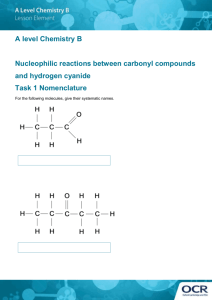
Residual cyanide distribution in a neutralized gold leach heap
advertisement

Residual cyanide distribution in a neutralized gold leach heap by James Gregory Poell A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Land Rehabilitation Montana State University © Copyright by James Gregory Poell (1994) Abstract: Kendall Mine Heap Leach Pad No. 1 is located in Fergus County northwest of Lewistown, Montana. The heap, constructed in the early 1980s, was most recently leached with sodium cyanide for gold in 1988. After leaching, Pad No. 1 was neutralized in spring, 1989 with fresh water rinsing followed by alkaline chlorination using calcium hypochlorite. Thirty months later, samples were taken from 15 boreholes across the heap at incremental depths in order to determine the speciation, concentration, and vertical and areal distribution of cyanide and associated metals. Two pore water samplers were used to monitor cyanide and metals levels near the heap base during the spring and summer of 1992. Analysis of solid ore samples showed significant increases in weak acid dissociable cyanide levels from the surface to middle of the heap. This may correspond to the interface of two lifts or benches of ore in the heap. Deeper in the heap, weak acid dissociable cyanide levels significantly decrease. Soluble . copper and nickel levels had a similar vertical distribution pattern. No significant differences with depth were found for total cyanide, pH, cadmium, iron, or zinc. Across the heap, significant differences were found between boreholes for total cyanide, weak acid dissociable cyanide, pH, cadmium, and copper. However, only cadmium levels, declining significantly from west boreholes to east boreholes, exhibited any areal pattern. These results indicate that areal patterns are not likely to be found across leach heaps. Pore water cyanide data indicate that total and weak acid dissociable cyanide degradation may have occurred as pore water drained the heap through spring and summer 1992. Pore water data suggest that soluble copper and nickel levels decreased over the same period. These changes may be due to the relative stability of metallo-cyanide complexes and competing degradation processes in the heap. Cadmium, iron, zinc and pH in pore water did not change significantly over time and distance. RESIDUAL CYANIDE DISTRIBUTION IN A NEUTRALIZED GOLD LEACH HEAP by James Gregory Poell A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Land Rehabilitation MONTANA STATE UNIVERSITY Bozeman, Montana March 1994 ^qz0I ii APPROVAL of a thesis submitted by James Gregory Poell This thesis has been read by each member of the thesis committee and has been found to be satisfactory regarding content, English usage, format, citations, bibliographic style, and consistency, and is ready for submission to the College of Graduate Studies. 7 A p r i l 1994 Date Chairperson, Graduate Committee Approved for the Major Department Approved for the College of Graduate Studies Date Graduate Dean iii STATEMENT OF PERMISSION TO USE In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Montana State University, I agree that the Library shall make it available to borrowers under rules of the Library. If I have indicated my intention to copyright this thesis by including a copyright notice page, copying is allowable only for scholarly purposes, consistent with "fair use" as prescribed in the U.S. Copyright Law. Requests for permission for extended quotation from or reproduction of this thesis in whole or in parts may be granted only by the copyright holder. Signat Date I IV ACKNOWLEDGEMENTS I would like to acknowledge the support and guidance of Dr. Frank Munshower, Dr. Doug Dollhopf, and Dennis Neuman as members of my graduate committee. Bob Vince and Kevin Ryan of Canyon Resources Corporation’s ■ Kendall Mine are thanked for their assistance and consultation in the conduct of my research. Thanks to Dr. William Schafer of Schafer and Associates for student employment that gave me invaluable technical experience. Special thanks are extended to Stuart Jennings, Bryce Romig, Gary Vodehnal, and. Murray Strong for their advice and friendship. Finally, I would like to thank my wife Martha for her support and kindness through my graduate education. TABLE OF CONTENTS Page ACKNOWLEDGEMENTS .............................. .. . ................... .............................. iv TABLE OF C O N TEN TS....... ..................................................................................... v LIST OF T A B L E S............................................ ............... .........................: ............. vii LIST OF F IG U R E S .............. : ......................... ...................... .. . . .......................... xi ABSTRACT .......................................................................... ...................................xii IN T R O D U C T IO N .......................................................... I LITERATURE REV IEW .................. 5 History Of The Cyanide Leach Process ....................... ; ................................ 5 Leach Heap Cyanidation.................................. 7 Leach Heap N eutralization............................................ ........................... .. . 10 Cyanide In The Neutralized H e a p ........................................................: . . . 11 Free Cyanide M easurem ent.......................................................... 17 WAD Cyanide M easurem ent.............................. 18 Total Cyanide Measurement . ................................... .................... ; . . . . . . 20 Measurement Of Cyanide In S o lid s ...................,.............................................20 Residual Cyanide Studies .............................. '. ............................ ..................21 STUDY A R E A ............................................ ..............i ........................................... 24 M E T H O D S .............. .........................; .'.................... .............................. I . . . . . . 27 Sampling Methods .......................................................... .. ............................... Analytical Methods. .......................................... 27 30 RESULTS AND D ISC U SSIO N ......... .................... Cyanide and Metals Depth Distributions .............................. . . .............. Cyanide and Metals Areal Distributions ................................. ....................... Pore W ater Behavior .......................................... ............................................ 34 34 37 39 SUMMARY AND CONCLUSIONS . ...................................................................... 43 Vi TABLE OF CQNTENTS--Contimied • Page LITERATURE CITED . ...................................................................................... 45 A PPE N D IC ES.................................................... .......................................................52 Appendix A - Data Tables .......................................................... .................. .5 3 Appendix B - Data Validation Tables .............. ............................................. 63 Appendix C - Statistical Data .............. ............................................................69 vii LIST OF TABLES Table 1. Page Selected Montana water quality standards applicable to cyanide heap leach effluents....................................................... 2 2. Relative stabilities of forms of cyanide in water. . T. ................. 13 3. Typical cyanide reactions in the abandoned heap leach environment......................... ........................... ......................................... 14 4. Analytical terms for cyanide measured in the leach heap environment....................................... .1 7 5. Cyanide levels after fresh water rinsing at Kendall Mine Heap Leach Pad No. I, March, 1989. . .................................................... 26 6. Location of suction lysifneters used in pore water sampling.............. 7. Effect of Iog10 data transformation on data skewness and kurtosis. . . . 32 8. Geometric mean levels of cyanide, pH and water soluble metals in spent ore through Kendall Mine Heap Leach Pad No. I. . . . . . . . . 35 9. Geometric mean levels of cyanide, pH and water soluble metals for boreholes acrossKendall Mine Heap Leach Pad No. 1....................... 38 29 10. Pore water cyanide and metals levels at Kendall Heap Leach Pad No. I, March - September, 1 9 9 2 ...................... .. . . ............ 40 11. Linear regression slope values and associated statistics for Kendall Heap Leach Pad No. I pore water.................................. .. 41 12. Total cyanide levels at Kendall Heap Leach Pad No. I, November I, 1991........................................................................................54 13. WAD cyanide levels at Kendall Heap Leach Pad No. I, November I, 1991. ........................................ .. . ............ ........................... 55 14. Free cyanide levels at Kendall Heap Leach Pad No. I, November I, 1991. ............................................ 56 viii LIST OF TABLES-Continued Table Page 15. pH levels at Kendall Heap Leach Pad No. I, November I, 1991..........57 16. Cadmium levels at Kendall Heap Leach Pad No. I, November I, 1991.................................................................... ............. .. 58 17. Copper levels at Kendall Heap Leach Pad No. I, ' November I, 1991. ...........................................................^ . . . . ............... 59 18. Iron levels at Kendall Heap Leach Pad No. I, November I, 1991............................................................................................60 19. Nickel levels at Kendall Heap Leach Pad No. I, November I, 1991.......................... 61 20. Zinc levels at Kendall Heap Leach Pad No. I,November I, 1991. . . . 62 y 21. Blind field replicate cyanide analysis re s u lts .......... ................................64 22. Laboratory replicate cyanide analysis results............................................ 65 23. Laboratory spiked cyanide analysis results. .......................... .................. 66 24. Blind field replicate pH and metals analysis results................... ■............67 25. Laboratory replicate pH and metals analysisresults....................................67 126. Laboratory pH and metals recovery results. . ............. 68 27. Analysis of variance for total cyanide (weighted, Iog10 transformed data). . ................................................. .. . . .......................... 70 28. Student-Newman-Keuls means separation tests for borehole total cyanide means (weighted, Iog10 transformed data). ........................71 29. Analysis of variance for WAD cyanide (weighted, Iog10 transformed data).................................. . ....................................................72 30. Student-Newman-Keuls means separation tests for depth WAD cyanide means (weighted, Iog10 transformed data). . ................... 72 <- . ix LIST OF TABLES-Contimied Table Page 31. ,Student-Newman-Keuls means separation tests for borehole WAD cyanide means (weighted, Iog10 transformed data)........................ 73 32. Analysis of variance for pH (weighted, Iog10 transformed data)............. 74 33. Student-Newman-Keuls means separation tests for borehole pH means (weighted, Iog10 transformed data)........................................... 75 34. Analysis of variance for cadmium (weighted, Iog10 transformed data). ..................................... 76 35. Student-Newman-Keuls means separation tests for borehole cadmium means (weighted, Iog10 transformed data)................ 77 36. Analysis of variance for copper (weighted, Iog10 transformed data). . . 78 37. Student-Newman-Keuls means separation tests for depth copper means (weighted, Iog10 transformed data).....................................78 38. Student-Newman-Keuls means separation tests for borehole copper means (weighted, Iog10 transformed data). ................... .. 79 39. Analysis of variance for iron (weighted, Iog10 transformed d a ta ).......... 80 40. Analysis of variance for nickel (weighted, Iog10 transformed data). . . . 80 41. Student-Newman-Keuls means separation tests for depth nickel means (weighted, Iog10 transformed data). . ............ .................. . 81 42. Analysis of variance for zinc (weighted, Iog10 transformed data)............. 81 43. Linear regression of pore water total cyanide data from borehole 4, Kendall Heap Leach Pad No. I .............................................82 44. Linear regression of pore water total cyanide data from borehole 8, Kendall Heap Leach Pad No. I ............................................. 83 45. Linear regression of pore water WAD cyanide data from borehole 4, Kendall Heap Leach Pad No. 1............................................. 84 tN X LIST OF TABLES--Continued Table Page 46. Linear regression of pore water WAD cyanide data from borehole 8, Kendall Heap Leach Pad No. I ................. ...................... . . 85 47. Linear regression of pore water pH data from borehole 4, Kendall Heap Leach Pad No. I ..................................................................86 48. Linear regression of pore water pH data from borehole 8, Kendall Heap Leach Pad No. I ............................................... ..................87 49. Linear regression of pore water cadmium data from borehole 4, Kendall Heap Leach Pad No. I ........................................... . 88 50. Linear regression of pore water cadmium data from borehole 8, Kendall Heap Leach Pad No. I ...................... .......................89 51. Linear regression of pore water copper data from borehole 4, Kendall Heap Leach Pad No. 1............................................. 90 52. Linear regression of pore water copper data from borehole 8, Kendall Heap Leach Pad No. I ........................................... . 91 53. Linear regression of pore water iron data from borehole 4, Kendall Heap Leach Pad No. I .......................... .................. 92 54. Linear regression of pore water iron data from borehole 8, Kendall Heap Leach Pad No. I. . ........................................ 93 55. Linear regression of pore water nickel data from borehole 4, Kendall Heap Leach Pad No. I ............................... 94 56. Linear regression of pore water nickel data from borehole 8, Kendall Heap Leach Pad No. 1............................................. 95 57. Linear regression of pore water, zinc data from borehole 4, Kendall Heap Leach Pad No. I. . ........................................ 96 58. Linear regression of pore water zinc data from borehole 8, Kendall Heap Leach Pad No. I ................................. .. . . . . 97 xi LIST OF FIGURES • - I Figure Page j 1. Relationship between HCN and CN' with pH .......................... 2. Detailed schematic showing potential geochemical conditions and cyanide reactions in and around an abandoned leach heap. .................15 3. Location of Kendall Mine. . ...................................................................... 24 4. Location of sampling boreholes I to 15 across the heap surface and sampling boreholes I to 7 and 8 to 15 in cross-section through Kendall Mine Heap Leach Pad No. I. ......................................28 5. Conceptual view of heap construction showing characteristics of material within the heap. ............................................................ 36 11 / xii Ab s t r a c t < Kendall Mine Heap Leach Pad No. I is located in Fergus County northwest of Lewistown5 Montana. The heap, constructed in the early 1980s, was most recently leached with sodium cyanide for gold in 1988. After leaching, Pad No. I was neutralized in spring, 1989 with fresh water rinsing followed by alkaline chlorination using calcium hypochlorite. Thirty months later, samples were taken from 15 boreholes across the heap at incremental depths in order to determine the speciation, concentration, and vertical and areal distribution of cyanide and associated metals. Two pore water samplers were used to monitor cyanide and metals levels near the heap base during the spring and summer of 1992. Analysis of solid ore samples showed significant increases in weak acid dissociable cyanide levels from the surface to middle of the heap. This may correspond to the interface of two lifts or benches of ore in the heap. Deeper in the heap, weak acid dissociable cyanide levels significantly decrease. Soluble . copper and nickel levels had a similar vertical distribution pattern. No significant differences with depth were found for total cyanide, pH, cadmium, iron, or zinc. Across the heap, significant differences were found between boreholes for total cyanide, weak acid dissociable cyanide, pH, cadmium, and copper. However, only cadmium levels, declining significantly from west boreholes to east boreholes, exhibited any areal pattern. These results indicate that areal patterns are not likely to be found across leach heaps. ( . Pore water cyanide data indicate that total and weak acid dissociable cyanide degradation may have occurred as pore water drained the heap through spring and summer 1992. Pore water data suggest that soluble copper and nickel levels decreased over the same period. These changes may be due to the relative stability of metallo-cyanide complexes and competing degradation processes in the heap. Cadmium, iron, zinc and pH in pore water did not change significantly over time and distance. I INTRODUCTION In the past 25 years there has been a wide expansion ,of the use of cyanide heap leaching by the mining industry. This process allows profitable recovery of gold and silver from low-grade ore. Heap leach pads are constructed on slight drainage grades and lined with an impervious clay and/or geotextile. Mined ore is stacked and leached in successive ten to thirty foot lifts or benches on the pad. Sprinkler systems set on the surface of the lift to be leached deliver a high pH sodium cyanide solution that, in its percolation through the ore, forms stable complexes with gold, silver, cobalt, iron, copper, nickel, zinc, and cadmium. This "pregnant" solution is then collected as it drains off the pad for precious metal I recovery and refinement. After leaching, the heap still contains interstitial and adsorbed cyanide species, including free cyanide and metal-complexed cyanides. The metalcomplexed cyanides are present as weak acid dissociable (WAD) complexes of cadmium, copper, nickel, and zinc, and extremely stable iron and cobalt cyanide complexes (Smith and Muddier 1991). These cyanides, in sufficient concentration, have the potential to degrade surface and ground water resources. The Montana W ater Quality Act directs the Montana Department of Health and Environmental Sciences (MDHES) to maintain standards for pollut­ ants threatening its nondegradation policy. The standards are referenced by the Montana Department of State Lands in mine operation and reclamation regula­ tion. Standards of interest to cyanide heap leach mining are listed in Table I. 2 Table I. Selected Montana water quality standards applicable to cyanide heap leach effluents (MDHES 1993). A cute A quatic Life Standards C hronic A quatic Life Standards H um an H ealth Stan d ard s for S urface W aters (AS I 1) (Ag I 1) (Ag I"1) WAD Cyanide 22.0 5.2 200.0 Cadmium 3.9' LI* 5.0 Copper 18.0* 12.0' 1000.0 P ollu tan t Iron — * 1000.0* 300.0 Nickel 1400.0* 160.0* 100.0 Zinc 120.0* 110.0' 5000.0 Water hardness dependent criteria (100 mg I"1 used). — No standard has been adopted. Cyanide in the heap will degrade naturally, if the heap is not disturbed. Natural degradation of cyanide can occur through volatilization, biodegradation, photodecomposition, and oxidation (Rolfes 1989). Other degradation mechanisms associated with cyanide chemistry include complexation with transition metals, precipitation of complex cyanide compounds, formation of thiocyanate, and the hydrolysis of free cyanide (Smith and Mudder 1991). How degradation will proceed, though, is site specific, depending on the interaction between geochemi­ cal conditions in the heap and site conditions such as precipitation, ore mineralo­ gy, and ore permeability (Struhsacker and Smith 1990, van Zyl et al. 1988). To speed this degradation process, most mines use fresh or chemically-treated water rinses. Rinsing is continued until effluent draining from the pad falls and remains below regulatory standard for a specified period of time. Currently, the Montana .3 Department of State Lands considers a spent heap to be neutralized for reclama­ tion when effluent WAD cyanide concentrations remain below 0.220 mg T1 through one full spring after rinsing. This WAD cyanide standard, commonly used by western state mine regulators, is 20 /Leg I4 above the MDHES human health standard (Table I). Despite acceptable effluent levels from abandoned leach heaps, later "spikes" or "slugs" of cyanide have been measured in effluent following large precipitation events or rinsing (Haight 1991, Struhsacker and Smith 1990). It is believed that residual cyanide in spent ore, pore spaces, and/or blind-off zones diffuses out into the heap over time. Subsequent wetting fronts generated by precipitation events may pick up this cyanide as they pass through the heap, degrading effluent water quality. Spent ore sampling that does not examine the full depth and breadth of a heap may not give a representative measure of I neutralization success. The concentration of cyanide throughout a decommissioned leach heap has not been thoroughly investigated. With funding supplied by the Montana Depart­ ment of Natural Resources and Conservation and the cooperation of the Montana Department of State Lands Hardrock Bureau and Canyon Resources Corporation, the Reclamation Research Unit of Montana State University sampled a decom­ missioned leach heap from October, 1991 through summer 1992. The objective of this study was to measure the concentration of residual cyanide species and associated metals throughout the heap, comparing the results for evidence of 4 vertical or areal distribution patterns. A second objective was to monitor cyanide and metals levels in pore water at the base of the heap through spring and summer 1992. This study was limited to one relatively small leach heap with limited construction and Operation documentation. No control data were available for ore leached in the heap. Rather than being a definitive work explaining the behavior of residual cyanide in neutralized leach heaps, this study will provide basic data to decision makers regarding the concentrations and distributions of cyanide species that may develop under similar conditions. Z .5 LITERATURE REVIEW History Of The Gyanide Leach Process The evolution of cyanidation technology in the pursuit of precious metals dates back 200 years in the historical record. In 1793, potassium cyanide was recognized as a gold solvent (Hiskey 1985). In 1844, Eisner investigated the reactions of various metals in a potassium cyanide solution, discovering that gold dissolution took place only in the presence of dissolved oxygen (Bosqui 1899). This dissolution reaction, known as Eisner’s Equation, is written as: 4 Au + 8 KCN + O2 +211,0 = 4 AuK(CN)2 + 4 KOH In 1896, Bodlaender found that the dissolution of gold was a two-step reaction with intermediate production of hydrogen peroxide (Hiskey 1985). This reaction proceeds as follows: 2 Au + 4 CN' + O2 + 2 H2O = 2 Au(CN)2' + 2 OH + H2O2 ' 2 Au + 4 CN + H2O2 = 2 Au(CN)2 + 2 OH The sum of this reaction sequence is equivalent to the dissolution reaction developed by Eisner. It is written as: 4 Au + 8 CN' + O2 + 2 H2O = 4 Au(CN)2" + 4 OH 6 The extraction of gold from gold ore using cyanidation was developed into a commercial process in Scotland by John MacArthur and Drs. Robert arid William Forrest (von Michaelis 1985). They were issued patents for their process in 1887 (Britain) and in 1889 (United States). The extraction is based on the agitation of finely ground gold ore in aerated potassium cyanide solution. Metals in the ore, including gold, complex with cyanide during the agitation. The gold cyanide complex is precipitated with zinc shavings (Eisele 1988). This precipita­ tion proceeds as follows: 2 Au(CN)2- + Zn = 2 Au + Zn(CN)42" The Merrill-Crowe process, as it is !mown in use today, improved gold recovery by removing oxygen from gold-bearing cyanide solutions before the addition of zinc (Hiskey 1985). After treatment with zinc, the solution is filtered under pressure to reitiove the gold precipitate for smelting. Carbon adsorption technology to recover dissolved gold from cyanide solutions was developed by Chapman in 1939 (Hiskey 1985). This technology did not require the filtration and deaeration of gold-bearing solutions as did the Merrill-Crowe process (Heinen et al. 1978). Activated charcoal, introduced to a cyanide-ore pulp (slurry), adsorbed the dissolved gold and floated away for collection (Hiskey 1985). As burning the enriched charcoal was the only known method to recover gold, carbon adsorption was not widely used (Eisele et al. 1984). 7 The Merrill-Crowe process was the predominant method of solution gold recovery when Zadra and .his associates (1952) developed a method to desorb gold from carbon. This process sent hot caustic cyanide solution through enriched carbon, stripping the gold for electrowinning onto stainless steel wool. .Despite lower costs, the low price of gold in the 1950s and 1960s precluded an expansion in the use of carbon adsorption and solution gold mining in general. Leach Heap Cyanidation Heap leaching may be defined as the percolation leaching of piles of lowgrade ores or mine waste that have been stacked or piled on specially prepared watertight drainage pads for the collection of precious metal-enriched "pregnant" solution (Heinen et al. 1978). Heap leaching of minerals dates back to sixteenth century Hungarian copper mines, with large-scale practice developed by the eighteenth century in Spanish copper mines (Hiskey 1985). Uranium producers have utilized heap lWching as an extraction technology for low-grade ores since the late 1950s (Dorey et al. 1988). Heap leach cyanidation of porous, low-grade, gold-bearing ores was first proposed in 1967 by U.S. Bureau of Mines metallur­ gists (Heinen et al. 1978). This treatment chemistry is the same as that of Bodlaender’s two-step dissolution reaction. , Gold is recovered from the pregnant solution draining from the heap leach using either Merrill-Crowe or carbon adsorption processes. In order ,to widen the applicability of cyanide heap leaching to include 8 impermeable high-clay ores, the U.S. Bureau of Mines developed an ore agglomeration treatment. After crushing, ore is mixed with 2 to 7 kg/metric ton (5 to 15 Ibs/short ton) of portland cement (to act as a binding and pH control agent), wetted with 8 to 16% (by weight) water, and subjected to mechanical tumbling to ensure adhesion of fines to coarser particles (Heinen et al. 1978, Heinen et al. 1979, McClelland and Eisele 1982). By 1983, it was estimated that agglomeration pretreatment allowed half of cyanide heap leach projects to operate (McClelland et al. 1983). The cyanide heap leach process is an efficient, low cost method of recover­ ing gold and silver from low-grade ore. Average ore grades recovered by heap leaching are 0.9 grams of gold per metric ton of ore (0.03 oz/short ton), with an extreme cutoff grade of 0.2 grams of gold per metric ton (0.006 oz/short ton) at the Round Mountain Mine in central Nevada (Spickelmier 1993). Relative ore grade dictates the choice of two heap leaching methodologies. Higher grade ores are crushed, agglomerated and stacked in short lifts of I to 3 in (3 to 10 ft) high on permanent asphalt or concrete leach pads. This method treats 900 to 9,000 metric tons (1,000 to 10,000 short tons) of ore for 7 to 30 days, after which the spent ore is neutralized and removed for disposal (Higgs and Gofmley 1992a, Rolfes 1989). Lower grade ores are typically not treated, being stacked as run-ofmine size (including boulders) in lifts of 3 to 9 m (10 to 30 ft) high on compacted clay and/or synthetic-lined pads. On these heaps, leaching of 9,000 to 1.8 million metric tons (10,000 to 2 million short tons) of ore will take months or years, after 9 which the spent ore heap is neutralized and abandoned (Hiskey 1985, Rolfes 1989). Pads under all leach heaps are constructed with a 2 to 5% slope to facili­ tate drainage of pregnant solution (Chamberlin and Pojar 1984). Leach heap pads are also designed to eliminate the loss of pregnant solution to the ground and prevent contamination of local water resources (Heinen et al. 1978). The objective of heap leach construction is to build a stable, porous heap that allows an even downward percolation of cyanide solution (Herzog 1990).. Heaps are commonly built in successive lifts using conveyor stacking, end dump­ ing, or truck and dozer techniques. The criteria in selecting a heap construction technique are to minimize layering, compaction, and ore particle segregation. (Dorey et al. 1988). The completed heap is put under cyanide leaching by a surface network of sprinklers or capillary tubes. Typical application rates, adjusted to result in a , partially-saturated flow of leachate through the heap, range from 0.002 to 0.003 1/s/m2 (0.003 to 0.005 g/min/ft2) (Dorey. et al. 1988). Though it is possible to leach gold with several types of solutions', all current commercial operations use 0.2 to 0.7 kilograms of sodium cyanide (NaCN) per metric ton of water (0.5 to 1.5 lbs of NaCN per short ton of water) (Chamberlin and Pojar 1984, Stanton et al. 1986). This produces a leach solution concentration of 200 to 700 mg T1 NaCN, or 125 to 350 mg I 1 free cyanide (Stanton et al. 1986). Leach solutions are maintained at pH 9.5 to 11 with lime (CaO) or caustic soda (NaOH) to ensure effective gold 10 dissolution (Chamberlin and Pojar 1984). Leaching consumes NaCN at a rate of 0.04 to 1.4 kg/metric ton (0.1 to 3.0 Ibs/short ton) of ore, depending on ore type, application method, porosity, solution pH, and the concentration of cyanide­ consuming metals and sulfides in the ore (U.S. EPA 1986, Stanton et al. 1986). Leach Heap Neutralization The consumption of NaCN is a major expense in heap leach operations that exploit low-grade ore (Barratt and McElroy 1990). To recover NaCN for use on other leach heaps and maximize precious metal recovery, leached ore is thoroughly rinsed with fresh water at the end of a leaching cycle (Heinen et al. 1978). This recovery action can be regarded as the initiation of heap neutraliza­ tion in reclaiming a heap leach project. The ultimate purpose of this washing or rinsing is to alter the chemistry of the reclaimed heap so that future drainage from the heap degrades neither surface or groundwater resources (Struhsacker and Smith 1990). Effective neutralization of spent ore heaps involves detoxifying residual cyanides and cyanide complexes found in interstitial solutions, adsorbed on ore particle surfaces, and diffused into the ore (Denton et al. 1992). 1' Active neutralization methodologies in use today include fresh water rinsing, alkaline chlorination, hydrogen peroxide treatment, sulfur dioxide oxida­ tion, bio treatment and acidification. These methods, are well documented by Scott (1984), Rolfes (1989), and Thompson and Gerteis (1990). 11 Cyanide In The Neutralized Heap Cyanides comprise a large class of compounds that are characterized by the presence of the cyanide ion (CN ) in their molecular structures (Higgs and Gormley 1992b). The forms of cyanide of particular interest to the heap leach process are free cyanide, simple cyanide and complex cyanide (U.S. EPA 1986). Free cyanide is the sum of the cyanide ion (CN ) and hydrocyanic acid (HCN) species released into an aqueous solution by the dissolution and dissociation of cyanide compounds (Mudder 1991). These two species coexist in solution, their relative proportions depending on pH and temperature. The relationship between solution pH and the ratio of CN' to HCN is illustrated in Figure I. Below a pH of 7.0, all free cyanide is present as HCN in the toxic gaseous state. Figure I. Relationship between HCN and CN' with pH (Higgs and Gormley 1992b). Percent HCN Percent CN 12 Simple cyanides are the salts of hydrocyanic acid that completely and easily dissociate in water (Maynard et al. 1986). These cyanides ionize in water, as sodium does .in Reaction [I]. . r ' NaCN = Na+ + CN' [1] The cyanide ion (CN") then hydrolyzes to form HCN in Reaction [2]. CN + HOH = HCN + OH [2] Again, the amount of HCN produced depends on solution pH and temperature. In the heap environment, the formation of gold and silver cyanide com­ plexes is the means of precious metal extraction from ores. Other transition metals present in the ore may also complex with cyanide. Most commonly, these metals are cadmium, copper, cobalt, iron, nickel and zinc (IEC 1979). The stability tif these metal-cyanide complexes depends on the stability of the particu­ lar metal. Cadmium and zinc are relatively unstable, easily dissociating to release the cyanide ion (CN"). The dissociation of complex cyanides is inversely related to solution pH and complex ion concentration (Huiatt et al. 1983). The toxicity of complex cyanides is related to their ability to release cyanide ions to solution, producing toxic HCN (Eisler 1991). Metals freed by. the dissociation of complex cyanides in a heap are not considered a threat to effluent water quality. Solubility of metals is limited at the high pH levels (7.5 to 9.5) normally observed in leach heaps rinsed of cyanide (Lindsay 1979). Further, once a complex cyanide 13 degrades, the metal would be expected to precipitate as a hydroxide or to adsorb onto solids surfaces (Schafer et al. 1991). The relative stabilities of the forms of cyanide common in leach heap solutions are shown in increasing order of stability in Table 2 (Huiatt 1984). Table 2. Relative stabilities of forms of cyanide in water (Huiatt 1984). Cyanide Form Free cyanide Typical Cyanide Compounds CN', HCN Simple cyanides: Readily soluble NaCN, KCN, Ca(CN)2, Hg (CN)2 Relatively insoluble Zn(CN)2, CuCN, Ni(CN)2, AgCN Weak complexes Zn(CN)42', Cd(CN)32-, Cd(CN)42" Moderately strong complexes Cu(CN)2', Cu(CN)32', Ni(CN)42-, Ag(CN)22" Strong complexes Fe(CN)64-, Co(CN)64", Au(CN)22' Regardless of the method of leach heap neutralization, natural degradation of residual cyanide and metal-cyanide complexes will occur after abandonment. Smith and Struhsacker (1988) have detailed a schematic showing potential geochemical conditions and cyanide reactions in and around an abandoned leach 14 heap. Table 3 lists the chemical reactions and their equations shown in schematic form in Figure 2. Table 3. Typical cyanide reactions in the abandoned heap leach environment (Smith and Struhsacker 1988). Hydrolysis (1) CN + H2O - HCN + OH Oxidation of HCN and CN (2) 2HCN + O2-* 2HCNO (3) 2CN + O2 + catalyst - 2CNO Hydrolysis of CNO (4) HCNO + H2O - NHw + COw Hvdrolvsis/Saponification of HCN (5) HCN + 2H20 -* NH4COOH (ammonium formate) or (6) HCN + 2HzO - NH3 + HCOOH (formic acid) Aerobic biodegradation of HCN (7) 2HCN + O2 + enzyme -» 2HCNO (4) HCNO + H2O - NH3 + CO2 Thiocyanate formation (8) S, + S2- + CN - S1., + CNS(9) S2O3 + CN- - SO32- + CNS Simple cyanide compound dissociation (10) NaCN - Na+ + CN Metal-cvanide complexation (11) Zn(CN)2 + 2CN" - Zn(CN)/ Anaerobic biodegradation of HCN and CN' (12) CN + H2S(aq) -» HCNS + H+ (13) HCN + HS - HCNS + H+ Figure 2. Detailed schematic showing potential geochemical conditions and cyanide reactions in and around an abandoned leach heap (Smith and Struhsacker 1988). Typical Cyanide Reactions Equation No. (see Table 2) No. 1-7, 10, 11 + 8 if sulfide present. precipitation OXIDIZED UNSATURATED SPENT ORE HEAP No. 2-7, 10, 11 if oxidized. SEEPAGE POTENTIAL No. 9, 10, 12, 13 if reduced. WEATHERED BEDROCK WATER TABLE REDUCED UNWEATHERED BEDROCK SATURATED 16 The degree and rate of natural cyanide degradation in the heap is a function of the type of cyanide species present, their concentration, ore mineralogy, available bacteria, heap permeability, and site conditions (tempera­ ture, precipitation, elevation) (Denton et al. 1992). Free cyanide in the heap degrades quicker than simple and complex cyanides. Cyanide reactions consuming free cyanide include hydrolysis and volatilization with decreasing pH, oxidation, hydrolysis/saponification, biodegradation, and thiocyanate formation (Smith and Struhsacker 1988). The mineralogy of ore in the heap determines the type and amount of metals available for metal-cyanide complexation and sulfides for the formation of thiocyanate. Biodegradation of free cyanide requires bacteria and an energy source. A permeable heap ensures an oxygen supply for oxidation and aerobic biodegradation reactions. It also ensures a water supply for hydrolysis, hydrolysis/saponification, and solute transport processes (Smith and Struhsacker 1988). Increases in temperature will speed reaction rates. Higher elevation locations will experience increased volatilization of HCN from the aqueous phase. Mine operators are required by regulators to neutralize spent leach heap ore to protect human health and the environment. Neutralization criteria vary from state to state, requiring analysis of heap effluent or leachate extracted from heap ore (Heriba 1991). The establishment of effective neutralization criteria is hampered by a confusion of terminology used to describe cyanide forms and the variety of analytical methods used to measure them (Smith and Struhsacker 1988). Huiatt (1984) classified cyanide and cyanide compounds into five forms 17 according to their stability (Table 2). Over the past decade, however, three analytical terms (total, WAD, and free cyanide) have come into common use in describing cyanide species in leach heaps. These terms and the cyanide com­ pounds they represent are listed in Table 4. Table 4. Analytical terms for cyanide measured in the leach heap environ­ ment (DeVries 1988). Cyanide Species Free cyanide Typical Cyanide Compounds CN", HCN, NaCN, KCN, Ca(CN)2, Hg (CN)2 WAD cyanide Free cyanide + Zn(CN)2, CuCN, Ni(CN)2, AgCN, Zn(CN)A Cd(CN)A Cd(CN)A Cu(CN)2", Cu(CN)A Ni(CN)A Ag(CN)22- Total cyanide WAD cyanide + Fe(CN)A Co(CN)A Au(CN)22" Free Cyanide Measurement The objective of free cyanide analysis is to measure the amount of CN' and HCN in a given sample (Maynard and Szczahor 1992). This measure will also include the readily soluble simple cyanides (Maynard et al. 1986). American 18 Society for Testing and Materials (ASTM) Procedure D4282 (1993) analyzes HCN which diffuses from a sample solution chamber (buffered at pH 6) to a NaOH solution chamber in four hours. Colorimetric analysis of the diffused solution is sensitive from 0.010 to 0.150 mg I"1 HCN. Diffusion methods for HCN in mine wastes suffer interference from the production of HCN by metal-cyanide complex dissociation (Maynard and Szczachor 1992). The American Public Health Associ­ ation (APHA) Cyanide-Selective Electrode Method 4500-CN-F (1992) measures CN concentrations in a range of 0.05 to 10 mg I 1 in sample solutions. Cyanide ion-selective electrode readings are compared to a cyanide standard calibration . curve, and a CN' concentration is calculated. This method is particularly sensitive to interference from the precipitation of sulfides, chlorides, and. mercury on the cyanide ion-selective electrode (Smith and Struhsacker 1988). WAD Cyanide Measurement The measurement of WAD cyanide in effluent solutions is most commonly performed using reflux distillation in APHA Standard Method 4500-CN-I (1992) or ASTM D-2036 Test Method C (1993). Distillation of sample solution (buffered at pH 4.5) for one hour liberates HCN from free cyanide and weak metal-cyanide complexes. Collected in a NaOH absorption solution, WAD cyanide is best estimated using direct colorimetric determination, accurate to 0.03 mg I 1 (ASTM D-2036 Test Method C 1993). The. reflux distillation method recovers all the cyanide from complexation with zinc and nickel, 70 percent from copper 19 complexes and 30 percent from cadmium complexes (Smith and Struhsacker . 1988). Cyanide is not recovered from its strongest complexes with iron, cobalt or gold. The appeal of this method is that it is relatively free of thiocyanate or sulfide interference, and other interferences can be removed before distillation (Mudder 1991). A less rigorous, less accurate method of measuring WAD cyanide concen­ trations in mine effluents is the picric acid method (Smith and Mudder 1991). This method, accurate to 0.5 mg I"1, involves the development of color with picric acid in the presence of nickel while heating over a water bath for 20 minutes, followed by colorimetric measurement (Smith and Mudder 1991). Though less accurate, it can be used more easily for on-site monitoring of effluents for WAD cyanide concentration changes (Maynard and Szczahor 1992). A new method for measuring WAD cyanide is found in Kelada’s procedure for cyanide speciation (Kelada et al. 1992). It was adopted by ASTM as Automat­ ed Methods for Determination of Total Cyanide, Dissociable Cyanide, and Thiocyanate (D4374) in September 1991 (ASTM 1993). This procedure uses acidification and thin film distillation to free cyanide from weak metal complexes, capturing HCN in a NaQH absorber for colorimetric measurement. It is free of thiocyanate interference and results are regarded as more reliable than distillation methods (Kelada et al. 1992). 20 Total Cyanide Measurement Analytical methods to measure total cyanide in wastewater can use manual or automated procedures. The most widely accepted procedure for determining total cyanide in mine effluents is the manual reflux distillation in APHA Standard Method 4500-CN-C (1992), ASTM D-2036 Test Method A (1993), or EPA 9010/ 9012 (1986). This procedure is almost the same as that used in reflux distillation for WAD cyanide measurement. However, the total cyanide reflux distillation procedure uses a strong acid (1:1 sulfuric acid) to free cyanide from stronger iron complexes. Interference may be caused by the presence of thiocyanate and sulfide (Smith and Mudder 1991). The ASTM D4374 (1.993) Automated Method for Determination of Total Cyanide, Dissociable Cyanide, and Thiocyanate uses ultraviolet radiation to free cyanide from the strongest iron, cobalt, gold and platinum complexes. As in the measurement of WAD cyanide, Kelada (1992) reports this method to be free from thiocyanate interference and regards it as more reliable than distillation methods in the measurement of total cyanide concentrations. Measurement Of Cyanide In Solids Cyanides can also exist on and in spent leach heap ores. Their measure­ ment in the past has typically involved homogenization and direct distillation of a solid sample or leachate using ASTM D-2036 (1993) or EPA 9010 (1986) methods (Hendrix et al. 1985, Durkin 1990, and Davis et al. 1991). Most recently, an 21 ongoing Bureau of Mines cyanide degradation study (Comba et al. 1992) adopted a sample preparation procedure whereby frozen ore is crushed to 10 to 20 mesh size. Subsequent cyanide analysis uses HO to 120 grams of the frozen sample in direct distillation using ASTM D-2036 (1993) procedures. A study to develop a standard solid sample analytical procedure (Davis et al. 1991) concluded that 60day bottle-roll extraction using 1.25 N NaOH solution, followed by leachate distillation, provided the most reliable WAD and total cyanide measurement. The study recommended further refinement of bottle roll extraction methods. Residual Cyanide Studies As more and more heap leach projects undertaken in the past 25 years near completion, effective neutralization of residual cyanide is of interest to both the mineral industry and regulators. Little information is available on residual cyanide levels following heap neutralization from the few projects decommissioned to date. Engelhardt (1985) found that approximately 85% of the free cyanide present in an untreated, inactive silver leach heap in California was degraded by natural processes over an 18 month period. The pH of the agglomerated ore declined from 10.5 to 9 through the course of this study. The Golden Maple-Gilt Edge gold heap leach project near Lewistown, Montana, was closed by regulators in late 1985 (Schafer et al. 1991). Leached ore had a high clay content but was not agglomerated. The heap was rinsed with calcium hypochlorite after closure. Subsequent inspection of the heap as it was excavated showed zones of high / 22 residual cyanide that were not effectively rinsed. The Borealis Mine in Hawthorne, Nevada was deactivated in 1987 after two year’s cyanide leaching of agglomerated ore (Schafer et al. 1991). Twenty-tWo months later, the heap was rinsed with fresh water to reduce effluent WAD cyan­ ide concentrations to less than 0.2 mg I'1. For five months following this neutral­ ization, effluent WAD cyanide levels remained less than 0.2 mg I 1 and the pH remained below 8.5 in most samples. Ore sampled from three backhoe pits had total cyanide levels below the 10 mg kg'1 limit set by the Nevada Division of Environmental Protection. In their study of the Gilt Edge Mine heap leach operation in Deadwood, South Dakota, Smith and Mudder (1990) concluded that sampling of ore is a superior method of assessing neutralization success compared to heap effluent monitoring. In a study of neutralization at Kendall Mine’s Heap Leach Pad No. I and Pad No. 2, Haight (1991) found total cyanide levels increasing from the surface to 6 m (20 ft) depth. He concluded that spent ore must be sampled at various depths and locations throughout a heap, as surface oi;e or effluent sam­ pling will not accurately characterize the cyanide concentrations within it. Schafer et al. (1991) conducted an intensive investigation of the behavior of cyanide and metals in a spent leach heap at the Landusky Mine near Malta, Montana. Ore in the heap was very permeable and was not agglomerated. Heap excavation and neutron probe data showed no evidence of blind-off zone develop­ ment. Natural degradation processes were predicted to reduce WAD cyanide 23 levels to less than 0.22 mg I"1 within 6 to 8 years. The addition of one pore volume of fresh water rinse reduced cyanide, nitrate and metal levels by 50 to 90 percent. Soluble metal levels were, found to be closely related to the level of cyanide in solution. WAD cyanide levels less than 0.22 mg I"1 in heap effluent had copper levels 4%, zinc levels 1%, iron levels 47%, and cadmium levels less than 10% of the drinking water standard for each metal (Schafer et al. 1991). The Snow Caps Mine gold leach heap near Independence, California was neutralized in 1991 using the INCO SO2ZAir process. Subsequent auger drill sampling found total cyanide levels below the regulatory level of 10 mg kg'1 in 9 of 10 samples (Young et al. 1992). In an ongoing study of natural degradation of cyanide forms in an inactive spent leach heap at the Trinity Silver Mine in Nevada (Comba et al. 1992), preliminary data indicate total and WAD cyanide levels are higher at lower heap depths. Natural cyanide degradation rates are slow, but are expected to increase as leach solution more completely drains from the heap. 24 STUDY AREA The Kendall Mine is located in the North Moccasin Mountains, approxi­ mately 32 kilometers (20 miles) northwest of Lewistown, Montana (Figure 3). Gold has been produced in this area since the late 1800s advent of cyanide Figure 3. Location of Kendall Mine (Canyon Resources Corporation 1991). .^M issouri Ri ver S. Moccasin Mountains Snowy . \ j Ij i 4I i I ' I i* \ Tr v 0 30 I L_ miles 25 vat leaching. Open pit heap leach mining of gold began in 1982: After several ownership transfers, Canyon. Resources Corporation acquired Kendall Mine in February 1988. The mine project is currently permitted for operation in Township 18N, Range I SE, Sections 29, 30, 31 and 32 and Township 17N, Range 18E, Section 6. Heap Leach Pad No. I, the focus of this investigation, is located in Township 18N, Range 18E, Section 31. Construction of Heap Leach Pad No. I was not thoroughly documented. The heap covers I hectare (2.4 acres) and contains 82,000 metric tons (90,000 short tons) of agglomerated, run of mine ore on a compacted clay liner (Haight 1991). The heap was constructed in two lifts using either end dump or truck and dozer techniques. The sandy gravel ore at the top of Pad No. I is comprised of 62% gravel, 24% sand, and 14% clay (HKM Associates 1982). In August of 1988, the new owners ripped the surface of the heap to increase permeability and r . ■ ' , subjected it to cyanide leaching during the next month. The heap was leached for 68 hours with approximately 760,000 liters (200,000 gallons) of sodium cyanide solution. No economically-recoverable gold was found and the. heap was left to drain before spring decommissioning. Active neutralization of Heap Leach Pad No. I began in March, 1989 w ith. one week of fresh water rinsing. Prior to chemical neutralization, ore at the surface was sampled for cyanide. Results are shown in Table 5. Effluent dis­ charge from the heap at this time measured 49.0 mg L total cyanide and had a pH of 8.6. i 26 Table 5. Cyanide levels after fresh water rinsing at Kendall Mine Heap Leach Pad No. I, March, 1989 (Haight 1991). Location Total Cyanide (mg I 1) WAD Cyanide (mg I 1) Free Cyanide (mg I 1) Pad No. I - East <0.5 <0.5 <0.5 Pad No. I 1- West 2.1 1.2 <0.5 ’ Neutralization of the heap with calcium hypochlorite followed the rinsing and continued through May, 1989. At this point, effluent draining the heap measured 0.1 mg I:1 total cyanide with a pH of 8.8. By the end of September, 1989, effluent draining the heap measured 0.02 mg. I"1 total cyanide with a pH of 8. 1. . / 27 METHODS Sampling Methods Ore samples were collected from Kendall Mine Heap Leach Pad No. I from October 29 to November 2, 1991, thirty months after active chemical neutralization ceased. Fifteen boreholes were located along two random transects across the heap in a randomized complete block field plot design (Figure 4). Boreholes were regarded as blocks and sampled depth increments as treatments. Block/depth interaction was assumed to be negligible. Samples were taken from nine to eleven depth increments: 0 to 0.2, 0.2 to 0.5, 0.6 to 1,2, 1.5 to 2.1, 3.0 to 3.7, 4.6 to 5.2, 6.1 to 6.7, 7.6 to 8.2, 9.1 to 9.8, 10.7 to 11.3, and 12,2 to 12.8 m (0 to 0.5, 0.5 to 1.5, 2 to 4, 5 to 7, 10 to 12, 15 to 17, 20 to 22, 25 to 27, 30 to 32, 35 to 37, and 40 to 42 ft), with incremental sampling halted at the clay liner. A truck-mounted drill rig using a hollow stem auger was used to bore into the heap, pausing at the top of each sampling depth to pound a split spoon sampler through the depth increment. Retrieved split spoon samples were halved along the length of the spoon and examined for lithological character­ ization. One half of the sample was bagged for cyanide analyses. The other half was bagged for metals analyses, Both samples were sealed in air-tight plastic bags, logged and stored in coolers. Sample splits for cyanide analyses were shipped to Energy Laboratories in Billings, Montana for measurement of total, WAD, and free cyanide. 28 Figure 4. Location of sampling boreholes I to 15 across the heap surface (top diagram) and sampling boreholes I to 7 and 8 to 15 in cross-section (bottom diagrams) through Kendall Mine Heap Leach Pad No. I. 25.9m WEST EAST 48.8m Boreholes 1 2 3 4 5 6 7 10.2m 10.7m 11.1m 11.7m 12.2m clay liner Boreholes 15 14 13 12 11 10 9 8 10.1m 10.7m 11.0m 11.9m clay liner 12.2m 29 Split spoons were decontaminated between samples with a wire brush and cloth wipe. Auger sections were decontaminated between boreholes by pressur­ ized steam cleaning. Any contact with the clay liner was backfilled with bentonite pellets. Boreholes were backfilled with drill cuttings after sampling was complete. Three boreholes were fitted with pore water samplers (suction lysimeters) prior to backfilling (Table 6). These three suction lysimeters were installed to monitor pore water quality near the heap base on a bimonthly basis through spring and summer 1992. The suction lysimeter installed in Borehole 13 was accidentally destroyed by a mine grader before the sampling program commenced. Table 6. Location of suction lysimeters used in pore water sampling. Borehole Number Lysimeter Installation Depth (meters) 4 9 8 10 13 8 Pore water samples for cyanide analyses were collected 6 to 8 hours after negatively pressurizing the suction lysimeters. These samples were collected directly into opaque sample bottles. They were preserved for cyanide analyses by the addition of sodium hydroxide to pH 12 and cold storage. After this sampling, the suction lysimeters were again negatively pressurized for collection of additional pore water samples 12 hours later. The second set of samples was used for metals analyses. Upon collection, these samples were measured for pH. Samples 30 s . were then preserved for metals analyses by the addition of nitric acid to pH 2 and . cold storage. Analytical Methods Because standard methods for a solids cyanide analysis applicable to mine ore were, as they remain, in a state of flux (Comba et al. 1992, Smith and Mudder 1991), ASTM (1993) D-2036 Test. Method C reflux distillation procedures for cyanide in liquids were modified to measure spent ore total and WAD cyanide levels. The modification consisted of adding one-half gram of < 2 mm air-dried ore sample to the reflux distillation solution prior to analysis. Free cyanide in the ore solutions was measured using a cyanide ion-specific electrode (APHA Method 45 OO-CN-F 1992). Saturated paste extracts of oven-dried ore samples were prepared for pH and soluble metals measurement using American Society of Agronomy Method 10-2.3.1 (Rhoades 1982). An Orion Model 901 meter/91-55 electrode was used to measure pH. Soluble cadmium, copper, iron, nickel, and zinc levels were mea­ sured in the saturated paste extracts by atomic absorption spectroscopy. Pore water samples were analyzed for total and WAD cyanide using ASTM D-2306 Test Method C (1993). Free cyanide was measured using APHA Method 4500-CN-F (1992). Atomic absorption spectroscopy was used to measure soluble cadmium, copper, iron, nickel and zinc concentrations in pore water samples. To monitor the precision of analyses, blind field replicates and laboratory 31 replicates were entered into the sample set at a 5 percent (I in 20) rate. Blind field replicate relative percent difference (RPD) averaged 18.4% total cyanide, 16.0% WAD cyanide, 3.1% pH, 19.1% cadmium, 23.4% copper, 22.8%, iron, 9.3% nickel, and 30.9% zinc. Laboratory replicate RPD averaged 23.2% total cyanide, 10.8% WAD cyanide, 1.6% pH, 11.8% cadmium, 21.9% copper, 6.2% iron, 20.1% nickel, and 19.5% zinc. Laboratory natural matrix spikes and laboratory digestion/extraction spikes were utilized at a 5 percent rate to monitor the accuracy of cyanide analyses. Natural matrix spike recoveries for total and WAD cyanide averaged 83.0% and 58.9%, respectively. Laboratory digestion/extraction spike recoveries for total and WAD cyanide averaged 98.4% and 96.1%, respectively. Accuracy of pH and metals analyses were monitored using laboratory standards at a 10 percent (I in 10) rate. Recoveries averaged 100.3% pH, 98.1% cadmium, 97.6% copper, 98.8% iron, 101.1% nickel, and 99.3% zinc. To clarify interpretation, the most complete data (from the upper nine of the eleven depth increments sampled for all boreholes) were combined into five depth categories. Measurements below analytical detection limit were adjusted for inclusion in the development of the five depth categories. This adjustment multiplied respective analytical detection limits by a factor of 0.7 to obtain a numerical value (Severson 1979). The five depth categories were 0 to 0.5, 0.6 to 2.1, 3.0 to 5.2, 6.1 to 8.2, and 9.1 to 9.8 m (0 to 1.5, 2 to 7, 10 to 17, 20 to 27, and 30 to 32 ft). 32 Skewness and kurtosis of untransformed data from the five depth catego­ ries (Table 7) indicate nonnormality in six of eight parameters at the 90% confi­ dence level. With a sample size of 75, skewness should lie within the range of -0.34 to +0.34 to indicate a normal data distribution at the 90% confidence level (Sachs 1982). Likewise, kurtosis should lie within the range of 2.40 to 3.50 (Sachs 1982). These data were normalized by a Iog10 transformation. Skewness and kurtosis of the transformed data indicate nonnormality in three of eight parame­ ters (Table 7). Transformed cadmium (skewness), nickel (skewness and kurtosis), and zinc (skewness and kurtosis) data were not improved enough to put them within the ranges reported by Sachs (1982). Despite indications of nonnormality, transformed cadmium, nickel, and zinc data were included in subsequent statistical analysis and interpretation. Table 7. Effect of Iog10 data transformation on data skewness and kurtosis. Parameter Untransformed Data Skewness Log10 Transformed Data Skewness Untransformed Data Kurtosis Log10 Transformed Data Kurtosis Total CN 1.69 -0.06 8.47 2.84 WAD CN 4.40 -0.04 31.42 3.25 pH 0.10 0.07 3.21 3.47 Cadmium 0.80 0.67 3.30 2.88 Copper -0.24 0.16 10.66 3.40 Iron 1.62 0.13 7.12 3.26 Nickel 2.33 0.92 15.36 5.44 Zinc 0.73 -0.57 4.69 3.63 33 Overall two way analysis of variance and its source components (depth and borehole) for normal total cyanide, WAD cyanide, pH, and metals data were evaluated at the 95. percent confidence interval using the PROC GLM procedure in SAS (SAS Institute 1987). Where the p-value for the observed F-statistic was less than or equal to 0.05, the hypothesis of equality of means was rejected. Data sets with unequal means were then subjected to the Student-Newman-Keuls means separation procedure at the 0;05 level of significance. ' Pore .water, data collected from suction lysimeters in boreholes 4 and 8 were subjected to linear regression at the 95 percent confidence interval. This regression was performed in order to identify trends in cyanide and metals levels in pore water near the clay heap pad through the spring and summer of 1992. 34 RESULTS AND DISCUSSION All solids analytical data are found in Appendix A. D ata validation tables are found in Appendix B. Statistical output from ANOVA, means separations, and linear regressions are provided in Appendix C. Data presented in this text are summaries of this information. . Many spent ore sample analyses showed cyanide at or below detection limits (Appendix A, Tables 12, 13, and 14). Samples which had total cyanide measurements below analytical detection limit were not further analyzed for WAD or free cyanide. Similarly, samples which, had WAD cyanide measurements below either WAD or free cyanide analytical detection limits were not further analyzed for free cyanide. With two exceptions, free cyanide was either below analytical detection limits or not analyzed at all. Cyanide and Metals Depth Distributions Depth category comparisons of cyanide, pH, and metals means are present­ ed in "Table 8. No significant differences exist for total cyanide, pH, cadmium, iron or zinc. Depth category three had significantly higher WAD cyanide concen­ trations than depth categories I, 2, and 5. WAD cyanide levels increase Mth depth to a peak in depth categories three and four, followed by a significantly lower concentration in depth increment 5. Soluble copper concehtrations were significantly higher in the upper three depth categories of the heap, subsequently declining through depth categories 35 T a b le 8. G e o m e tr ic m e a n lev els1 o f c y an id e, p H a n d w a te r so lu b le m e ta ls in s p e n t o re th ro u g h K e n d a ll M in e H e a p L e a c h P a d N o . I. _______ Copper Iron Nickel Zinc O tg r 1) O tg r 1) O t g r 1) O tg r 1) O t g r 1) 8A2 113A2 264A2 93AB2 112A2 IOA 144A 302A 113A 127A 7.7A 9A 113A 360A 61B 140A 0.6AB 7.7A IlA 76B 255A 64B 166A 0.2D 7.7A 8A 54C 140A 28C 139A D cplh Cnlegory D epth (m ) ToialCN WADCN (A ee"') (M g -1) I 0-0.5 L lA 2 0.4C2 7.9A2 2 0.6-2.1 1.3A 0.5BC 7.7A 3 3.0-5.2 2.2A 0.7A 4 6.1-8.2 1.5A 5 9.1-9.8 0.8A pH 1 Mean values are based on N = 10, 11, 12, 13, 14 or 15 observations. 2 Multiple means comparisons were performed using Student-Newman-Keuls Test on Iog10 transformed data. Means followed by the same letter in columns are not significantly different (p s 0.05). 4 and 5. Soluble nickel concentrations in depth category 2 were significantly higher than concentrations in depth categories 3, 4, and 5. The peak of WAD cyanide concentrations in depth category three and four (3.0 to 8.2 m) may be attributable to textural changes and/or compaction at a lift interface. These depth categories, approximately halfway through the heap, would be inclusive of the interface between the two lifts comprising Heap Leach Pad No. I. Schafer et al. (1991) detailed the concept behind particle segregation in heap lift construction that can lead to textural changes at lift interfaces. Coarser ore end-dumped on the second lift rolls to the base where it accumulates over finer ore particles at the top of the first lift (Figure 5). The gradation of each lift, therefore, would change from coarse at the bottom to finer at the top. The texture of the upper several feet of each lift may also be made finer by mechani­ cal weathering and particle disaggregation during leaching. Porosity and 36 Figure 5. Conceptual view of heap construction showing characteristics of material within the heap (Schafer et al. 1991). DETAIL Fine ore Coarse or Coarser % v * '0 • 0 interface Ripper track permeability could be further limited in this zone by compaction caused by lift construction traffic (Chamberlin 1981). Changes in flow paths at this interface or the development of blind-offs under the compacted ore may protect residual cyanide from neutralizing rinses and natural degradation processes. Evidence of the existence of the coarse/fine interface might be found in an examination of the lithological descriptions from split spoon samples from each depth category. Sampling in the interface zone would find rock fragments immediately above fine material. Sampling spoon lithological descriptions from Heap Leach Pad No. I do not contain any notation of this distinction. They do indicate rock fragments in 97% of depth categories sampled. However, the 37 descriptions do not differentiate undisturbed whole rock fragments from rock fragments cored from larger rocks and stones by the force of the pounded split spoon. . Cyanide and Metals Areal Distributions The comparisons of mean cyanide, pH, and soluble metals concentrations for each borehole across Heap Leach Pad No. I are summarized in Table 9. . Significant differences exist between boreholes for all parameters except soluble iron, nickel, and zinc. However, with one exception, no discernible areal pattern (e.g. mean concentrations increasing north to south across the heap) is apparent among parameters with significant differences between boreholes. Only mean borehole cadmium levels exhibited an areal pattern, declining significantly moving west to east across the heap. The distribution pattern of significant differences, where they exist, for each of the parameters is predominantly random. This would indicate that areal pat- . terns have not developed in the neutralized leach heap environment. An explana­ tion or hypothesis for these random patterns and the areal pattern of cadmium levels is not possible without control data from the heap prior to leaching and neutralization. T a b le 9. Borehole G e o m e tric m e a n lev els1 o f c y an id e, p H a n d w a te r so lu b le m e ta ls fo r b o re h o le s a cro ss K e n d a ll M in e H e a p L e a c h P a d N o . I. Total CN (Mg g l) WAD CN (Mg g"‘) pH Cadmium (Mg I*1) Copper (Mg I 1) Iron (Mg I'1) Nickel (Mg I 1) Zinc (Mg I'1) I 1.05CDEF2 0.39BCD2 8.16ABC2 19A2 95CDE2 160A2 65A2 142A2 2 1.17DEF 0.47ABCD 8.22AB 12ABCD 101CD 368A 54A 87A 3 2.32ABC 0.78A 8.29A 5F 179B 280A 136A IlOA 4 3.46A 0.69AB 7.89BCD 280A 206A 172A 130A 5 1.10CDEF 0.38BCD 7.69DE 6EF 130BC 170A 58A 225A 6 1.25CDE 0.43ABCD 7.02F 5F 81CDE 284A 39A 200A 7 0.48F 0.24D 7.61DE 6EF 57DE 113A 31A 182A 8 0.53EF 0.35BCD 7.56DE 7DEF 49E 219A 35A 15IA 9 1.09CDEF 0.35BCD 7.92BCD 70CDE 312A 79A 76A 10 0.48F 0.40BCD 7.67DE 8CDEF 61DE 373A 43A 86A 11 2.93AB 0.36BCD 7.46E 7DEF 7ICDE 186A 38A 172A 12 1.5 IBCD 0.31CD 7.81CDE IOBCDE 95CDE 24IA 48A 104A 13 0.81EFD 0.29CD 7.72DE 16AB 84CDE 146A 73A 135A 14 0.75EFD 0.26D 7.87BCD 13ABC 81CDE 248A 79A 108A 15 1.62BCD 0.59ABC 8.19AB 15AB 88CDE 387A 173A 86A 8CDEF 6EF 1 Mean values are based on N = 3, 4 or 5 observations. Multiple means comparisons were performed using Student-Newman-Keuls Test on Iog10 transformed data. Means followed by the same letter in columns are not significantly different (p < 0.05). 39 Pore Water Behavior Pore water samples were collected every two weeks, March through September, 1992. Data from this sampling effort are summarized in Table 10. Pore water WAD cyanide concentrations exceeded Montana’s effluent water quality standard level of 0.22 mg I"1 once over the course of sampling. This measurement, from borehole 4 on August 9, 1992, showed a WAD.cyanide concentration of 0.31 mg I'1. The only measure of free cyanide (0.2 mg I'1) was, notably, also registered here. The sample collected from borehole 8 on this date was of insufficient volume for WAD or free cyanide analysis. The previous borehole 4 pore water WAD cyanide concentration measured 0 J 9 mg I1 on July 24, 1992. Between July 24 and August 9, borehole 4 pore water pH decreased from 7.8 to 6.4, the lowest pH recorded in the sampling program. Linear regression analysis was used to describe concentrations of parame­ ters in the pore water as functions of time. Results of these analyses and regres­ sion coefficients are presented in Appendix C, Tables 43 to 58. Table 11 displays the slope of each regression and associated F and p values. If the slope is different (p < 0.05) than zero, then changes in concentration of that parameter over the sampling period are demonstrated. Pore water samples collected from the upgradient position of the heap (borehole 4) showed increased concentrations of total and WAD cyanide, but decreased levels of nickeh No changes were found for these parameters in pore water collected from the downgradient position of the heap (borehole 8). T a b le 10. P o r e w a te r cy an id e a n d m e ta ls levels a t K e n d all H e a p L e a c h P a d N o. I, M a rc h - S e p te m b e r, 1992. Total Cyanide (mg I"1) WAD Cyanide (mg I"1) Free Cyanide (mg I 1) PH Cadmium Copper 4 0.48 — 0.69 0.76 0.96 0.94 0.99 0.97 1.08 1.14 0.64 1.99 0.07 — 0.12 0.15 0.15 0.17 0.14 0.15 0.19 0.31 0.15 0.19 BD — BD BD BD BD BD BD BD 0.2 ISV ISV 6.8 6.7 6.5 7.4 7.6 7.7 7.1 6.8 7.8 6.4 7.8 7.6 ISV 33 26 26 20 26 33 26 33 26 26 ISV ISV 48 19 38 87 0 0 29 0 0 0 ISV 8 0.69 0.88 0.41 0.33 0.36 0.40 0.44 0.55 0.55 0.52 0.35 0.41 0.05 0.06 0.06 0.04 0.05 0.05 0.05 0.06 0.07 ISV 0.03 ISV BD BD BD BD BD BD BD BD BD ISV ISV ISV 6.7 6.7 6.6 7.3 7.4 7.4 7.2 7.2 7.4 6.4 8.0 7.5 ISV 66 26 20 26 26 26 33 33 33 26 ISV ISV 67 29 19 9 38 29 19 19 0 9 ISV Sampling Date Borehole 3/19/92 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 9/06/92 3/19/92 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 9/06/92 BD ISV Below analytical detection limit. Insufficient sample volume for analysis. Sample destroyed in transit. Iron (Mg I"1) Nickel Zinc ISV 649 375 1134 ISV 537 862 500 458 575 500 ISV ISV 152 108 86 86 65 108 65 65 65 43 ISV ISV 442 97 299 75 32 245 372 140 ISV ISV ISV ISV 757 416 416 208 458 291 125 208 125 612 ISV ISV 130 43 86 65 108 43 86 65 86 65 ISV ISV ISV 420 194 215 311 406 500 ISV 240 256 ISV 41 Table 11. Linear regression slope values and associated statistics for Kendall Heap Leach Pad No. I pore water. Borehole P aram eter Slope F V alue p V alue 4 Total CN 0.0050 7.290 0.024 WAD CN 0.0007 7.208 0.025 PH 0.0038 1.800 0.209 Cadmium -0.0002 4.327 E-05 0.995 Copper -0.3810 4.315 0.071 Iron -1.5030 0.688 0.434 Nickel -0.5647 15.521 0.004 Zinc -0.6733 0.161 0.702 Total CN -0.0012 2.011 0.187 WAD CN -4.067 E-05 0.248 0.632 PH 0.0042 3.352 0.097 Cadmium -0.0850 0.791 0.400 Copper -0.2830 6.867 0.031 Iron -1.8980 1.601 0.241 Nickel -0.1544 0.557 0.477 Zinc -0.1214 0.013 0.913 8 Concentrations of copper decreased over the sampling period in pore waters at the downgradient position. Pore water pH from both gradient positions in the heap did not change. Interpretation of these data is difficult because they are restricted to one sampling season, and no attempts were made to describe the variation within a particular sampling data or at other positions within the heap. Variation could be measured if multiple pore water samplers were installed in various locations and 42 depths within the heap. The limited data do,, however, indicate that upgradient cyanide concentrations increased over the sampling period while downgradient cyanide levels did not change. Cyanide may have become attenuated as pore water drained downgradient over the course of the spring and summer. It is interesting to note that the slopes of the regressions for all metals were negative (Table 11). Factors influencing these decreasing trends may be related to complex stabilities and competing degradation processes described in the literature review. Additional data from subsequent sampling periods and from additional pore water samplers could be used to verify these trends. 43 SUMMARY AND CONCLUSIONS In Montana, the use of heap leach cyanidation for gold recovery has become widespread. These heaps, after gold extraction, must be neutralized to state effluent standards to assure nondegradation of ground or surface water quality. However, cyanide may still exist within a heap, protected from the full effect of neutralization or natural degradation processes. Subsequent drainage from these heaps, particularly in association with large precipitation events, represent a possible threat to downstream water quality. Cyanide and metal concentrations in ore and pore water from a decommis­ sioned leach heap (Kendall Mine Heap Leach Pad No. I) were measured and compared. Thirty months after active neutralization with calcium hypochlorite, WAD cyanide levels increased significantly from the heap surface to the middle of the heap, and significantly declined toward the heap bottom. Copper and nickel exhibited a similar vertical distribution. These concentration changes may be attributable to altered flowpaths or .blind-off development at a lift interface within the heap. Significant areal distribution patterns could not be derived for cyanide, pH, or metals, with the exception of cadmium, which significantly decreased west to east across the heap. On balance, the data indicate that areal patterns did not develop in the neutralized heap environment. Pore water data indicate that total and WAD cyanide concentrations near the bottom of the heap may have degraded as pore water drained downgradient over the course of spring and summer sampling. Pore water soluble metals data 44 indicate that copper and nickel levels declined over the sampling period. These changes might be due to relative metallo-cyanide. complex stabilities and compet­ ing degradation processes. A more extensive and intensive pore water sampling design inclusive of all depth categories sampled is recommended for future heap investigations of cyanide or metals distributions. Conclusions regarding the distribution of cyanide within this heap cannot be made without recognition of the difficulty in measuring cyanide concentrations in heterogeneous spent ore. Development and standardization of collection proto­ cols and analysis procedures for cyanide in spent leach heap ore, pore Water, and effluent would be helpful to both industry and regulators in the evaluation of neutralization efficacy. 45 LITERATURE CITED r 46 x American Public Health Association. 1992. Cyanide, pp. 4-18 to 4-31 In: Standard Methods for the Examination of Water and Wastewater, Washington, D.C. American Society for Testing and Materials. 1993. Test Methods for Cyanides in Water, pp. 85-119 In Annual Book of ASTM Standards^ Section'll, Water and Environmental Technology, Volume 11.02 Water (II), Philadelphia, PA. Barratt, D. and R. McElroy. 1990. Heap leaching for precious metals. Engineering and Mining J. 191(6):40-46. Bosqui, F. 1899. Practical Notes on the Cyanide Process. The Scientific Publishing Co. New York and London. 201 p. Canyon Resources Corporation. 1991. 1990 Annual Report. Golden, CO. 36 p. Chamberlin, P.D. 1981. Heap leaching and pilot testing of gold and silver ores. Mining Congress J. 67(4):47-52. Chamberlin, P.G. and M. Pojar. 1984. Gold and silver leaching practices in the United States. U.S. Dept, of Interior, Bur. of Mines. Inform. Circ. 8969. Washington, D.C. 47 p. Comba, P., R. Dix, and S. McGill. 1992. A field study to assess natural degradation of cyanide species in an inactive leached ore heap. U.S. Dept, of Interior, Bur. of Mines. Internal Report. Reno, NV. 9 p. Davis, A., A. Heribu and B. Davis. 1991. Development of a solid sample analytical procedure for cyanide in spent ore: Final technical report. South Dakota School of mines and Technology. Rapid City, SD. 227 p. Denton, D., S. Iverson and B. Gosling. 1992. HEAPREC - A methodology for determining cyanide heap leach reclamation performance bonds. U.S. Dept, of Interior, Bur. of Mines. Inform. Circ. 9328. Washington, D.C. 79 p. ^ DeVries, F. 1988. Environmental aspects of cyanide in mineral processing. pp. 79-84 In: Eighth High Altitude Revegetation Workshop Proc. W.R. Keammerer and L. Brown (eds.). Colorado State Univ. Fort Collins, CO. 47 Dorey, R., D. van Zyl and J. Kiel. 1988. Overview of heap leaching technology, pp. 1-22 In: Introduction to Evaluation, Design and Operation of Precious Metal Heap Leaching Projects, Chapter I. D. van Zyl, I. Hutchinson and J. Kiel (eds.). Soc, of Mining Engineers, Inc. Littleton, CO. Durkin, T. 1990. Neutralization of spent ore from cyanide heap leach.gold mine facilities in the Black Hills of South Dakota - Current practices and requirements, pp. 29-40 In: Proc. of the 4th Western Regional Conf. on Precious Metals and the Environment. Lead, SD. Eisele, J. 1988. Gold metallurgy - a historical perspective. Can. Metall. Q. . 27(4):287-291. Eisele, J., A. Colombo and G. McClelland. 1984. Recovery of gold and silver from ores by hydrometallurgical processing, pp. 387-395 In: Precious metals: Mining, Extraction and Processing. Proc. of an Internt’l. AIME Symp. V. Kudiyk (ed.). The Metallurgical Soc. of AIME, Littleton, CO. x Eisler, R. 1991. Cyanide hazards to fish, wildlife and invertebrates: A synoptic review. U.S. Dept, of Interior, Fish and Wildlife Serv. Biol. Rept. 85(1.23). Washington, D.C. 55 p. Ehgelhardt, P. 1985. Long-term degradation of cyanide in an inactive leach heap. pp. 539-545 In: Cyanide and the Environment. D. van Zyl (ed.). Colorado State Univ. Fort Collins, CO. HKM Associates. 1982. Slope stability analyses for the North Moccasin Mine. Prepared for Geo West, Inc. Billings, MT. 40 p. Haight, S. 1991. Cyanide heap neutralization at the Kendall Mine. pp. 14.5514.65 In: Proc. of the U.S. Forest Service Heap Leach Technology Workshop. D. van Zyl, M. Henderson and W. Schafer (eds.). Reno, NV. Heinen, H., G. McClelland and R. Lindstrom. 1979. Enhancing percolation rates in heap leaching of gold-silver ores. U.S. Dept, of Interior, Bur. of Mines. Rept. of Investigation 8388. Washington, D.C. 20 p. Heinen, H., D. Peterson and R. Lindstrom. 1978. Processing gold ores using heap leach-carbon adsorption methods. U.S. Dept, of Interior, Bur. of Mines. Inform. Circ. 8770. Washington, D.C. 21 p. ^ Hendrix, J., J. Nelson and M. Ahmadiantehrani. 1985. Fate of cyanide tailings - An update, pp. 265-272 In: Cyanide and the Environment. D. van Zyl (ed.). Colorado State Univ. Fort Collins, CO. 48 Heriba, A. 1991. Factors in the Development of a Standard Test for Cyanide in Neutralized, Spent Ore. Ph.D. Dissertation, South Dakota School of Mines and Technology. Rapid City, SD. 73 p. Herzog, D. 1990. Cyanide leach technology and its applicability to Alaskan conditions. U.S. Dept, of Interior, Bur. of Mines. OFR 39-90. Washington, D.C. 31 p. Higgs, T. and L. Gormley. 1992a. Cyanide process chemistry and technology, pp. 2.1-2.31 In: Technical Guide for .the Environmental Management of Cyanide in Mining. T. Higgs and K. Lunde (eds.). British Columbia Technical and Research Committee on Reclamation. Vancouver, B.C., Canada. Higgs, T. and L. Gormley. 1992b. Cyanide treatment technology, pp. 7.2-7.17 In: Technical Guide for the Environmental Management of Cyanide in Mining. T. Higgs and K. Lunde (eds.). British Columbia Technical and Research Committee on Reclamation. Vancouver, B.C., Canada. Hiskey, J. 1985. Gold and silver extraction: the application of heap-leaching cyanidation. Arizona Bur. of Geol. and Mineral Technology Fieldnotes 15(4):1-5. Huiatt, J. 1984. Cyanide from mineral processing: Problems and research needs; pp. 65-81 In: Cyanide and the Environment. D. van Zyl (ed.). Colorado State Univ. Fort Collins, CO. Huiatt, J., J. Kerrigan, F. Olson and G. Potter (eds.). 1983. Proc. of a workshop: Cyanide from mineral processing. Utah Mining and Mineral Resources Research Inst. College of Mines and Minerals Industries. Salt Lake City, UT. 122 p. International Environmental Consultants, Ltd. 1979. Factors affecting natural degradation of free and metal-complexed cyanides from gold milling effluents. Fisheries and Environ. Canada. Burlington, Ontario, Canada. 45 p. . Kelada, N., C. Lue-Hing, W. Willbom and K. Rao. 1992. Cyanide speciation , by automated Kelada methods and comparison with manual standard methods for total cyanide, pp. 385-393 In: Randol Gold Forum Proc. Vancouver, B.C., Canada. Lindsay, W. 1979. Chemical Equilibria in Soils. Wiley-Interscience, New York, NY. 449 p. 49 Maynard, A. and B. Szczahor. 1992. Cyanide measurement, pp. 4.1-4.19 In: Technical Guide for the Environmental Management of Cyanide in Mining. T. Higgs and K. Lunde (eds.). British Columbia Technical and Research Committee on Reclamation, Vancouver, B.C., Canada. Maynard, A., P. Chapman and S. Cross. 1986. Evaluation study of the inland , waters directorate database for total cyanide measurements in western Canada (1974-1983) and the analytical methodology used to derive this database. Environ. Canada. Regina, Saskatchewan, Canada. 55 p. "\ McClelland, G. and J. Eisele. 1982. Improvements in heap leaching to recover silver and gold from low-grade resources. U.S. Dept, of Interior, Bur. of Mines. Rept. of Investigation 8612. Washington, D.C. 26 p. McClelland, G., D. Pool and J. Eisele. 1983. Agglomeration-heap leaching operations in the precious metals industry. U.S. Dept, of Interior, Bur. of Mines. Inform. Circ. 8945. Washington, D.C. 16 p. Montana Department of Health and Environmental Sciences. 1993. Montana numeric water quality standards. W ater Quality Bureau, Special Projects Section. Circular WQB-7. Helena, MT. 19 p. Mudder, T. 1991. Cyanide chemistry, pp. 1.1-4.6 In: Heap Leach Technology . Workshop Proc. U.S. Dept, of Agric., Forest Serv. Washington, D.C. Rhoades, J. 1982. Saturation extract and other aqueous extracts, pp. 168-170 In: Methods of Soil Analysis, Part 2, Monograph No. 9. American Society of Agronomy, Inc., Soil Science Society of America, Inc. Madison, WL Rolfes, H. 1989. Methods of Neutralizing Cyanide in Mining Wastes and Waste Waters. M.S. Thesis, Montana State Univ., Bozeman, MT. 63 p. Sachs, L. 1982. Applied Statistics. Springer-Verlag, New. York, NY. 706 p. SAS Institute Inc. 1987. SAS/STAT User’s Guide, Release 6.03 Edition. Cary, N G 1028 p. Schafer, W., J. Goering, S. Smith and J. Liu. 1991. Cyanide degradation and rinsing behavior in Landusky heaps. Unpublished report prepared for Zortman Mining Inc. Bozeman, MT. 158 p. Scott, J. 1984. An overview of cyanide treatment methods for gold mill effluents pp. 307-330 In: Cyanide and the Environment. D. van Zyl (ed.). Colorado State Univ. Fort Collins, CO. 50 Severson, R. 1979. Regional soil chemistry in the Bighorn and Wind River Basins of Wyoming and Montana. U.S. Geological Survey Professional Paper No. 1134B. U.S. Geological Survey. Denver, CO. 9 p. Smith, A., and T. Mudder. 1990. An evaluation of the effectiveness of rinsing procedures on cyanide removal from spent heap leach ore: Brohm Mining Corporation, Lead, South Dakota. Prepared for South Dakota Department of W ater and Natural Resources and Brohm Mining Corporation. 16 p. Smith, A., and T. Mudder. 1991. Chemistry and Treatment of Cyanidation Wastes. Mining Journal Books. London, England. 345 p. Smith, A. and D. Struhsacker. 1988. Cyanide geochemistry and detoxification regulations, pp. 275-292 In: Introduction to Evaluation, Design and Operation of Precious Metal Heap Leaching Projects. D. van Zyl, I. Hutchinson and J. Kiel (eds.). Soc. of Mining Engineers, Inc. Littleton, CO. Spickelmier, K. 1993. Round Mountain halves its cutoff grade. Mining Engineering 194(l):41-48. Stanton, M., T. Colbert and R. Trenholme. 1986. Environmental handbook for cyanide leaching projects. U.S. Dept, of Interior, National Park Serv., Energy, Mining, and Minerals Div. Denver, CO. 57 p. Struhsacker, D. and A. Smith. 1990. Cyanide neutralization and reclamation of heap leach projects, pp. 83-93 In: Fifth Billings Symposium on Disturbed Land Rehabilitation Proceedings, Volume I. Billings, MT. Thompson, L. and R. Gerteis. 1990. New technologies for mining waste management biotreatment processes for cyanide, nitrates and heavy metals, pp. 271-278 In: Mining and Mineral Processing Wastes. Proc. of the Western Regional Symp. on Mining and Mineral Processing Wastes. F. Doyle (ed.). Soc. for Mining, Metallurgy and Exploration of AIME. Littleton, CO. U.S. Environmental Protection Agency. 1986. Test methods for evaluating solid waste. U.S. EPA Office of Solid Waste and Emergency Response. SW. 846, Vol. I, Sect. C., Washington, D.C. van Zyl, D., I. Hutchinson, and J. Kiel (eds.). 1988. Introduction to Evaluation, Design and Operation of Precious Metal Heap Leaching Projects. Society of Mining Engineers, Inc. Littleton, CO. 372 p. 51 von Michaelis, H. 1985. Role of cyanide in gold and silver recoveiy.* pp. 51-64 In: Cyanide and the Environment. D. van Zyl (ed.). Colorado State Univ. Fort Collins, CO. Young, A., M. Smith, G. Sintay and G. Bakker. 1992. The Snow Caps Mine - A case history, pp. 369-372 In: Randol Gold Forum Proceedings, Vancouver, B.C., Canada. Zadra, J., A. Engel and H. Heinen. 1952. Process for recovering, gold and silver from activated carbon by leaching and electrolysis. U.S. Dept, of Interior, Bur. of Mines. Rept. of Investigations 4843. Washington, D.C. 32 p. 52 APPENDICES .53 APPENDIX A Data Tables T a b le 12. T o ta l c y an id e levels (/tg g'1) a t K e n d a ll H e a p L e a c h P a d N o . I, N o v e m b e r I, 1991. Depth Interval (meters) I 2 3 4 5 6 7 0 -0 .2 0.7 1.1 2.2 2.8 BD LI 1.2 0.2 - 0.5 1.2 0.8 2.6 4.5 1.6 0.8 0.6 - 1.2 1.7 BD LI 2.3 2.1 1.5 - 2.1 1.7 2.8 2.3 1.7 3.0 - 3.7 8.8 BD 13.8 4.6 - 5.2 4.8 BD 6.1 - 6.7 13.2 7.6 - 8.2 9.1 - 9.8 9 10 11 12 13 14 15 1.2 BD BD 1.5 BD 1.5 0.8 0.5 BD 0.7 1.6 BD 0.8 1.4 1.0 1.8 1.3 2.7 BD BD BD 0.8 1.8 1.3 2.2 2.0 1.7 1.8 2.0 BD BD BD 1.3 1.9 2.9 1.9 1.7 2.0 3.0 2.2 2.2 1.2 0.9 BD 1.6 1.4 3.3 BD BD 3.0 10.4 2.5 2.4 2.6 BD BD 1.5 4.3 6.1 4.1 3.1 3.8 7.6 5.2 BD 2.4 BD 2.5 BD BD BD BD 3.3 2.4 BD 5.9 4.6 LI 4.2 2.7 0.9 BD BD 0.5 BD BD BD 10.3 LI 4.5 2.0 2.2 0.3' 0.5 1.7 4.8 0.9 1.2 BD BD 2.2 BD 10.3 1.6 0.2' 0.1' 3.7 BD BD 0.8 0.6 0.6 0.2' — — Liner 0.2' Liner 0.2' Below Liner 0.8 10.7 - 11.3 — — — 12.2 - 12.8 — — — BD 1 Borehole 8 — —— •— — — — —— Below analytical detection limit (<0.5 /ng g"1). No sample from the depth indicated. Analytical detection limit lowered to 0.1 /Ag g"1 due to minimal limestone interference with sample analysis. —— T a b le 13. W A D c y a n id e levels (/Ag g"1) a t K e n d a ll H e a p L e a c h P a d N o . I, N o v e m b e r I, 1991. Depth Interval (meters) I 2 3 4 5 6 7 0 -0 .2 BD 0.5 1.3 LI NA BD 0.21 0.2 - 0.5 0.6 BD 1.2 1.6 BD BD 0.6 - 1.2 BD NA BD 0.6 BD 1.5 - 2.1 0.8 0.9 0.7 0.6 3.0 - 3.7 1.9 NA 10.0 4.6 - 5.2 2.0 NA 6.1 - 6.7 2.2 7.6 - 8.2 Borehole 8 9 10 11 12 13 14 15 BD NA NA BD NA BD BD BD NA BD BD NA BD 0.2' BD BD 0.6 BD NA NA NA BD BD BD BD BD 0.6 BD BD NA NA NA BD BD 0.5 0.6 0.6 0.7 0.8 0.9 BD BD BD NA 0.6 BD 1.0 BD NA 0.7 2.1 BD 0.6 0.6 NA NA BD BD 0.5 0.6 1.0 0.9 1.2 1.5 NA BD NA 1.3 NA NA NA NA BD BD NA 0.9 0.9 BD 0.9 BD BD NA NA BD NA NA NA BD BD 1.2 0.4' 0.3' 9.1 - 9.8 0.T BD BD BD BD NA NA NA BD NA BD BD 0.1' BD *■ 10.7-11.3 — — — — BD NA NA BD BD BD 0.1' — —— —— 12.2-12.8 — — — — -- -- Liner 0.1' Liner 0.1' Below Liner 0.5 BD NA 1 — - —— — —— Below analytical detection limit (<0.5 /Ag g'1). Not analyzed. No sample from the depth indicated. Analytical detection limit lowered to 0.1 /Ag g"1 due to minimal limestone interference with sample analysis. T a b le 14. Depth Interval (meters) F r e e c y an id e levels (jx g g"1) a t K e n d a ll H e a p L e a c h P a d N o . I, N o v e m b e r I, 1991. 1 2 3 4 5 6 7 Borehole 8 9 10 11 12 13 14 15 0 -0 .2 NA NA BD BD NA NA NA NA NA NA NA NA NA NA NA 0.2 - 0.5 NA NA BD BD NA NA NA NA NA NA NA NA NA NA NA 0.6 - 1.2 NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA 1.5 - 2.1 NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA 3.0 - 3.7 BD NA 3.0 NA NA NA NA NA NA NA NA BD NA NA BD 4.6 - 5.2 BD BD 2.0 NA NA NA NA NA NA NA NA NA BD NA BD 6.1 - 6.7 BD BD NA NA NA BD NA NA NA NA NA NA NA NA NA 7.6 - 8.2 NA NA NA NA NA NA NA NA NA NA NA NA BD NA NA 9.1 - 9.8 NA NA NA NA NA NA NA NA NA NA NA NA NA NA — — 10.7-11.3 — — — — NA NA NA NA NA NA NA — — — — — 12.2-12.8 — — — — — — — — — — — — — — — BD NA Below analytical detection limit (<1.0 /u-g g"1). Not analyzed. No sample from the depth indicated. T a b le 15. p H levels a t K e n d a ll H e a p L e a c h P a d N o. I, N o v e m b e r I, 1991. Depth Interval (meters) I 2 3 4 5 6 7 0 -0 .2 8.0 8.6 8.1 8.0 6.7 7.2 8.0 0.2 - 0.5 8.6 8.7 8.9 8.3 8.1 7.6 0.6 - 1.2 7.9 ISV 8.0 7.9 7.6 1.5 - 2.1 7.6 8.6 8.5 8.0 3.0 - 3.7 8.7 7.9 8.0 4.6 - 5.2 7.0 7.9 6.1 - 6.7 8.2 7.6 - 8.2 Borehole 8 9 10 11 12 13 14 15 7.3 8.2 7.6 7.8 7.9 7.9 7.8 8.1 7.8 7.3 7.9 7.6 7.0 7.5 7.7 8.2 8.2 6.9 7.5 6.6 7.8 7.3 7.2 7.6 7.6 8.1 8.3 7.7 5.9 7.5 6.8 8.3 7.2 7.6 7.2 8.3 7.8 8.7 7.4 7.3 6.7 7.4 8.0 7.2 7.4 ISV 8.4 7.7 7.8 8.3 7.8 7.9 7.5 7.4 7.4 7.8 7.7 7.6 7.6 7.8 7.5 7.8 8.1 7.7 7.5 ISV 7.6 7.8 7.4 7.4 ISV 7.4 7.9 7.8 7.5 7.8 7.6 7.5 7.4 8.2 8.0 7.6 ISV 6.9 7.7 ISV 7.9 7.1 8.1 7.9 7.9 8.1 9.1 - 9.8 8.2 7.8 8.1 7.5 7.9 6.5 7.5 8.2 7.9 8.0 7.7 8.1 7.6 7.6 10.7-11.3 — — — — 7.9 7.7 7.3 8.0 7.7 7.6 7.8 — — — 12.2-12.8 — — — — — — Liner 7.5 Liner 7.8 Below Liner 7.9 — —— —— — — ISV Insufficient sample volume. No sample from the depth indicated. — T a b le 16. C a d m iu m levels ( jig I"1) a t K e n d a ll H e a p L e a c h P a d N o . I, N o v e m b e r I, 1991. Depth Interval (meters) 1 2 3 4 5 6 7 Borehole 8 9 10 11 12 13 14 15 0 -0 .2 15 BD1 BD1 BD1 BD1 BD1 11 BD2 BD2 BD2 BD2 14 17 8 13 0.2 - 0.5 10 10 BD1 BD1 BD1 BD1 8 BD2 BD2 BD2 12 9 14 14 22 0.6 - 1.2 12 ISV ISV BD1 28 BD1 BD1 25 BD2 22 14 14 14 BD2 11 1.5 - 2.1 32 10 BD1 8 BD1 BD1 BD1 22 BD2 11 14 14 12 8 11 3.0 - 3.7 20 BD1 28 BD1 13 BD1 BD1 BD2 11 BD2 ISV 12 7 BD2 8 4.6 - 5.2 17 12 BD1 14 BD1 BD1 BD1 BD2 BD2 18 12 14 14 8 11 6.1 - 6.7 28 35 25 ISV BD1 25 BD1 BD2 ISV 14 7 12 14 8 14 7.6 - 8.2 12 BD1 10 7 17 ISV 28 BD2 ISV BD2 3 3 BD2 BD2 8 9.1 - 9.8 32 21 BD1 21 BD1 BD1 BD1 BD2 BD2 BD2 3 7 25 25 —— 10.7-11.3 — — — — 7 17 17 BD2 BD2 22 44 — — — 12.2-12.8 — — — — — — Liner 11 Liner BD2 Below Liner BD2 — — - — BD1 Below analytical detection limit (<5 /ig I"1). BD2 Below analytical detection limit (<8 /Ag I"1). ISV Insufficient sample volume. No sample from the depth indicated. — — T a b le 17. C o p p e r levels (/tg I"1) a t K e n d a ll H e a p L e a c h P a d N o . I, N o v e m b e r I, 1991 Depth Interval (meters) 1 2 3 4 5 6 7 Borehole 8 9 10 11 12 13 14 15 0 - 0.2 94 138 568 645 131 88 43 62 119 81 62 175 64 62 62 0.2 - 0.5 116 61 383 1665 179 61 43 68 93 56 70 111 88 118 86 0.6 - 1.2 173 ISV 155 122 127 87 HO 228 62 HO 76 70 169 137 106 1.5 - 2.1 109 355 161 172 174 100 77 HO 62 157 100 226 361 193 275 3.0 - 3.7 173 77 161 238 355 77 77 56 126 138 ISV 706 86 75 81 4.6 - 5.2 111 230 750 54 122 72 66 31 31 63 58 41 86 75 62 6.1 - 6.7 145 90 100 ISV 83 109 55 25 ISV 181 29 35 118 112 HO 7.6 - 8.2 105 66 45 72 72 ISV 72 31 ISV 43 142 150 50 BD1 BD1 9.1 - 9.8 56 72 55 81 88 89 61 25 43 31 71 35 56 50 10.7-11.3 — — — — 54 45 45 37 63 78 142 — — — 12.2-12.8 — — — — — — Liner 94 Liner 56 Below Liner BD2 — — — — BD1 Below analytical detection limit (<20 /ng I"1). BD2 Below analytical detection limit (<31 /ig I'1). ISV Insufficient sample volume. No sample from the depth indicated. — —— — T a b le 18. Iro n levels (jug I"1) a t K e n d a ll H e a p L e a c h P a d N o. I, N o v e m b e r I, 1991. Depth Interval (meters) I 2 3 0 -0 .2 114 274 0.2 - 0.5 142 0.6 - 1.2 4 5 6 7 309 330 335 171 182 183 471 345 283 246 121 ISV 705 316 493 1.5 - 2.1 298 429 302 119 3.0 - 3.7 2212 316 974 4.6 - 5.2 633 938 6.1 - 6.7 3237 7.6 - 8.2 9.1 - 9.8 9 10 11 12 13 14 15 272 272 545 236 395 246 134 259 163 145 281 481 208 111 119 857 475 263 187 402 454 559 111 104 246 726 144 216 410 203 571 218 280 119 365 627 211 221 225 140 104 633 245 329 136 ISV 111 619 736 537 274 558 133 380 31 BD3 145 475 82 134 1512 1543 1018 2358 233 ISV BD1 220 BD2 BD3 ISV 170 74 141 619 1236 893 107 522 298 584 38 ISV 103 54 ISV 136 195 158 1189 57 BD3 50 415 105 64 105 417 46 363 381 327 256 417 BD3 BD3 — — 207 194 51 436 73 85 97 Liner 31 Liner BD3 Below Liner 72 10.7-11.3 — — — — 12.2-12.8 — — — — BD1 BD2 BD3 ISV Borehole 8 - - — Below analytical detection limit (<28 f i g I"1). Below analytical detection limit (<30 f i g I"1). Below analytical detection limit (<40 /ug I"1). Insufficient sample volume. No sample from the depth indicated. — — — — — ■■ — — — — T a b le 19. N ick el levels (/Ltg I'1) a t K e n d a ll H e a p L e a c h P a d N o. I, N o v e m b e r I, 1991. Depth Interval (meters) I 2 3 4 5 6 7 0 -0 .2 BD5 118 913 1787 BD4 BD4 BD1 0.2 - 0.5 175 42 203 1044 139 BD4 0.6 - 1.2 74 ISV BD2 194 162 1.5 - 2.1 108 262 169 127 3.0 - 3.7 83 BD2 1897 4.6 - 5.2 BD5 BD5 6.1 - 6.7 81 7.6 - 8.2 9.1 - 9.8 9 10 11 12 13 14 15 34 465 BD3 BD3 74 BD6 141 326 BD1 BD3 81 58 BD6 BD6 140 187 212 151 BD4 75 69 75 101 55 166 206 217 81 BD4 BD4 62 93 100 129 175 263 195 217 161 152 BD4 BD4 BD3 62 69 ISV 368 75 BD1 43 1248 BD4 33 BD4 BD4 BD3 46 75 BD6 BD6 137 BD1 43 121 54 ISV BD2 782 49 34 ISV 37 BD6 BD6 62 326 162 46 BD5 BD4 BD4 BD4 ISV 67 34 ISV 58 25 50 BD1 BD1 BD1 BD5 BD4 BD2 BD4 BD2 BD4 49 46 BD3 BD3 50 BD6 BD1 BD1 —— BD4 BD4 BD4 BD3 BD3 100 162 Liner 40 Liner 58 Below Liner 58 10.7-11.3 — — — — 12.2-12.8 — — -- — BD1 BD2 BD3 ISV Borehole 8 — -- Below analytical detection limit (<32 /ng I 1). Below analytical detection limit (<33 /Hg I"1). Below analytical detection limit (<34 /tg I"1). Insufficient sample volume. —— —— — — — ■■ BD4 Below analytical detection limit (<35 /Hg I"1). BD5 Below analytical detection limit (<36 /Hg I"1). BD6 Below analytical detection limit (<40 /Hg I"1). No sample from the depth indicated. —— T a b le 20. Z in c levels (/Ag I"1) a t K e n d a ll H e a p L e a c h P a d N o. I, N o v e m b e r I, 1991. Depth Interval (meters) I 2 3 4 5 6 7 0 -0 .2 173 63 70 148 162 222 0.2 - 0.5 75 61 46 145 156 0.6 - 1.2 121 ISV 304 90 1.5 - 2.1 120 100 70 3.0 - 3.7 56 88 4.6 - 5.2 39 6.1 - 6.7 Borehole 8 9 10 11 12 13 14 15 103 156 55 89 73 81 60 88 46 no 96 375 81 73 104 243 118 190 116 153 137 248 248 77 186 155 169 123 76 84 131 339 180 183 159 34 0 148 164 1099 98 40 165 178 262 243 67 65 188 160 ISV 89 405 48 61 71 163 79 281 241 287 284 83 155 456 164 304 46 30 74 51 307 ISV 417 176 181 112 ISV 1000 131 183 230 25 218 7.6 - 8.2 426 160 127 169 451 ISV 378 126 ISV 89 371 97 69 92 135 9.1 - 9.8 256 125 171 111 272 322 347 55 79 79 274 34 159 108 10.7-11.3 — — — — 123 245 202 91 133 0 284 — — — 12.2-12.8 — — — — Liner 232 Liner 149 Below Liner 81 — —— —— —— TO ISV — Insufficient sample volume. No sample from the depth indicated. -- — —— 63 APPENDIX B Data Validation Tables 64 T a b le 21. B lin d field re p lic a te cy an id e an aly sis re su lts. Relative Percent DifTerence (%) BD1 BD2 NA Sample Number Total Cyanide WAD Cyanide Free Cyanide 115/116 BD1 BD2 NA 143/144 66.7 BD1 NA 153/155 BD2 BD2 NA 178/179 18.2 BD1 NA 197/198 BD2 BD2 NA 224/225 37.8 BD1 NA 240/241 0.0 BD2 NA 259/260 16.2 0.0 NA 272/273 6.9 52.6 NA 299/300 BD1 BD2 NA 325/326 BD2 BD2 NA 340/342 9.5 0.0 NA 356/358 22.8 6.5 NA 382/383 0.0 13.3 NA 407/408 BD2 BD2 NA 425/427 5.5 23.3 BD1 Both sample analyses were below analytical detection limit. One sample analyses was below analytical detection limit, precluding RPD calculation. Not analyzed. 65 T a b le 22. L a b o ra to ry re p lic a te c y an id e an aly sis resu lts. Relative Percent Difference (%) Sample Number BD NA Total Cyanide WAD Cyanide Free Cyanide 102 25.0 BD NA 122 66.7 0.0 NA 143 8.7 BD NA 163 17.7 BD NA 185 0.0 BD NA 206 18.8 18.2 NA 228 14.6 0.0 NA 248 25.6 40.0 NA 271 4.9 0.0 NA 292 120.0 BD NA 312 10.5 BD NA 333 11.8 BD NA 354 3.5 20.0 BD 376 0.0 0.0 BD 395 42.9 8.3 NA 417 0.0 BD NA Below analytical detection limit. Not analyzed. 66 T a b le 23. Sample Number NA L a b o ra to ry s p ik e d cy an id e an aly sis re su lts. Total Cyanide % Recovery WAD Cyanide Free Cyanide 104 86' NA NA 124 101" NA NA 144 91" 63', 105" NA 166 69', 93" NA NA 187 98" 106" NA 208 75' 62', 95" 105 231 100" 52', 92" NA 251 98' NA NA 272 92' 61', 93" NA 293 73', 102" NA NA 315 73', 97" 39', 92" NA 335 81' 51', 95" NA 356 75' 76' 82" 377 101" 67, 85" 95" 398 108' 98" NA 418 103" 100" NA The soil was spiked with cyanide before distillation. The soil distillate was spiked with cyanide before colorimetric analysis. Not analyzed. 67 Table 24. Blind field replicate pH and metals analysis results. Relative Percent Difference (% ) Sample Number pH Cadmium Copper Iron Nickel Zinc 111/112 9.4 BD 83.0 BD BD 21.4 183/184 1.3 19.4 12.8 11.9 15.0 12.1 204/205 1.3 15.4 0.0 40.0 5.6 72.6 226/227 0.0 15.4 4.8 6.1 6.3 40.9 238/239 0.0 27.3 49.7 BD BD 20.3 257/258 0.0 BD BD 17.1 BD 8.3 286/287 2.7 BD 22.2 67.1 BD 17.0 330/331 1.5 BD 36.3 36.7 BD 68.0 338/339 6.7 BD 6.5 13.0 10.4 47.4 357/359 3.7 BD 5.2 12.0 BD 38.7 412/413 6.5 BD 0.0 16.8 BD 23.8 432/433 3.9 18.2 37.3 6.8 BD 0.7 Table 25. Laboratory replicate pH and metals analysis results. Relative Percent Difference (% ) Sample Number PH Cadmium Copper Iron Nickel Zinc 175 2.3 BD 9.2 7.3 BD 12.8 200 1.5 0.0 9.3 0.0 46.7 14.9 244 2.5 25.0 9.8 11.7 12.5 23.5 283 1.4 15.8 26.3 5.3 BD 6.6 309 0.3 BD 6.6 14.4 BD 20.3 375 3.4 BD 7.3 4.4 1.1 25.0 436 0.1 6.5 85.1 0.0 BD 33.2 BD Below analytical detection limit. 68 T a b le 26. Sample Lot 101-175 ICV LCS CCVl CCV2 CCV3 177-227 ICV LCS CCVl CCV2 L a b o ra to ry p H a n d m e ta ls re c o v e ry re su lts. ---- 100.7 100.6 100.6 100.1 ———— 100.6 100.6 286-339 ICV LCS CCVl CCV2 99.7 — — 100.3 100.6 •••■ 99.7 100.7 341-405 ICV LCS CCVl CCV2 100.1 406-436 ICV LCS CCVl CCV2 100.0 100.0 100.4 SL1 100.0 SL1 SL2 ICV LCS CCVl CCV2 ICV LCS CCVl CCV2 106.0 100.0 96.0 93.0 93.0 93.0 100.0 100.0 100.0 106.0 100.0 100.7 96.2 90.8 95.3 98.4 100.4 106.5 106.5 103.9 101.0 101.7 101.0 108.5 102.2 97.5 102.0 96.5 97.5 100.0 98.8 97.1 98.1 100.0 100.0 100.9 99.3 101.0 99.5 103.3 104.7 100.3 100.0 103.0 103.0 97.0 98.5 94.0 94.0 100.0 93.0 98.9 98.9 100.8 104.1 101.6 103.6 95.6 97.9 102.4 99.5 101.0 98.0 100.0 97.5 94.0 97.0 94.0 94.0 98.0 97.4 96.3 96.3 98.5 100.0 98.5 97.0 100.0 99.0 99.0 101.6 101.0 99.3 100.3 101.0 100.0 100.0 97.5 96.0 97.6 96.0 95.4 92.3 100.0 98.1 97.8 96.3 102.9 101.7 103.6 109.3 99.0 98.4 101.6 100.7 97.5 92.0 96.5 97.5 100.0 99.1 100.0 99.1 100.7 100.3 98.4 97.7 99.3 99.0 107.4 100.0 100.0 98.6 100.5 97.5 109.0 104.0 104.0 100.0 95.2 95.2 95.2 90.6 94.0 96.4 102.3 100.0 101.1 97.7 104.5 96.5 97.0 98.0 93.0 91.0 106.5 103.0 96.5 93.0 102.3 100.0 96.7 93.7 101.2 97.4 96.1 97.4 100.0 100.0 100.0 95.6 98.5 93.0 93.0 97.0 100.0 100.0 SL2 N ickel Cadm ium 237-285 ICV LCS CCVl CCV2 ICV LCS CCVl CCV2 % Recovery C opper Iron pH ———— 99.7 100.4 ———— ———— 100.9 101.0 100.0 ———— 100.7 100.3 Zinc Samples 104, 106, 132, 146, 156, 158, 163, 166, 172, 192, 195, 197, 216, 231, 232, 234, 244, 267, and 277. Samples 297, 302, 320, 328, 335, 348, 352, 366, 372, 373, 385, 388, 392, 409, 414, 422, and 430. Initial calibration verification. Laboratory control standard. Continuing calibration verification following ICV and ten sample analyses. Continuing calibration verification following CCVl and ten sample analyses. 69 APPENDIX C Statistical Data 70 T a b le 27. A nalysis o f v a ria n c e fo r to ta l c y an id e (w e ig h te d , Iog10 tra n s fo rm e d d a ta ). D e p e n d e n t V a ria b le : T O T A L C Y A N ID E Source DF Sum of Squares Mean Square Model Error Corrected Total 18 55 73 1.998775 2.011509 4.010284 0.111043 0.036573 R-Square 0.498412 Source DEPTH BOREHOLE C.V. 401.2983 DF 4 14 Root MSE 0.1912 Type III SS 0.3648411 1.6150962 F Value Pr > F 3.04 0.0008 CYANIDE Mean 0.0477 Mean Square F Value Pr > F 0.0912103 0.1153640 2.49 3.15 0.0534 0.0012 71 T a b le 28. S tu d e n t-N e w m a n -K e u ls m e a n s s e p a ra tio n te sts fo r b o re h o le to ta l cy an id e m e a n s (w e ig h te d , Iog10 tra n s fo rm e d d a ta ). 1 Borehole N Transformed Mean SNK Grouping 4 5 0.5393 A1 11 5 0.4662 AB 3 5 0.3653 ABC 15 4 0.2103 BCD 12 5 0.1795 BCD 6 5 0.0966 CDE 5 5 0.0426 CDEF 9 5 0.0356 CDEF I 5 0.0213 CDEF 2 5 -0.0670 DEF 13 5 -0.0930 DEF 14 5 -0.1242 DEF 8 5 -0.2753 EF 7 5 -0.3149 F 10 5 -0.3181 F Means with the same letter are not significantly different. Alpha = 0.05 df = 55 MSE = 0.036573 Number of Means I 2 I 3 I 4 ! 5 I 6 - - - - - - - - - - - - - - - - - - - 1- - - - - - - - - - - - 1- - - - - - - - - - - - 1- - - - - - - - - - - H- - - - - - - - - h - - - - - - — Critical Range j 0.2444 | 0.2938 j 0.3231 j 0.3440 j 0.3601 Number of Means ! 7 ! 8 I 9 ! 10 I 11 - - - - - - - - - - - - - - - - - - - j- - - - - - - - - - - + - - - - - - - - - - - 1- - - - - - - — — + - - - - - - - - - - f—- - - - - - - Critical Range j 0.3731877 j 0.3842 j 0.3937 j 0.4021 | 0.4095 Number of Means I 12 I 13 ! 14 I 15 --------------------------- 1---------------- 4— ——--------H----------- ----- + -------------- 4 Critical Range I 0.4161 j 0.4222 j 0.4278 I 0.4329 72 T a b le 29. A nalysis o f v a ria n c e fo r W A D c y an id e (w e ig h te d , Iog10 tra n s fo rm e d d a ta ). D e p e n d e n t V a ria b le : W A D C Y A N ID E Source DF Sum of Squares Mean Square Model Error Corrected Total 18 42 60 0.9867030 0.7378051 1.7245081 0.0548168 0.0175668 R-Square 0.572165 C.V. -33.23712 Source DF DEPTH BOREHOLE Table 30. Pr > F 0.0012 3.12 Root MSE 0.1325 Type III SS 4 14 F Value CYANIDE Mean -0.3988 Mean Square F Value Pr > F 0.1338824 0.0359721 7.62 2.05 0.0001 0.0371 0.5355296 0.5036090 Student-Newman-Keuls means separation tests for depth WAD cyanide means (weighted, Iog10 transformed data). 1 Depth N Transformed Mean SNK Grouping 3 14 -0.1439 A1 4 11 -0.2101 AB 2 12 -0.3181 BC I 14 -0.3795 C 5 10 -0.6346 D Means with the same letter are not significantly different. Alpha = 0.05 df = 42 MSE = 0.017567 Number of Means ! 2 I 3 I Critical Range j 0.1093 j 0.1315 j - - - - - - - - - - - - - - - - - - + - - — - - - - - i-— 4 ! 5 0.1448 ] 0.1543 -------- — + -------- 73 T a b le 31. S tu d e n t-N e w m a n -K e u ls m e a n s s e p a ra tio n te sts fo r b o re h o le W A D c y an id e m e a n s (w e ig h te d , Iog10 tra n s fo rm e d d a ta ) . 1 B orehole N T ransform ed M ean S N K G rouping 3 5 -0.1069 A1 4 5 -0.1619 AB 15 4 -0.2286 ABC 2 4 -0.3253 ABCD 6 4 -0.3689 ABCD 10 2 -0.4007 BCD I 5 -0.4132 BCD 5 4 -0.4246 BCD 11 5 -0.4486 BCD 8 2 -0.4559 BCD 9 3 -0.4559 BCD 12 5 -0.5127 CD 13 5 -0.5336 CD 14 5 -0.5927 D 7 3 -0.6261 D Means with the same letter are not significantly different. Alpha = 0.05 I l I 2 I ---------------------------------- + --------------- --— + — Critical Range j 0.1970 j Number of Means df = 42 MSE = 0.017567 l 3 l ! l 4 ! ------------ +---------- + — 0.2371 j 0.2611 j 5 I --- + — 0.2781 | 6 ----- 0.2914 Number of Means S 7 I 8 I 9 I 10 I 11 ----------------------------j------------- — I----------------- H------------------1---------------- 1------------Critical Range | 0.3021 | 0.3112 | 0.3190 j 0.3259 j 0.3320 Number of Means ! 12 I 13 ! 14 I 15 --------—- ——-------- 1-------------------1------------------1-------- — — I------- --------- 1 Critical Range j 0.3375 I 0.3425 I 0.3471 j 0.3513 74 T a b le 32. A nalysis o f v a ria n c e fo r p H (w e ig h te d , Iog10 tra n s fo rm e d d a ta ). D e p e n d e n t V a ria b le : P H Source DF Sum of Squares Mean Square Model Error Corrected Total 18 54 72 0.0087106 0.0062246 0.0149351 0.0004839 0.0001153 R-Square 0.583226 Source DEPTH BOREHOLE C.V. 1.204340 DF 4 14 Root MSE 0.0107 Type III SS 0.0006444 0.0080161 F Value Pr > F 4.20 0.0001 PH Mean 0.8915 Mean Square F Value Pr > F 0.0001611 0.0005726 1.40 4.97 0.2472 0.0001 75 T a b le 33. S tu d e n t-N e w m a n -K e u ls m e a n s s e p a ra tio n te sts fo r b o re h o le p H m e a n s (w e ig h te d , Iog10 tra n s fo rm e d d a ta ). 1 Borehole N Transformed Mean SNK Grouping 3 5 0.9185 A1 2 5 0.9149 AB 15 4 0.9132 AB I 5 0.9118 ABC 9 4 0.8987 BCD 4 5 0.8969 BCD 14 5 0.8958 BCD 12 5 0.8926 CDE 13 5 0.8875 DE 5 5 0.8860 DE 10 5 0.8848 DE 7 5 0.8811 DE 8 5 0.8783 DE 11 5 0.8728 E 6 5 0.8463 F Means with the same letter are not significantly different. Alpha = 0.05 I df = 54 i MSE = 0.000115 i i i Number of Means ! 2 ! 3 I 4 I 5 ! 6 -------------------------- -I— ----- —— + ------------- — I— —---------- + — ----------+ ------------Critical Range j 0.0138 j 0.0166 j 0.0183 j 0.0195 j 0.0204 Number of Means I 7 ! 8 ! 9 I 10 I 11 —--- ——--- ——4------------ 1------------H------------+-------—+-------— Critical Range g 0.0211 j 0.0218 g 0.0223 j 0.0228 g 0.0232 Number of Means ------ Critical Range ! 12 ! 13 I 14 I 15 1-------------1----- ----- 4---------- —I--------- -H j 0.0236 j 0.0239 j 0.0242 I 0.0245 76 T a b le 34. A n aly sis o f v a ria n c e fo r c a d m iu m (w e ig h te d , Iog10 tra n s fo rm e d d a ta ). D e p e n d e n t V a ria b le : C A D M IU M Source DF Sum of Squares Mean Square Model Error Corrected Total 18 54 72 0.8635915 0.9072893 1.7708807 0.0479773 0.0168017 R-Square 0.487662 Source DEPTH BOREHOLE C.V. 13.95810 DF 4 14 Root MSE 0.1296 Type III SS 0.0571687 0.8041337 F Value Pr > F 2.86 0.0015 CADMIUM Mean 0.9286 Mean Square F Value Pr > F 0.0142922 0.0574381 0.85 3.42 0.4995 0.0006 77 T a b le 35. S tu d e n t-N e w m a n -K e u ls m e a n s s e p a ra tio n te sts fo r b o re h o le c a d m iu m m e a n s (w e ig h te d , Iog10 tra n s fo rm e d d a ta ). 1 Borehole N Transformed Mean SNK Grouping I 5 1.2704 A1 13 5 1.2071 AB 15 4 1.1708 AB 14 5 1.1068 ABC 2 5 1.0751 ABCD 12 5 0.9812 BCDE 4 5 0.8928 CDEF 10 5 0.8765 CDEF 8 5 0.8499 DEF 11 5 0.8403 DEF 7 5 0.7962 EF 9 4 0.7925 EF 5 5 0.7395 EF 3 5 0.7095 F 6 5 0.6826 F Means with the same letter are not significantly different. Alpha = 0.05 df = 54 MSE = 0.016802 I Number of Means ! 2 ! 3 ■ 4 I 5 I 6 0.2462 Critical Range I I I 0.1671 I I I 0.2008 I I I 0.2209 I I I 0.2352 I I I Number of Means I I 7 I I 8 I I 9 I I 10 I I 11 Critical Range I I I 0.2552 I I I 0.2627 I I I 0.2692 I I I 0.2749 I I I 0.2800 Number of Means I I 12 I I 13 I I 14 I I 15 0.2887 I I I 0.2926 I I I 0.2961 Critical Range I I I 0.2846 I I I 78 T a b le 36. A nalysis o f v a ria n c e fo r c o p p e r (w e ig h te d , Iog10 tra n s fo rm e d d a ta ). D e p e n d e n t V a ria b le : C O P P E R Source DF Sum of Squares Mean Square Model Error Corrected Total 18 54 72 1.4687654 0.9461275 2.4148928 0.0815981 0.0175209 C.V. 6.752258 R-Square 0.608211 Source DF Table 37. Type III SS 4 14 DEPTH BOREHOLE Root MSE 0.1324 F Value Pr > F 4.66 0.0001 COPPER Mean 1.9603 Mean Square F Value Pr > F 0.1514624 0.0628274 8.64 3.59 0.0001 0.0003 0.6058495 0.8795838 Student-Newman-Keuls means separation tests for depth copper means (weighted, Iog10 transformed data). 1 Depth N Transformed Mean SNK Grouping 2 15 2.1577 A1 I 15 2.0542 A 3 15 2.0534 A 4 14 1.8819 B 5 14 1.7356 C Means with the same letter are not significantly different. Alpha = 0.05 I df = 54 i i 2 i ----------------------- + — — -------1— Critical Range j 0.0983 j Number of Means MSE = 0.017521 i i 3 ! 0.1181 j 4 ! 5 0.1299 j 0.1383 — — —+ — ------ 79 T a b le 38. S tu d e n t-N e w m a n -K e u ls m e a n s s e p a ra tio n te s ts fo r b o re h o le c o p p e r m e a n s (w e ig h te d , Iog10 tra n s fo rm e d d a ta ). 1 Borehole N Transformed Mean SNK Grouping 4 5 2.4472 A1 3 5 2.2518 B 5 5 2.1134 BC 2 5 2.0051 CD 12 5 1.9783 CDE I 5 1.9780 CDE 15 4 1.9424 CDE 13 5 1.9237 CDE 6 5 1.9108 CDE 14 5 1.9059 CDE 11 5 1.8526 CDE 9 4 1.8443 CDE 10 5 1.7884 DE 7 5 1.7540 DE 8 5 1.6936 E Means with the same letter are not significantly different. Alpha = 0.05 • Number of Means I df = 54 i 2 ! MSE = 0.017521 I 3 I I 4 I 5 ! 6 ------------------- 1----- ——H-------- —+---- -----—+------ —+------— Critical Range j 0.1706 j 0.2051 j 0.2256 j 0.2402 j 0.2514 Number of Means ! 7 I 8 I 9 ! 10 I 11 --------- -------- 1-------- —4---------- -+—------—4-------- —4-------- Critical Range j 0.2606 I 0.2683 j 0.2749 | 0.2808 | 0.2859 Number of Means I 12 I 13 I 14 I 15 --------------- --- I---- ----—H--- --------H—--------- 1--- ----—H Critical Range j 0.2906 j 0.2949 j 0.2987 j 0.3023 80 T a b le 39. A n aly sis o f v a ria n c e fo r iro n (w e ig h te d , Iog10 tra n s fo rm e d d a ta ). D e p e n d e n t V a ria b le : IR O N Source DF Sum of Squares Mean Square Model Error Corrected Total 18 54 72 1.0212741 2.1967981 3.2180722 0.0567374 0.0406814 R-Square 0.317356 Source C.V. 8.548673 DF DEPTH BOREHOLE Table 40. Root MSE 0.2017 Type III SS 4 14 0.4564189 0.5284874 F Value Pr > F 1.39 0.1724 IRON Mean 2.3594 Mean Square F Value Pr > F 0.1141047 0.0377491 2.80 0.93 0.0345 0.5358 Analysis of variance for nickel (weighted, Iog10 transformed data). Dependent Variable: NICKEL Source DF Sum of Squares Model Error Corrected Total 18 54 72 2.4619855 2.4450649 4.9070504 C.V. 11.83867 R-Square 0.501724 Source DEPTH BOREHOLE DF 4 14 Mean Square 0.1367770 0.0452790 Root MSE 0.2128 Type III SS 1.2189305 1.1185310 F Value Pr > F 3.02 0.0009 NICKEL Mean 1.7974 Mean Square F Value Pr > F 0.3047326 0.0798951 6.73 1.76 0.0002 0.0693 81 T a b le 41. S tu d e n t-N e w m a n -K e u ls m e a n s s e p a ra tio n te sts fo r d e p th n ic k el m e a n s (w e ig h te d , Iog10 tra n s fo rm e d d a ta ) . 1 Depth N Transformed Mean SNK Grouping 2 15 2.0513 A1 I 15 1.9679 AB 4 14 1.8057 B 3 15 1.7830 B 5 14 1.4471 C Means with the same letter are not significantly different. Alpha = 0.05 df = 54 I I MSE = 0.045279 I I S 2 S 3 ! 4 S 5 —--- ----------- -4---- ------- 1------ -----4----------—f —----Critical Range j 0.1580 j 0.1899 j 0.2089 j 0.2224 Number of Means Table 42. Analysis of variance for zinc (weighted, Iog10 transformed data). Dependent Variable: ZINC Source DF Sum of Squares Model Error Corrected Total 18 54 72 0.5369455 0.9080027 1.4449482 R-Square 0.371602 Source DEPTH BOREHOLE C.V. 6.160244 DF 4 14 Mean Square 0.0298303 0.0168149 Root MSE 0.1297 Type III SS 0.0604987 0.4642185 F Value Pr > F 1.77 0.0540 ZINC Mean 2.1050 Mean Square F Value Pr > F 0.0151247 0.0331585 0.90 1.97 0.4708 0.0383 82 I T a b le 43. L in e a r re g re ss io n o f p o r e w a te r to ta l cy an id e d a ta fro m b o re h o le 4, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) I 2 3 4 5 6 7 8 9 10 11 3/19/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 9/06/92 0 37 49 62 77 92 HO 125 141 155 169 Total Cyanide Level (mg I'1) 0.48 0.69 0.76 0.96 0.94 0.99 0.97 1.08 1.14 0.64 1.99 Number of points = 11 P aram eter Expected V alue Standard Error Lower 95% C l Upper 95% C l Slope 0.00497 0.00184 0.00081 0.00913 Y intercept 0.50790 0.19400 0.06915 0.94660 X intercept -102.22 Correlation coefficient (r) = 0.6690. r squared = 0.4475. Standard deviation of residuals from line (Sy.x) = 0.3089. Test: Is the slope significantly different from zero? F = 7.290 The p value is 0.0244, considered significant. This result was obtained from the following ANOVA table: S ou rce o f variation D egrees o f freedom Sum o f squares M ean square Linear regression (Model) I 0.6955 0.6955 Deviations from linearity (Residual) 9 0.8587 0.0954 Total 10 1.5542 Runs test: Do the number of runs indicate a linear model? There are 5 points above the line, 6 below, and 4 runs. The p value is 0.1104, considered not significant. There is not a significant departure from linearity. 83 T a b le 44. L in e a r re g re s s io n o f p o r e w a te r to ta l c y an id e d a ta fro m b o re h o le 8, K e n d a ll H e a p L e a c h P a d N o . I . Observation Number Sampling Date Time (Days) 1 3/19/92 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 9/06/92 0 22 37 49 62 77 92 no 125 141 155 169 2 3 4 5 6 7 8 9 10 11 12 Total Cyanide Level (mg I"1) 0.69 0.88 0.41 0.33 0.36 0.40 0.44 0.55 0.55 0.52 0.35 0.41 Number of points = 12 Parameter Expected V alue Standard E rror Lower 95% C l Upper 95% C l Slope -0.00121 0.00085 -0.00311 0.00069 Y intercept 0.59550 0.08620 0.40350 0.78760 X intercept 492.53 Correlation coefficient (r) = -0.4092. r squared = 0.1675. Standard deviation of residuals from line (Sy.x) = 0.1542. Test: Is the slope significantly different from zero? F = 2.011 The p value is 0.1865, considered not significant. This result was obtained from the following ANOVA table: S ou rce o f variation D egrees o f freedom Sum o f squares M ean square Linear regression (Model) I 0.0478 0.0478 Deviations from linearity (Residual) 10 0.2379 0.0238 Total 11 0.2857 Runs test: Do the number of runs indicate a linear model? There are 6 points above the line, 6 below, and 5 runs. The p value is 0.1753, considered not significant. There is not a significant departure from linearity. 84 T a b le 45. L in e a r re g re s s io n o f p o r e w a te r W A D cy an id e d a ta fro m b o re h o le 4, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) I 2 3 4 5 6 7 8 9 10 11 3/19/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 9/06/92 0 37 49 62 77 92 no 125 141 155 169 WAD Cyanide Level (mg I"1) 0.07 0.12 0.15 0.15 0.17 0.14 0.15 0.19 0.31 0.15 0.19 Number of points = 11 Parameter Expected Value Standard Error Lower 95% Cl Upper 95% Cl Slope 0.00074 0.00028 0.00012 0.00137 Y intercept 0.09418 0.02910 0.02835 0.16000 X intercept -127.04 Correlation coefficient (r) = 0.6669. r squared = 0.4447. Standard deviation of residuals from line (Sy.x) = 0.0464. Test: Is the slope significantly different from zero? F = 7.208 The p value is 0.0250, considered significant. This result was obtained from the following ANOVA table: Source of variation Degrees of freedom Sum of squares Mean square Linear regression (Model) I 0.0155 0.0155 Deviations from linearity (Residual) 9 0.0193 0.0021 Total 10 0.0348 Runs test: Do the number of runs indicate a linear model? There are 5 points above the line, 6 below, and 5 runs. The p value is 0.2619, considered not significant. There is not a significant departure from linearity. 85 T a b le 46. L in e a r re g re ss io n o f p o r e w a te r W A D c y an id e d a ta fro m b o re h o le 8, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) I 2 3 4 5 6 7 8 9 10 3/19/92 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/23/92 0 22 37 49 62 77 92 HO 125 155 WAD Cyanide Level (mg I"1) 0.05 0.06 0.06 0.04 0.05 0.05 0.05 0.06 0.07 0.03 Number of points = 10 Parameter Expected Value Standard Error Lower 95% Cl Upper 95% Cl Slope -4.067 E-05 8.165 E-05 -0.00023 0.00015 Y intercept 0.05496 0.00704 0.03874 0.07119 X intercept 1351.50 Correlation coefficient (r) = -0.1734. r squared = 0.0301. Standard deviation of residuals from line (Sy.x) = 0.0119. Test: Is the slope significantly different from zero? F = 0.2481 The p value is 0.6318, considered not significant. This result was obtained from the following ANOVA table: Source of variation Degrees of freedom Sum of squares Mean square Linear regression (Model) I 3.489 E-05 3.489 E-05 Deviations from linearity (Residual) 8 0.0011 0.0001 Total 9 0.0012 Runs test: Do the number of runs indicate a linear model? There are 4 points above the line, 6 below, and 5 runs. The p value is 0.4048, considered not significant. There is not a significant departure from linearity. 86 T a b le 47. L in e a r re g re s s io n o f p o r e w a te r p H d a ta fro m b o re h o le 4, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) pH I 2 3 4 5 6 7 8 9 10 11 12 3/19/92 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 9/06/92 0 22 37 49 62 77 92 HO 125 141 155 169 6.8 6.7 6.5 7.4 7.6 7.7 7.1 6.8 7.8 6.4 7.8 7.6 Number of points = 12 Parameter Expected V alue Standard Error Lower 95% C l Upper 95% C l Slope 0.00376 0.00280 -0.00249 0.01001 Y intercept 6.85800 0.28360 6.22600 7.48900 X intercept -1822.70 Correlation coefficient (r) = 0.3906. r squared = 0.1525. Standard deviation of residuals from line (Sy.x) = 0.5073. Test: Is the slope significantly different from zero? F = 1.800 The p value is 0.2094, considered not significant. This result was obtained from the following ANOVA table: S ou rce o f variation D egrees o f freedom Sum o f squares M ean square Linear regression (Model) I 0.4632 0.4632 Deviations from linearity (Residual) 10 2.5730 0.2573 Total 11 3.0362 Runs test: Do the number of runs indicate a linear model? There are 6 points above the line, 6 below, and 6 runs. The p value is 0.3918, considered not significant. There is not a significant departure from linearity. 87 T a b le 48. L in e a r re g re s s io n o f p o r e w a te r p H d a ta fro m b o re h o le 8, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) pH I 2 3 4 5 6 7 8 9 10 11 12 3/19/92 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 9/06/92 0 22 37 49 62 77 92 HO 125 141 155 169 6.7 6.7 6.6 7.3 7.4 7.4 7.2 7.2 7.4 6.4 8.0 7.5 Number of points = 12 Parameter Expected V alue Standard E rror Lower 95% C l Upper 95% C l Slope 0.00423 0.00231 -0.00092 0.00937 Y intercept 6.78400 0.23350 6.26400 7.30400 X intercept -1604.60 Correlation coefficient (r) = 0.5010. r squared = 0.2510. Standard deviation of residuals from line (Sy.x) = 0.4177. Test: Is the slope significantly different from zero? F = 3.352 The p value is 0.0970, considered not quite significant. This result was obtained from the following ANOVA table: S ource o f variation D egrees o f freedom Sum o f sq u ares M ean square Linear regression (Model) I 0.5849 0.5849 Deviations from linearity (Residual) 10 1.7450 0.1745 Total 11 2.3299 Runs test: Do the number of runs indicate a linear model? There are 7 points above the line, 5 below, and 6 runs. The p value is 0.4242, considered not significant. There is not a significant departure from linearity. 88 T a b le 49. L in e a r re g re s s io n o f p o r e w a te r c a d m iu m d a ta fro m b o re h o le 4, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) I 2 3 4 5 6 7 8 9 10 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 22 37 49 62 77 92 no 125 141 155 Cadmium Level ( j i g I"1) 33 26 26 20 26 33 26 33 26 26 Number of points = 10 Parameter Expected V alue Standard Error Lower 95% C l Upper 95% C l Slope -0.00022 0.03299 -0.07630 0.07586 Y intercept 27.51900 3.20100 20.13800 34.9000 X intercept 126807.00 Correlation coefficient (r) = -0.0023. r squared = 5.408 E-06. Standard deviation of residuals from line (Sy.x) = 4.4790. Test: Is the slope significantly different from zero? F = 4.327 E-05 The p value is 0.9949, considered not significant. This result was obtained from the following ANOVA table: S ou rce o f variation D egrees o f freedom Sum o f sq u ares M ean square Linear regression (Model) I 0.0009 0.0009 Deviations from linearity (Residual) 8 160.5000 20.062 Total 9 160.5009 Runs test: Do the number of runs indicate a linear model? There are 3 points above the line, 7 below, and 6 runs. The p value is 0.8333, considered not significant. There is not a significant departure from linearity. 89 T a b le 50. L in e a r re g re s s io n o f p o r e w a te r c a d m iu m d a ta fro m b o re h o le 8, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) I 2 3 4 5 6 7 8 9 10 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 22 37 49 62 77 92 no 125 141 155 Cadmium Level Og I"1) 66 26 20 26 26 26 33 33 33 26 Number of points = 10 Parameter Expected Value Standard Error Lower 95% Cl Upper 95% Cl Slope -0.08502 0.09559 -0.30540 0.13540 Y intercept 38.89600 9.27300 17.51200 60.28100 X intercept 457.52 Correlation coefficient (r) = -0.3000. r squared = 0.0900. Standard deviation of residuals from line (Sy.x) = 12.9770. Test: Is the slope significantly different from zero? F = 0.7910 The p value is 0.3997, considered not significant. This result was obtained from the following ANOVA table: Source of variation Degrees of freedom Sum of squares Mean square Linear regression (Model) I 133.2200 133.2200 Deviations from linearity (Residual) 8 1347.3000 168.4100 Total 9 1480.5200 Runs test: Do the number of runs indicate a linear model? There are 5 points above the line, 5 below, and 3 runs. The p value is 0.0397, considered significant. There are significantly fewer runs than expected, suggesting that the data follow a curve rather than a line. 90 T a b le 51. L in e a r re g re s s io n o f p o re w a te r c o p p e r d a ta fro m b o re h o le 4, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) I 2 3 4 5 6 7 8 9 10 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 22 37 49 62 77 92 no 125 141 155 Copper Level ( f i g I'1) 48 19 38 87 0 0 29 0 0 0 Number of points = 10 P aram eter Expected V alue Standard Error Lower 95% C l Upper 95% C l Slope -0.38100 0.18340 -0.80390 0.04195 Y intercept 55.24400 17.79300 14.21400 96.27400 X intercept 145.01 Correlation coefficient (r) = -0.5919. r squared = 0.3504. Standard deviation of residuals from line (Sy.x) = 24.8990. Test: Is the slope significantly different from zero? F = 4.315 The p value is 0.0714, considered not quite significant. This result was obtained from the following ANOVA table: S ource o f variation D egrees o f freedom Sum o f sq u ares M ean square Linear regression (Model) I 2675.20 2675.20 Deviations from linearity (Residual) 8 4959.70 619.97 Total 9 7634.90 Runs test: Do the number of runs indicate a linear model? There are 5 points above the line, 5 below, and 7 runs. The p value is 0.8333, considered not significant. There is not a significant departure from linearity. 91 T a b le 52. L in e a r re g re s s io n o f p o r e w a te r c o p p e r d a ta fro m b o re h o le 8, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) I 2 3 4 5 6 7 8 9 10 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 22 37 49 62 77 92 HO 125 141 155 Copper Level (/ig I"1) 67 29 19 9 38 29 19 19 0 9 Number of points = 10 Parameter Expected V alue Standard E rror Lower 95% C l Upper 95% C l Slope -0.28300 0.10800 -0.53200 -0.03397 Y intercept 48.4200 10.47700 24.26100 72.57900 X intercept 171.10 Correlation coefficient (r) = -0.6796. r squared = 0.4619. Standard deviation of residuals from line (Sy.x) = 14.6610. Test: Is the slope significantly different from zero? F = 6.867 The p value is 0.0306, considered significant. This result was obtained from the following ANOVA table: Source of variation Degrees of freedom Sum of squares Mean square Linear regression (Model) I 1476.10 1476.10 Deviations from linearity (Residual) 8 1719.50 214.94 Total 9 3195.60 Runs test: Do the number of runs indicate a linear model? There are 6 points above the line, 4 below, and 5 runs. The p value is 0.4048, considered not significant. There is not a significant departure from linearity. 92 T a b le 53. L in e a r re g re s s io n o f p o re w a te r iro n d a ta fro m b o re h o le 4, K e n d a ll H e a p L e a c h P a d N o. I. Observation Number Sampling Date Time (Days) Iron Level (/Ag I 1) I 2 3 4 5 6 7 8 9 4/10/92 4/25/92 5/07/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 22 37 49 77 92 HO 125 141 155 649 375 1134 537 862 500 458 575 500 Number of points = 9 Parameter Expected V alue Standard Error Lower 95% C l Upper 95% C l Slope -1.5030 1.8120 -5.7880 2.7810 Y intercept 756.0500 181.4400 326.9400 1185.2000 X intercept 503.01 Correlation coefficient (r) = -0.2992. r squared = 0.0895. Standard deviation of residuals from line (Sy.x) = 241.28. Test: Is the slope significantly different from zero? F = 0.6884 The p value is 0.4341, considered not significant. This result was obtained from the following ANOVA table: S ou rce o f variation D egrees o f freedom Sum o f sq u ares M ean square Linear regression (Model) I 40073.0 40073.0 Deviations from linearity (Residual) 7 407500.0 58214.0 Total 8 447573.0 Runs test: Do the number of runs indicate a linear model? There are 3 points above the line, 6 below, and 7 runs. The p value is 1.0000, considered not significant. There is not a significant departure from linearity. 93 T a b le 54. L in e a r re g re ss io n o f p o r e w a te r iro n d a ta fro m b o re h o le 8, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) Iron Level Og I"1) I 2 3 4 5 6 7 8 9 10 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 22 37 49 62 77 92 HO 125 141 155 757 416 416 208 458 291 125 208 125 612 Parameter Expected Value Standard Error Lower 95% Cl Upper 95% Cl Slope -1.8980 1.5000 -5.3580 1.5610 Y intercept 526.7700 145.5600 191.1000 862.4400 X intercept 277.47 Number of points = 10 Correlation coefficient (r) = -0.4084. r squared = 0.1668. Standard deviation of residuals from line (Sy.x) = 203.70. Test: Is the slope significantly different from zero? F = 1.601 The p value is 0.2414, considered not significant. This result was obtained from the following ANOVA table: Source of variation Degrees of freedom Sum of squares Mean square Linear regression (Model) I 66434.0 66434.0 Deviations from linearity (Residual) 8 331948.0 41494.0 Total 9 398382.0 Runs test: Do the number of runs indicate a linear model? There are 3 points above the line, 7 below, and 5 runs. The p value is 0.5833, considered not significant. There is not a significant departure from linearity. 94 T a b le 55. L in e a r re g re s s io n o f p o r e w a te r n ic k el d a ta fro m b o re h o le 4, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) Nickel Level (jug I"1) I 2 3 4 5 6 7 8 9 10 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 22 37 49 62 77 92 no 125 141 155 152 108 86 86 65 108 65 65 65 43 Number of points = 10 P aram eter Expected V alue Standard Error Lower 95% C l Upper 95% C l Slope -0.5647 0.1433 -0.8953 -0.2342 Y intercept 133.4300 13.9070 101.3600 165.5000 X intercept 236.28 Correlation coefficient (r) = -0.8123. r squared = 0.6599. Standard deviation of residuals from line (Sy.x) = 19.46. Test: Is the slope significantly different from zero? F = 15.521 The p value is 0.0043, considered very significant. This result was obtained from the following ANOVA table: S ou rce o f variation D egrees o f freedom Sum o f squares M ean square Linear regression (Model) I 5878.20 5878.20 Deviations from linearity (Residual) 8 3029.90 378.74 Total 9 8908.10 Runs test: Do the number of runs indicate a linear model? There are 4 points above the line, 6 below, and 6 runs. The p value is 0.6905, considered not significant. There is not a significant departure from linearity. 95 T a b le 56. L in e a r re g re s s io n o f p o r e w a te r n ic k el d a ta fro m b o re h o le 8, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) Nickel Level Og I"1) I 2 3 4 5 6 7 8 9 10 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 8/09/92 8/23/92 22 37 49 62 77 92 no 125 141 155 130 43 86 65 108 43 86 65 86 65 Number of points = 10 Parameter Expected V alue Standard Error Lower 95% C l Upper 95% C l Slope -0.1544 0.2069 -0.6315 0.3277 Y intercept 91.1330 20.0730 44.8440 137.4200 X intercept 590.22 Correlation coefficient (r) = -0.2551. r squared = 0.0651. Standard deviation of residuals from line (Sy.x) = 28.09. Test: Is the slope significantly different from zero? F = 0.5569 The p value is 0.4769, considered not significant. This result was obtained from the following ANOVA table: S ou rce o f variation D egrees o f freedom Sum o f sq u ares M ean square Linear regression (Model) I 439.44 439.44 Deviations from linearity (Residual) 8 6312.70 789.08 Total 9 6752.14 Runs test: Do the number of runs indicate a linear model? There are 5 points above the line, 5 below, and 10 runs. The p value is 1.0000, considered not significant. There is not a significant departure from linearity. 96 T a b le 57. L in e a r re g re s s io n o f p o r e w a te r zin c d a ta fro m b o re h o le 4, K e n d a ll H e a p L e a c h P a d N o. I. Observation Number Sampling Date Time (Days) Zinc Level (/xg I 1) I 2 3 4 5 6 7 8 4/10/92 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 7/24/92 22 37 49 62 77 92 HO 125 442 97 299 75 32 245 372 140 Number of points = 8 Parameter Expected V alue Slope -0.6733 Y intercept 261.0600 X intercept 387.75 Lower 95% C l Upper 95% C l 1.6780 -4.7800 3.4340 132.9700 -64.3200 586.4300 Standard Error Correlation coefficient (r) = -0.1616. r squared = 0.0261. Standard deviation of residuals from line (Sy.x) = 159.50. Test: Is the slope significantly different from zero? F = 0.1609 The p value is 0.7022, considered not significant. This result was obtained from the following ANOVA table: S ou rce o f variation D egrees o f freedom Sum o f sq u ares M ean square Linear regression (Model) I 4093.70 4093.70 Deviations from linearity (Residual) 6 152638.00 25440.0 Total 7 156731.70 Runs test: Do the number of runs indicate a linear model? There are 4 points above the line, 4 below, and 6 runs. The p value is 0.8857, considered not significant. There is not a significant departure from linearity. 97 T a b le 58. L in e a r re g re s s io n o f p o r e w a te r zinc d a ta fro m b o re h o le 8, K e n d a ll H e a p L e a c h P a d N o . I. Observation Number Sampling Date Time (Days) Zinc Level (fig I"1) I 2 3 4 5 6 7 8 4/25/92 5/07/92 5/20/92 6/06/92 6/21/92 7/09/92 8/09/92 8/23/92 37 49 62 77 92 no 141 155 420 194 215 311 406 500 240 256 Number of points = 8 Standard Error Lower 95% C l Upper 95% C l P aram eter Expected V alue Slope -0.1214 1.0700 -2.7400 2.4970 Y intercept 328.7200 105.6600 70.1660 587.2800 X intercept 2708.00 Correlation coefficient (r) = -0.0463. r squared = 0.0021. Standard deviation of residuals from line (Sy.x) = 120.45. Test: Is the slope significantly different from zero? F = 0.0129 The p value is 0.9134, considered not significant. This result was obtained from the following ANOVA table: S ource o f variation D egrees o f freedom Sum o f squares M ean square Linear regression (Model) I 186.73 186.73 Deviations from linearity (Residual) 6 87047.00 14508.00 Total 7 87233.73 Runs test: Do the number of runs indicate a linear model? There are 3 points above the line, 5 below, and 4 runs. The p value is 0.4286, considered not significant. There is not a significant departure from linearity. MONTANA STATE UNIVERSITY LIBRARIES 3 1762 10217023 8 HOUCHEN LTO bindery tmCA/OMAHA