 0.152 1.45E+05 0.265 1.14E+04 0.323 1.15E+05 0.193 1.48E+06 0.208 1.12E+06 0.222 1.26E+06 0.288 1.29E+06 0315 1.37E+06 0.176 Cell Count data I 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Plate counts, where each count represents agit. rate/hc ■ I 2 3 4 5 init abs .204 19 11 17 17 13 abs .065 dil I 13 11 5 7 8 abs .04 dil I 9 14 10 9 9 60/.2/10 10 16 10 13 13 60/.2/40 6 8 6 7 9 60/1/10 5 8 7 8 5 60/1/40 32 28 31 37 41 100/. 6/20 13 13 17 14 10 140/.2/10 26 17 20 21 25 140/.2/40 35 41 45 43 30 140/1/10 11 14 23 18 16 140/1/40 8 12 6 19 13 100/. 6/20 14 11 12 12 8 60/10 19 18 11 16 16 60/40 9 10 15 16 11 100/20 18 16 9 16 8 140/10 14 16 7 11 13 140/40 15 11 14 11 16 one pi\. sample. 6 7 18 22 12 9 6 12 9 9 8 6 9 9 34 22 13 14 25 21 47 50 15 12 8 12 11 11 14 14 12 11 9 10 10 22 16 14 8 15 6 13 12 8 6 27 13 21 37 10 10 10 17 10 12 13 17 9 15 9 14 6 10 7 29 13 23 37 12 12 16 14 9 16 10 10 10 Averag Std. dev 10 15.70 3.68 8 8.80 2.57 16 11.20 3.08 9 10.70 2.83 7.56 1.42 8 7.20 1.48 47 32.80 7.30 10 13.00 2.00 25 22.40 2.88 37 40.20 6.11 14 14.50 3.84 14 11.40 3.69 10 11.50 2.22 9 14.80 3.08 9 11.20 2.49 12 12.60 3.63 13 12.90 4.07 13 13.70 2.41 93 Experiment 29 Date: 10/19/94 C:N ratio: 15:1 V Results plate Time in Seconds Agitatio HC volt 10 20 60 0.2 0.042 0.052 100 0.6 0.051 0.046 140 0.2 0.044 0.044 140 I 0.043 0.036 40 0.051 0.047 0.103 0.034 Control Agitatic 60 100 140 Time in seconds 10 20 0.101 40 0.085 I 2 3 4 5 6 7 8 9 10 11 12 13 Dil. fact. 1.00E+05 1.00E+05 1.00E+04 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+04 1.00E+03 1.00E+05 1.00E+05 1.00E+05 0.104 ToLCC Std. dev. 1.34E+06 0.193 1.21E+06 0.402 4.54E+05 0.102 8.40E+05 0.219 3.08E+05 0.160 8.70E+04 0.372 1.25E+05 0.197 3.01E+04 0.130 6.70E+04 0.234 8.40E+03 0.298 1.30E+06 0.249 1.57E+06 0.238 9.70E+05 0.266 Cell Count data Plate counts, where each count represents a one pi\. sample. agit. rate/hc ■ I 2 3 4 5 6 7 I init abs .204 12 10 14 11 16 17 17 2 abs .075 dil I 8 16 21 17 15 9 8 3 abs .042 dil I 47 36 48 48 45 45 40 4 60/.2/10 5 9 7 9 10 11 6 5 60/.2/40 34 23 36 36 36 26 28 6 100/. 6/20 13 7 5 14 8 7 7 7 140/.2/10 10 10 13 16 12 14 14 8 140/.2/40 34 24 36 29 27 27 34 9 140/1/10 9 6 9 7 5 5 7 10 140/1/40 10 11 9 6 10 12 7 11 60/40 15 15 13 10 19 II 14 12 100/20 10 14 19 20 12 20 14 13 140/10 11 14 8 11 6 10 11 8 14 12 52 9 26 5 10 28 5 9 7 12 12 9 12 7 49 9 34 12 16 29 6 5 13 19 7 10 Averag Std. dev 11 13.40 2.59 8 12.10 4.86 44 45.40 4.62 9 8.40 1.84 29 30.80 4.94 9 8.70 3.23 10 12.50 2.46 33 30.10 3.90 8 6.70 1.57 5 8.40 2.50 13 13.00 3.23 17 15.70 3.74 7 9.70 2.58 94 Experiment 30 Results Time in Seconds Agitatio HC volt 10 20 60 0.2 0.055 0.058 60 I 0.054 0.051 100 0.6 0.033 0.029 140 0.2 0.033 0.031 140 I 0.031 0.027 Date: 10/24/94 C:N ratio: 15:1 V plate 40 0.043 0.045 0.028 0.028 0.27 Control Agitatic 60 100 140 Time in seconds 10 20 0.133 0.125 0.14 40 0.104 0.103 Cell Count data agit. rate/hc' 1 init abs .204 2 abs .065 dil I 3 abs .04 dil I 4 60/.2/10 5 60/.2/40 6 60/1/10 7 60/1/40 8 100/.6/20 9 140/.2/10 10 140/. 2/40 11 140/1/10 12 140/1/40 13 60/10 14 60/40 15 100/20 16 140/10 17 140/40 Experiment 31 Date: 10/26/94 C:N ratio: 15:1 Interference Plate counts, where I 2 5 5 14 18 10 6 12 19 6 14 13 12 7 6 24 24 27 37 5 8 17 16 13 13 22 16 11 9 9 8 16 10 14 23 Dil. fact. I 1.00E+06 2 1.00E+05 3 1.00E+05 4 1.00E+05 5 1.00E+05 6 1.00E+05 7 1.00E+05 8 1.00E+05 9 1.00E+04 10 1.00E+04 11 1.00E+04 12 1.00E+03 13 1.00E+05 14 1.00E+05 15 1.00E+05 16 1.00E+05 17 1.00E+05 each count represents a one ftl. sample. 3 4 5 6 7 9 9 10 6 6 21 16 14 20 17 5 8 8 8 6 16 15 19 23 28 11 9 18 11 13 14 8 10 13 10 10 6 9 7 7 24 19 21 22 24 34 39 31 41 38 12 5 9 12 10 22 18 27 22 15 13 15 12 9 22 24 22 19 27 25 6 6 7 8 8 11 16 15 9 12 16 8 9 15 8 28 17 17 15 18 Results plate Time in Seconds Agitatio HC volt 10 20 40 60 I 0.066 0.066 0.06 140 I 0.061 0.056 0.053 Control Time in seconds Agitatic 10 20 60 100 0.104 140 0.101 40 0.111 1 2 3 4 5 6 7 8 9 10 8 7 14 5 16 11 8 6 19 43 13 21 15 18 9 8 11 18 Dil. fact. 1.00E+05 1.00E+05 1.00E+04 1.00E+04 1.00E+04 1.00E+04 1.00E+03 1.00E+05 1.00E+04 1.00E+05 ToLCC Std. dev. 7.80E+06 0.289 1.73E+06 0.170 6.80E+05 0.238 1.81E+06 0.268 1.05E+06 0376 1.10E+06 0.212 7.70E+05 0.229 2.09E+06 0.162 3.66E+05 0.139 8.70E+04 0.351 1.89E+05 0.218 1.37E+04 0.251 2.24E+06 0.173 8.30E+05 0.241 1.15E+06 0.263 1.22E+06 0.323 1.83E+06 0.228 9 11 17 6 15 7 8 18 42 5 13 11 23 7 12 10 17 10 Averag' Std. dev 10 7.80 2.25 22 17.30 2.95 6 6.80 1.62 18.11 4.86 5 10.50 3.95 11.00 2.33 11 7.70 1.77 14 20.90 3.38 34 36.60 5.10 8 8.70 3.06 18 18.90 4.12 14 13.70 3.43 28 22.40 3.86 12 8.30 2.00 15 11.50 3.03 19 12.20 3.94 16 18.30 4.16 ToL CC Std. dev. 8.10E+05 0.299 6.60E+05 0.228 2.27E+05 0.168 2.85E+05 0.162 1.75E+05 0.158 1.09E+05 0.261 7.80E+03 0.240 6.11E+05 0.172 3.66E+05 0.077 6.44E+05 0.175 Cell Count data agit. rate/hc' 1 init abs .200 2 abs .086 dil I 3 abs .038 dil I 4 60/1/10 5 60/1/40 6 140/1/10 7 140/1/40 8 60/40 9 100/20 10 140/10 Plate counts, where each count represents a one fil. sample. I 2 3 4 5 6 7 6 6 12 9 9 11 7 4 7 8 9 6 5 6 25 20 18 26 16 24 25 31 25 26 23 25 29 32 17 22 16 15 20 17 13 7 8 16 10 8 12 10 6 6 7 9 8 10 11 6 5 6 7 8 7 6 30 40 37 38 39 35 36 6 5 7 8 6 5 7 8 23 33 17 9 5 8 27 37 17 12 12 8 5 39 8 8 5 36 6 6 6 10 Averag Std. dev 10 8.10 2.42 7 6.60 1.51 22.67 3.81 24 28.50 4.62 21 17.50 2.76 14 10.90 2.85 5 7.80 1.87 6.11 1.05 36.60 2.84 6.44 1.13 95 APPENDIX B IMAGE ANALYSIS RESEARCH 96 Image Analysis System Experimental Design The initial experimental design made use of the image analysis system at the Center for Biofilm Engineering (CBE). Initially the proposed project would determine the kinetics of bacterial attachment at the hydrocarbon-water interface. Design of the experimental system went through a series of modifications as different methods were tried and subsequently rejected. The scope of research was altered after it became obvious that finding a working system using image analysis was unattainable with materials available for the research project. The original project design incorporated cell surface hydrophobicity as measured by adhesion of bacteria cells to hydrocarbon, which was to be compared to results from the kinetics of attachment as determined from the image analysis results. Setup The experimental system was similar to that of R. Mueller (1990). The image analysis system was not changed. In order to obtain slow flow rates a syringe pump was utilized. A flow rate of 4.8 ml per hour was used in all the experiments. This flow rate corresponded to a Renolds number of 0.1077 and a mean bulk fluid velocity of 6.15 * IO"5 meters per second. The flow cell dimensions were: depth 0.180 cm, width 1.204 cm and length 4.054 cm. The flow cell consisted of a shallow groove cut into polycarbonate which was covered by a glass slide that was pressed against a rubber seal by the compression of a stainless steel plate. The plate was screwed onto the base of the polycarbonate. The hydrocarbon-water interface could be observed with a light microscope connected to the image analysis system. 97 Design for containment of the hydrocarbon, which was to be observed by the image analysis system, proved to be troublesome. Methods were tried and discarded and it became apparent that the design of the system was very important to the final success of the project. One of the first prototypes for containment was a simple hole punched through a thin Teflon plate. The hydrophobic surface of the Teflon should have kept the hydrocarbon intact in the hole while water flowed past. Observation of the interface of this system was easy. The microscope focused on the hole and the interface was found under higher magnification. It became apparent that the hydrocarbon was not to be contained by the hydrophobic attraction to the Teflon. Water replaced the hydrocarbon soon after the syringe pump was turned on. Other hydrophobic materials (polystyrene, polycarbonate and glass) were also tested, and produced similar results. In another method a thin layer (monolayer) of hydrocarbon was placed on the surface of the water in the flow cell and the coverslip replaced. Flow was started and the interface was observed. In this experiment the oil film slipped between the surface of the coverslip and the polycarbonate flow cell. Not all of the hydrocarbon became dispersed with the flow and droplets of hydrocarbon could be observed adhering to the glass coverslip. The last method used the observation that small droplets of hydrocarbon will adhere to the glass coverslip. After cleaning the surface of the glass to remove any surfactants small droplets of hydrocarbon were placed on the coverslip. The coverslip was inverted and placed on top of the flow cell. Procedure In a typical experiment the cell suspension was removed from the water bath during the stationary growth phase. The cells were prepared as described earlier in the methods section. The autoclaved elements essential to the experiment were prepared. Two systems were initially employed, one in which the cell suspension flowed directly to the flow cell. 98 The second design decreased the pulsation of the fluid flow observed in the first system. In the second design two bottles were placed in series with the syringe pump before the cell suspension entered the flow cell. Sterilized water from the syringe pump entered the first bottle forcing air from the head space into a tube connected to the next bottle. The air from the first bottle entered the second bottle at the top and subsequently forced the cell suspension out of the bottle through a glass tube. This system had another advantage over the first because it allowed mixing of the cell suspension in the bottle. From the glass tube the cell suspension flowed through tybex tubing and into the flow cell. The flow cell was prepared as described above. After carefully turning the cover slip over it was placed onto water that was contained by the groove in the flow cell. The flow cell was covered by the metal clamp and tightened down. The connections to the rest of the system were made and any trapped air was purged from the system through a relief valve. The syringe pump was turned on and the interface was observed under the microscope. Observations The results of the image analysis experiments indicated that further exploration of the problem is needed. Finding the hydrocarbon-water interface before the bacterial suspension had entered the flow cell proved to be difficult. The interface was transparent and unless something adhered between the phases the exact location of the hydrocarbonwater interface was not easy to find. In some cases a little dust or some other outside object could be observed and in such cases observation of the interface could begin. All of the hydrophobic surfaces that were tested failed to hold the hydrocarbon in place when subjected to fluid flow. In some cases the hydrocarbon would spread as soon as it came in contact with the water. Droplets of hydrocarbon on the surface of hydrophobic materials other than glass did not work because of interference of the light entering the objective lens. 99 Once it was determined that hydrocarbon droplets on the glass slide could be observed, an experiment was initiated to determine the kinetics of bacteria attachment to hydrocarbon. Initially the experiment progressed as desired. The location of the hydrocarbon-water interface was determined. The flow was started after which images were taken at fifteen minute intervals. The suspended bacteria could be observed flowing through the cell. Some of the bacteria could be observed interacting with the hydrocarbon. Cells that came in contact with the hydrocarbon would have a slower rate of flow than those which did not. The flow rate nearest the glass surface was slower then fluid flow towards the center of the groove. After the cells came into contact with the hydrocarbon another problem developed. The surface of the hydrocarbon began to rotate. The direction of the movement was hard to determine. Cells that were observed at the interface moved slowly along with the flow of the hydrocarbon. In some cases cells rotated towards the glass surface only to disappear from view as they came into contact with the glass. Once the cells contacted the glass surface they could not be observed any longer. Two different processes could be taking place. In one case the cell would desorb from the hydrocarbon and enter the bulk fluid only to exit the flow cell. The other possibility is that the cell moves with the interface as it rotates, and if the hydrocarbon flows inward and under the glass the cell moves with it. In this case the cell disappears from view as it gets covered by the hydrocarbon. The exact rotational movement of the hydrocarbon was not determined. No observation of an attached cell desorbing into the bulk fluid was observed. Therefore it could not be determined if the glass forced the cells into the bulk liquid or if the bacteria remained attached only to resurface else where on the spherical hydrocarbon droplet. 100 As the experiment progressed it became apparent that the tendency of the cells to clump was interfering with the experiment. The cells were entering the flow cell in small visible clumps which moved along the lower surface of the channel. Attachment kinetics could be influenced by the tendency of the cells to clump together. Clumped cells were never observed attaching to the interface further complicating the determination of the kinetics of attachment at the interface. The kinetics of bacterial attachment at the hydrocarbon-water interface could not be determined using this experimental design. The system was fraught with flaws and any data obtained from the experiments would be very difficult to interpret. A method for maintaining a hydrocarbon-water interface stationary is needed. MONTANA STATE UNIVERSITY LIBRARIES »