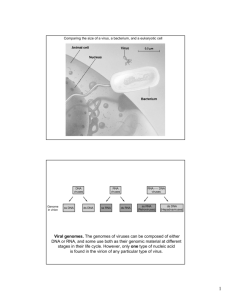
Bovine virus diarrhoea virus RNA synthesis by Peter Clayton Roberts
advertisement

Bovine virus diarrhoea virus RNA synthesis by Peter Clayton Roberts A thesis submitted in partial fulfillment of the requirements for the degree Doctor of Philosophy in Microbiology Montana State University © Copyright by Peter Clayton Roberts (1990) Abstract: The size of the genomic RNA of the pestivirus bovine virus diarrhoea virus (BVDV) is unclear. Early estimates ranged from 8.2-10.5 kilobases (kb). More recently a size of 12.5-13 kb has been reported. Very little is known concerning the synthesis of BVDV RNA in infected cells. The goals of the project were: to accurately size the genomic RNAs of cytopathic (cBVDV) and noncytopathic (ncBVDV) BVDV strains; and to compare the synthesis of BVDV RNA in cells infected with different BVDV strains. The BVDV genome was sized by comparison of BVDV-specific intracellular and virion RNAs to vesicular stomatitis virus mRNAs and denatured bacteriophage λ DNA restriction fragments. This resulted in a size estimate of 12.6 kb for the BVDV genome. No significant difference in genome size was detected between the strains studied. For the study of RNA synthesis, total cell RNA was prepared from infected cells at various times postinfection. The RNA was dot blotted onto duplicate nitrocellulose filters, that were probed with either plus or minus sense 32P-labelled BVDV RNA transcribed from a BVDV cDNA. Quantitation of the RNAs was achieved by scanning autoradiographs with a densitometer. The concentration of BVDV RNA was calculated using BVDV-specific RNA calibrations. The quantity of BVDV RNA varied markedly between cells infected with different strains of the virus. There was a lag of 5-10 h before BVDV RNA was detected. With cBVDV isolates the quantity of both plus and minus sense RNA increased until 22 h postinfection. In cells infected with ncBVDV, plus and minus sense RNA levels varied throughout the course of the experiment. The ratio of plus to minus sense RNA was higher in cells infected with ncBVDV as compared to cells infected with cBVDV. The conclusions to be drawn from this work are: 1) the BVDV genome is approximately 12.6 kb in size; 2) the synthesis of BVDV RNA follows a similar time course to that observed in cells infected with flaviviruses; and 3) cBVDV isolates have lost control of minus sense RNA synthesis, possibly due to an inability to encapsidate viral plus sense RNA efficiently. BOVINE VIRUS DIARRHOEA VIRUS RNA SYNTHESIS by Z Peter Clayton Roberts A thesis submitted in partial fulfillment of the requirements for the degree Doctor of Philosophy in Microbiology MONTANA STATE UNIVERSITY Bozeman, Montana May 1990 ^ 5^-3 S' ii APPROVAL of a thesis submitted by Peter Clayton Roberts This thesis has been read by each member of the thesis committee and has been found to be satisfactory regarding content, English usage, format, citations, bibliographic style and consistency, and is ready for submission to the College of Graduate Studies. /7 Date Chairperson, Graduate Committee Approved for the Major Department S_T_ Tkoz /fro ^ Dme Head, Major Department Approved for the College of Graduate Studies ~ Date a Graduate Dean iii STATEMENT OF PERMISSION TO USE In presenting this thesis in partial fulfillment of the requirements for a doctoral degree at Montana State University, I agree that the library shall make it available to borrowers under rules of the library. I further agree that copying this thesis is allowable only for scholarly purposes, consistent with "fair use" as prescribed in the U.S. Copyright Law. Requests for extensive copying or reproduction of this thesis should be referred to University Microfilms International, 300 North Zeeb Road, Ann Arbor, Michigan 48106, to whom I have granted "the exclusive right to reproduce and distribute copies of the dissertation in and from microfilm and the right to reproduce and distribute by abstract in any format." Signature Date /7 , TABLE OF CONTENTS Page TABLE OF C O N T E N T S........................................................................................ v LIST OF T A B L E S......... ....................................................................................... viii LIST OF F IG U R E S ................................................................................. ............. ix ABSTRACT .............................................. xi INTRODUCTION .................................................................................................. I The Pestiviruses............................................................................................. History ........................................................................................................... The D isease.................................................................................................... Acute In fe c tio n .................................................................................... Haematological Changes ..................................................... ............. Immunosuppression............................................................................ TransplacentalJnfection...................................................................... Persistent Infections............................................................................. Mucosal Disease ........... Epidemiology .................. Control of BVDV ............................................................................... The Virus ............' ........................................................................................ M orphology............................................................ .................. .. . . . . The G e n o m e ......... .............................................................................. Structural P ro te in s........................................................! .................... Physicochemical Properties ............................................................... Viral Replication and M orphogenesis........................................................ The Virus R e c e p to r......... ................................................................... Growth Phases ............................................................................ RNA Species and Synthesis............................................................... Intracellular Polypeptides................................................................... Genome Organisation . ...................................................................... Putative Protein F unctions................................................... . ; . . . . Morphogenesis ......................................................: ........................... Overview of the P ro je c t............................................................................... 2 3 6 6 8 9 11 14 15 19 20 23 23 24 27 29 30 30 31 32 32 35 37 38 39 MATERIALS AND METHODS ........................................................................ 41 Biologicals and B uffers......................................... Cells, Viruses and Plasmids ........................................................................ Preparation of BVDV A ntiserum ............................................................... Infectious Virus Assays ............................................................................... 41 44 45 46 vi TABLE OF CONTENTS-Continued Page End Point Dilution Microtitration Assay (EPDMA) ..................... 46 Plaque A ssa y ................................................................................. . . . 47 Biological Cloning of BVDV Isolates ............................' .......................... 47 Virus Growth C u rv e s.................................................................................... 48 Preparation of Cellular R N A ...................................................................... 49 Cytoplasmic RNA ............................................................. 49 Nuclear Fraction R N A ........................................................................ 50 Total Cell R N A .................................................................................... 50 Virus Purification and RNA E xtraction..................................................... 51 Radiolabelling of Viral RNA ................................ ............................. . . . . 52 Preparation of X DNA Molecular Weight M a rk e rs ....................... .. 53 Agarose Gel Electrophoresis of R N A ........................................................ 53 Formamide Gels ............; ................................................................... 53 Glyoxal Gels .............................................................................................54 Northern Transfers ...................................................................................... 54 RNA Dot Blots ............................................................................................. 55 Agarose Gel Electrophoresis of D N A .................................................' . . . 55 Preparation of Plasmid D N A ...................................................................... 55 Transformation of Escherichia coli D H 5 .......................................... 55 Screening of T ransform ants............................................................... 56 Large Scale Plasmid Purification ............................................ 57 Purification of the pBV4-p80 BVDV cDNA segment .................. 58 Preparation of BVDV-specific P ro b e s............................................ '.......... 58 Nick Translation of c D N A ................................................................. 58 In vitro Transcription of pBV 4-p80.............................. 58 Hybridisations ............................................................................................... 59 Detection of 3’ Polyadenosine Sequences ................................................. 60 Quantitation of BVDV-specific R N A ...................................................: . 61 R E S U L T S ............'................................. ' ........................................■. •....... ........... 63 Selection of Cell L in e s ............................i ................................................... 63 Cytopathic Effect of the BVDV Strains .......................... ........................ 64 Titration of Infectious V iru s ........................................................................ 65 Raising of BVDV-specific Antiserum ................................. ■ ............. 66 End Point Dilution Microtitration A ssay.......................................... 67 Comparison With Plaque Assay . ............................ ......................... 69 Biological Cloning of BVDV S tra in s.......................................................... 70 Virus Growth K in e tic s......... ............................................i ......................... 71 Purification of BVDV ................................................................................. 71 Sizing of the BVDV Genomic R N A .......................................................... 75 Northern Blot Comparisons ......................................................................■. 80 Time Course of BVDV RNA Synthesis ................................................... 81 BVDV NADL RNA Synthesis........................................................... 86 BVDV Singer RNA Synthesis .......................................................... 89 BVDV Oregon C24V RNA Synthesis.............................................. 89 V ll TABLE OF CONTENTS-Contouerf Page BVDV New York-1 RNA Synthesis ................................. ................ 92 BVDV TGAC RNA S ynthesis............................................................ 94 BVDV TGAN RNA Synthesis............................................................ 94 Comparison of Plus Sense RNA Levels With Levels of Infectious Virus .' ...■........................................................... Comparison of the Ratio of Plus to Minus Sense R N A ................ 97 DISCUSSION ......................................................................................................... Virus Strains .................................................................................................. Development of the Experimental System .............................................. BVDV RNA: Size and Synthesis . . ........................................................ 98 98 99 104 LITERATURE CITED ................ ..................................................................... Ill A P P E N D IX ........................................................................................................... 137 Abbreviations ................................................... .......................■................. 137 9 viii LIST OF TABLES Table ' Page 1. Members of the Pestivinis genus and their host species . . ..................... 2. 3 Possible combinations of BVDV and BVDV-specific antibody in cattle and their significance................................................................... .. 21 Size and sedimentation coefficient estimates of the genomic RNA of BVDV ................................................................................................. 25 4. Reported sizes of the structural polypeptides of BVDV ....................... 28 5. Molecular weights of BVDV-specific polypeptides detected in cells infected with BVDV ..................................... ..................................... 33 6. Composition of buffers, reaction mixtures and media ............................ 42 7. BVDV strains studied ................................................................................. 45 8. Comparison of BVDV titres determined by plaque assayand EPDMA 9. Recovery of BVDV during concentration................................................. 3. 10. Reanalysis of the size of the genomic RNA of four strains of BVDV . . 70 73 105 ix LIST OF FIGURES Figure Page 1. Possible outcomes of transplacental BVDV infection of the bovine foetus .................................................................................................. 2. 3. 13 Epidemiology of BVDV: routes and possible outcomes of infection of c a ttle ........................................................................................... 20 Putative genomic organisation of the NADL cBVDV is o la te ................. 36 4. Plasmid pB V 4-p80........................................................................................ 59 5. 6. Growth of MDBK cells in KClOO serum-free medium or DME supplemented with either 10% equine or 10% foetal bovine se ru m ........................................................................................... 64 Plaques of BVDV strain Oregon C24V: comparison of staining with neutral red and crystal violet ............................................................... 66 7. Focus of infection of the ILLC strain of BVDV on MDBK c e l ls ......... 68 8. One step multiplication curves of four BVDV strains ........................ 72 9. Comparison of sedimentation of NADL strain of BVDV on potassium tartrate and glycerol-tartrate density gradients ........................ 74 10. Sizing of the genome RNA of BVDV: comparison of viral intracellular and virion RNA with denatured X Hindlll and Sail restriction fragments ............................................................................................. 78 11. Sizing of BVDV genomic RNA: comparison of BVDV strain Singer mRNA with VSV m R N A ................................................................. 79 12. Quantitation of BVDV intracellular R N A ..................................... 84 13. Calibration curves for BVDV NADL plus and minussense RNA . . . . 85 14. Time course of RNA synthesis of BVDV NADL ................................... 87 15. Northern blots of total cell RNA from MDBK cells infected with BVDV N A D L ...................................................................................... 88 16. Time course of RNA synthesis of BVDV S in g e r............................ ' . . . . 90 X LIST OF FIG U R E S-C onrzm ^ Figure Page 17. Time course of RNA synthesis of BVDV Oregon C24V .................... 91 18. Time course of RNA synthesis of BVDV New York-1 ....................... 93 19. Time course of RNA synthesis of BVDV TGAC ................................ 95 20. Time course of RNA synthesis of BVDV TGAN . . . .......................... ■. 96 ABSTRACT The size of the genomic RNA of the pestivirus bovine virus diarrhoea virus (BVDV) is unclear. Early estimates ranged from 8.2-10.5 kilobases (kb). More recently a size of 12.5-13 kb has been reported. Very little is known concerning the synthesis of BVDV RNA in infected cells. The goals of the project were: to accurately size the genomic RNAs of cytopathic (cBVDV) and noncytopathic ' (ncBVDV) BVDV strains; and to compare the synthesis of BVDV RNA in cells infected with different BVDV strains. The BVDV genome was sized by comparison of BVDV-specific intracellular and virion RNAs to vesicular stomatitis virus mRNAs and denatured bacterio­ phage X DNA restriction fragments. This resulted in a size estimate of 12.6 kb for the BVDV genome. No significant difference in genome size was detected between the strains studied. For the study of RNA synthesis, total cell RNA was prepared from infected cells at various times postinfection. The RNA was dot blotted onto duplicate nitrocellulose filters, that were probed with either plus or minus sense 32P-Iabelled BVDV RNA transcribed from a BVDV cDNA. Quantit­ ation of the RNAs was achieved by scanning autoradiographs with a densitometer. The concentration of BVDV RNA was calculated using BVDV-specific RNA calibrations. The quantity of BVDV RNA varied markedly between cells infected with different strains of the virus. There was a lag of 5-10 h before BVDV RNA was detected. With cBVDV isolates the quantity of both plus and minus sense RNA increased until 22 h postinfection. In cells infected with ncBVDV, plus and minus sense RNA levels varied throughout the course of the experiment. The ratio of plus to minus sense RNA was higher in cells infected with ncBVDV as compared to cells infected with cBVDV. The conclusions to be drawn from this work are: I) the BVDV genome is approximately 12.6 kb in size; 2) the synthesis of BVDV RNA follows a similar time course to that observed in cells infected with flaviviruses; and 3) cBVDV isolates have lost control of minus sense RNA synthesis, possibly due to an inability to encapsidate viral plus sense RNA efficiently. I INTRODUCTION Bovine virus diarrhoea virus (BVDV) is the etiological agent of one of the most complex disease syndromes in veterinary medicine (78). The virus appears to be ubiquitous, since there is a high incidence of seropositive animals throughout the world (147,197). Only recently, through a better understanding of its epidemi­ ology, has the full economic impact of BVDV become appreciated (78). The infection of animals may not be diagnosed for several months after the introduc­ tion of the virus into a susceptible herd and losses may continue for several years (6,79). The virus is now considered to be one of the major causes of economic loss to the cattle industry (130). Field isolates of BVDV are initially characterised by their cytopathic effect in cell culture. They are usually described as either cytopathic (cBVDV) or noncytopathic (ncBVDV) (93); intermediate cytopathologies also occur (93,94,115,186). Fernelius (93) felt that these differences were genetic in nature and labelled them biotypes. They appear to be genetically stable traits in bovine cell lines, but can be altered by passage in non-bovine cell lines (93) or in rabbits (94). The virus occurs as a single serotype (55), though subtle but distinct antigenic differences are detected between BVDV strains (40-42,55,58,62,81,92,114,115,117,136,142, 163,173,296). These antigenic differences extend to presumed identical strains from different laboratories (42,55). No definite correlation has been found between a specific strain of BVDV and specific clinical symptoms, though such a correlation has been described for the closely related Border disease virus (BDV) (215). 2 The Pestiviruses Bovine virus diarrhoea virus is a small, spherical, enveloped virus with a single stranded, positive polarity RNA genome. It is the type species of the genus Pestivinis, at present classified in the family Togaviridae (299) but it is likely that the genus will be reclassified as being in the family Flaviviridae (52,55). Other members of the Pestivinis genus are hog cholera virus of swine (HCV) (also referred to as classical or European swine fever virus) and BDV of sheep. There is some evidence that a human pestivirus exists (101,221,303,305) that may cause infantile gastroenteritis associated with respiratory inflammation (305). The cBVDV strain Oregon C24V has been designated the type strain of the genus ( 299). The pestiviruses are serologically related (211,216), share similarities in their pathogenicity, especially for the foetus (125), and have a lower sedimentation coefficient and buoyant density than members of other togavirus genera (157). The pestiviruses are generally species specific but interspecies infections do occur (81,273). The three pestiviruses can be differentiated by panels of monoclonal antibodies (42,81,111,129,190,213,273,293-295,307). Monoclonal antibodies specific for pestiviruses in general, HCV alone or BVDV alone are available; however, a monoclonal antibody that is specific for BDV has not yet been described. BVDV and BDV seem to be very similar and are increasingly being referred to as the ruminant pestiviruses. They are antigenically diverse, have a wide host range (Table I) and a similar range of biotypes (125,134,158). In contrast, HCV has a limited host range (Table I), strains form a compact antigenic group and all field isolates are noncytopathic (120,134). There is about 85% homology at the amino acid level between BVDV and HCV (193). HCV also differs from the 3 Table I. Members of the Pestivirus genus and their host species. Virus Host species Bovine virus diarrhoea virus Cattle, sheep, goats, swine, wild ruminants, rabbits3 (5,76,78,94,119) Border disease virus Sheep, goats, cattle, swine (125) Hog cholera virus Swine (120) a. Rabbits have not been shown to be infected outside the laboratory. other pestiviruses in that virulent and avirulent strains occur (120). Virulent HCV strains are antigenically different from BVDV and BDV (120); however, avirulent HCV strains have been described that are antigenically more diverse: they appear to be more closely related serologically to BVDV than to virulent HCV (120,158). BVDV, BDV and avirulent HCV may prove to be the same virus, since they are only truly differentiated on the basis of their host species. History It is likely that the first description of the bovine virus diarrhoea-mucosal disease complex was that of Simonds and Brown (268) in England in 1871. Their description probably went unnoticed by many later researchers, as they attributed the syndrome they observed to acorn poisoning. In 1946, Olafson et al. (208) described a highly contagious and transmissible disease of cattle in New York, of high morbidity but low mortality. They called the disease virus diarrhoea (VD), as no bacteria could be isolated from infectious material and diarrhoea was a common clinical symptom (209). A similar but more severe disease syndrome, X disease, was described by Childs (44) in Saskatchewan the same year. Both 4 acute and chronic forms of X disease were described. The low morbidity and high mortality of X disease differentiated it from VD. In .1953, Ramsey and Chivers (232) described a syndrome in Iowa and surrounding states they called mucosal disease (MD). They believed that the syndrome had not previously been reported but their description is very similar to that of Child’s X disease, a fact Ramsey later acknowledged (cited in reference (223)). These early descriptions led to a belief that, although they were similar in general symptoms, VD and MD were separate disease entities. At that time the nature of the etiological agent of VD was still in question. In 1954, Baker et al. (4) conducted an extensive study of infectious material from two cases of VD. The results concurred with the earlier studies of Olafson et al. (208,209) i.e., the etiological agent of VD was a virus as no other agent was detected. This conclusion was supported the following year by the filtration studies of Pritchard et al. (224) and Schipper et al. (261). In 1957, Lee and Gillespie (160) successfully propagated in vitro one of Baker’s isolates, New York-1. The virus was found to be noncytopathic. The same year, Underdahl et al. (282) propagated in tissue culture two cytopathic viruses isolated from the tissues of animals from separate outbreaks of MD. Cross-neutralisation studies showed that the two viruses were related. In addition, cattle from herds with no history of MD were found to have serum neutralising antibody to the two viruses. Kniazeff (cited in reference (223)) later showed that UnderdahTs viruses were related to New York-1. In 1960, Gillespie et al. (103) described a cytopathic virus that was isolated from the spleen of a cow reported to have VD (a later report indicates that the cow actually had MD (197)). This isolate, Oregon C24V, has been designated the Pestivirus type strain (299). Cross-neutralisation studies, performed both in vivo and in vitro, showed that Oregon C24V was antigenically 5 related to New York-1. The following year the same group showed that viruses isolated from cases of VD and MD were antigenically related (104). This finding was supported by comparisons of VD and MD virus isolates from Europe and the United States (152,223). The ability to grow BVDV in tissue culture greatly facilitated studies of the bovine virus diarrhoea-mucosal disease complex. It provided irrefutable evidence that BVDV was the etiological agent of VD. Although virus neutralisation studies implied that VD and MD were caused by the same virus, proof of this remained elusive. A major problem was the inability to reproduce MD experimentally. In their original description of MD, Ramsey and Chivers (232) described attempts to transmit the disease to healthy calves. The only response they detected was a transient pyrexia. Similar results were obtained in other studies (102,224,261). Tyler and Ramsey (281) failed to detect any significant differences in the pathologic, immunologic and clinical responses to VD and MD virus infections in healthy calves. The shroud of mystery surrounding the pathogenesis of MD began to be lifted in 1964 when Thomson and Savan (276) observed that only animals without an antibody titre to BVDV succumbed to MD. In 1968, Malmquist (177) published a landmark paper. He observed that animals suffering from MD had a persistent BVDV viraemia but failed to develop neutralising antibodies to the virus. He hypothesised that the affected animals had been infected in utero prior to the attainment of immunocompetence and were subsequently born immunotolerant to the virus. Although there was circumstantial evidence that BVDV could infect the foetus, direct proof was provided in 1969 by Ward et al. (289). They demonstrated that BVDV could cross the placenta and that it was a teratogenic agent. In 1974, Liess et al. (161) reproduced MD experimentally. Having also observed that only seronegative animals died of MD, they selected four 6 seronegative cattle from herds where the majority of animals were immune to BVDV. The four animals were challenged by intranasal inoculation with a cBVDV strain and two subsequently died of MD. In 1978, Coria and McClurkin (59) described an apparently healthy bull with a persistent ncBVDV viraemia, proving the presence of persistent BVDV infections in animals other than those with MD. In recent years, several groups have shown that MD only occurs in persistently infected animals (25,34,99,248). The syndrome has been reproduced by superinfection of persistently infected animals with cBVDV but not ncBVDV (25,34). Brownlie et al. (34) proposed the hypothesis that MD is produced by superinfection of persistently infected cattle with cBVDV. This was later modified when it became obvious that the cytopathic virus infecting animals with MD was always antigenically homologous to the original persisting ncBVDV (36). The hypothesis is now that MD arises in persistently infected animals either through mutation of ncBVDV to cBVDV or superinfection with an antigenically homolo­ gous cBVDV. Current research is aimed at elucidating the mechanism of induction of MD. The Disease Acute Infection Seronegative and immunocompetent animals postnatally infected with BVDV undergo an acute infection. The primary site of infection is probably the respiratory tract, in particular the bronchiolar epithelia and tonsils (10). From there the virus is disseminated to epithelial and lymphoid tissues throughout the animal, especially those of the alimentary tract and skin. This dissemination may be achieved by transport of the virus in cells of the reticuloendothelial system (10). 7 Most acute infections are subclinical. They are characterised by a transient pyrexia that is often biphasic, a profound leucopenia and a viraemia that may persist for 2 weeks (78,223). During the period of viraemia, virus is shed at low levels in body secretions and excretions; however, transmission to other animals is not considered to be very efficient (78). Two weeks after infection animals start to produce serum neutralising antibody (38). This response peaks after 10-12 weeks and is generally considered to protect the animal for life (38,78); in two cases, however, immunity was shown to only last for 3-4 months (127,224). Clinical acute infections are rarely encountered. They generally occur in animals 6 months to 2 years old. Prior to six months of age, colostrum-derived neutralising antibody protects the calf from BVDV infection (267). Severe, clinical epizootics are diagnosed as bovine virus diarrhoea (BVD). Affected animals may present a variety of symptoms other than those described above: inappetence, rapid respiration, depression, oculonasal discharges and occasional ulceration and erosion of the oral mucosa (5). Mucus is sometimes present in the faeces, often as strands that can be nearly an inch thick and several feet long (223). Diarrhoea, when present, appears 1-7 days after the febrile period (223). It may be explosive in character and present continuously or intermittently (78,223). In the later stages large quantities of bright red blood may be present in the faeces (223). Diarrhoeic animals are often severely dehydrated and can lose as much as 25% of their total bodyweight (223). Affected dairy cows have a marked reduction in milk yield (6,78). The lesions of BVD are less severe than would be expected from the symptoms (208). There are diffuse reddened areas in the mucosa of the entire alimentary tract but they are more prevalent in the upper regions. These areas may develop into erosions and shallow, punched-out ulcers, especially in the oral 8 cavity, oesophagus, abomasum and caecum (208,223). In nonfatal cases the ulcers heal very rapidly (208). The morbidity rate in infected herds is generally close to 100% of the susceptible animals present when based on seroconversion. The mortality rate ranges from 0-20% (223). Death is. due to the effects of either the primary infection, especially dehydration and emaciation, or secondary infections by opportunistic pathogens (212). There is some evidence that acute BVDV infection of neonatal calves can result in a severe, sometimes fatal, enteritis (5). This has been reproduced experimentally, with both colostrum-fed and colostrum-deprived animals (153). The importance of the virus in neonatal calf diarrhoea is still an open question (5). Older, colostrum-fed calves undergo acute infections, as described above (207). Another syndrome that has been associated with neonatal infection with BVDV is hyena disease, a disorder of skeletal development (86). Haematological Changes The most obvious haematological change after BVDV infection is the transient ieucopenia. Bolin et al. (23) showed that 4 days after infection the total white blood cell count is significantly reduced. The most marked reduction is that of T lymphocytes. Most cell populations return to or exceed pre-exposure levels within 3 days but lymphocyte numbers do not recover until 2 weeks postinfection. Flow cytofluorimetric analysis showed that helper T cells, cytotoxic/suppressor T cells, B lymphocytes and neutrophils are depleted (83). The numbers of other lymphocytes and monocytes are not affected. There are some indications that Ieucopenia, involving both neutropenia and lymphopenia, correlates with a more severe clinical course than lymphopenia alone (212). 9 Several recent reports have described the association of thrombocytopenia, with resultant haemorrhagic conditions, with severe outbreaks of BVD (57,212, 233). The thrombocytopenia has been reproduced experimentally (56,57). Platelet destruction appears to be due to direct interaction with the virus (56). As the incidence of thrombocytopenia is low (233) and can be reproduced experimen­ tally with specific strains of ncBVDV (56,57) it is likely that it is induced by a limited number of BVDV strains. Immunosuppression Although acute infections with BVDV are generally innocuous, there is strong evidence that they involve a profound, transient immunosuppression. The most obvious manifestation of this is the transient leucopenia. BVDV infections also result in the destruction of lymphoid tissues (2,10). The most severely affected lymphoid tissues are those in the submucosa of the intestine, especially the Peyefs patches and the area distal to the ileocaecal valve. The tonsils and lymph nodes exhibit a loss of differentiation between the cortex and the medulla, with general loss of lymphocytes, especially from the germinal centres (2,10). There is often a depletion of thymocytes in the thymus (2,10). In the spleen, the periarteriolar lymphoid sheath is severely affected (10). In both lymphoid and nonlymphoid tissues BVDV antigen is present predominantly in cells of the reticuloendothelial system (10). In vitro studies have shown that the virus can replicate in several different lymphoid cell populations: B and T lymphocytes (2,3,18,257,279); neutrophils (257,258); and monocytes and macrophages (2,18,278,279). The virus has been isolated from, and can replicate in, the leucocytes of actively immunised animals (2,196,279). BVDV infection can result in the suppression of cellular functions. 10 The B lymphocyte response to pokeweed mitogen is suppressed (2,3,155). This results in a significant reduction in plasma cell development and IgG and IgM synthesis. Antibody secretion but not synthesis is affected in persistently infected animals (202). The mitogenic response of T lymphocytes is depressed by BVDV infection (2,28,100,143,154,155,201,256,257). A more marked depression of the blastogenic response to phytohemagglutinin compared with that to ^concanavalin A has been noted (28,257). The blastogenic response can be partially restored by addition of isoprinosine (100). In two cases, however, BVDV infection did not affect the mitogenic response of lymphocytes (39,269). Interleukin-2 receptor expression may be compromised in BVDV infected lymphocytes (100). Infection of monocytes results in a significant decrease in their random locomotion and chemotaxis (151). Infected neutrophils exhibit a defect in the myeloperoxidase, hydrogen peroxide, halide system and antibody dependent cell-mediated cytotox­ icity (257,258). Active BVDV infection of cells is required to produce the defects in cellular response and function; heat-killed or ultraviolet-irradiated virus has no effect (2,3,83,151,201,257,258). Apart from the direct action of BVDV on lymphoid cell populations it also impacts on the immune system in other ways. Bovine foetal lung cells infected with BVDV release substances that suppress concanavalin A stimulation of bovine leucocytes (180). The agent responsible has been tentatively characterised as a prostaglandin. The serum of animals suffering from MD has been shown to suppress the mitogenic response of lymphocytes (155,269). BVDV also affects the production and action of interferon (IFN). Cells infected with BVDV do not produce IFN (66) but can be induced to produce IFN by treatment with polyriboinosinic-polyribocytidylic acid (253). The production of IFN by phytohemaggluti­ nin stimulated lymphocytes infected with BVDV is reduced (234). In contrast, 11 animals infected both in utero and postnatally have been shown to produce IFN in response to BVDV infection (234,240). BVDV-infected tissue culture cells are refractory to IFN action (66,255); however, pretreatment of cells with IFN prior to infection results in reduced virus yields (15,66,97). Recombinant bovine IFN-Cn1I, IFN-y and tumour necrosis factor alpha, when added to the growth medium of BVDV-infected cells, cause enhanced cBVDV cytopathogenesis and a cytopathic effect in ncBVDV infected cells (15). A major effect of BVDV-induced immunosuppression is the potentiation of opportunistic pathogens (5). Evidence is accumulating that the virus plays a major role in bovine respiratory tract disease (5,181,270). It is believed to act synergistically with several organisms, the most important of which is Pasteurella haemolytica (5,218,220,235,280). Though BVDV infection can impair bacterial clearance from the lungs (220), the clearance of P. haemolytica may actually be enhanced (280). It has been suggested that the more severe pulmonary lesions observed in dual infections of BVDV and P. haemolytica are a product of neutrophil degranu­ lation (280). Bacterial blood clearance mechanisms are also impaired, resulting in an endogenous bacteraemia in 85% of animals with acute BVDV infections (219). Other pathogens that BVDV may act synergistically with are infectious bovine rhinotracheitis virus (82,235), parainfluenza-3 virus (33), respiratory syncytial virus (33), bovine leukosis virus (244), Coxiella burnetii (222), Leptospira interrogans serovar hardjo (222) and salmonella (304). Transplacental Infection BVDV infection of pregnant, seronegative, immunocompetent cattle general­ ly results in transplacental transmission of the virus to the foetus (reviewed in reference (285))i Such infections are the major source of economic losses due to 12 BVDV (13,121,249); 78-90% of total losses (13). Embryonic death has been produced early in gestation after intrauterine infections with BVDV; however, this is unlikely to be a common occurrence in the field (285). Exposure of bovine embryos, with or without the zona pellucida, to BVDV in vitro did not result in embryonic death (9). The virus has also been shown to impair fertilisation (109). Placental attachment, which occurs after about 35 days of gestation, appears to be necessary for transfer of BVDV from the dam to the foetus (150). After this early, refractive period, the virus is thought to cross the placenta by sequential infection of adjacent cells (285). Transplacental infections do not occur in seropositive animals, limiting the number of foetuses at risk. The outcome of foetal infection with BVDV is dependent primarily on the developmental age of the foetus, though the genotype of the host and the virus strain may also play a role (12,285). Congenital infections can result in prenatal death, stillbirth, malformations, lesions or the birth of calves with a persistent viraemia (Figure I) (285). Abortions are common early in gestation, though they can occur as a consequence of foetal infection and death at any time (70,285). Foetal mummification may also occur (38,70). During the second trimester of pregnancy, congenital malformations are the most frequent sequelae to foetal infection. Common lesions are cerebellar hypoplasia, dysmyelination of the central nervous system, ocular defects such as retinopathy and cataracts, hydranencephaly, intrauterine growth retardation of the whole animal or specific organs (especially the brain, thymus and lungs) and skeletal defects (19,285). The outcome of BVDV teratism is the birth of calves that may be stillborn, stunted, ataxic, blind or that manifest various neuromuscular disorders. In sheep and cattle the generation of malformations appears to coincide with the initial development of the immune system (164,250,251). The bovine foetus 13 Prenatal effect Foetal death Postnatal corollary Abortion, stillbirth Persistent infections Mucosal disease I Ocular lesions Blindness Cerebellar hypoplasia Ataxia Immune response ............... Organogenesis Iiiiiii Neutralising antibody Birth 100 200 Gestational age (days) Probability of occurrence: high Figure I. 300 low | Possible outcomes of transplacental BVDV infection of the bovine foetus. (Adapted from reference (78)). becomes immunologically competent to BVDV by day 100 of gestation; however, neutralising antibody to the virus is not produced before day 180-200 of gestation (17,30,32,262). The production of neutralising antibody coincides with elimination of BVDV from the foetus (30). This suggests that there can be a long lag before the virus is cleared from the foetus. During this period the immature foetal immune system could act against virus-infected cells causing the various terato­ genic effects. In the third trimester of pregnancy BVDV infection has little effect on the foetus, due to the ability of the foetus to mount a normal immune response to the virus. Abortions may still occur at this stage of gestation. One outcome of 14 infection of the foetus late in gestation is the birth of calves with the typical lesions of BVD (289). Persistent Infections Transplacental infection of the foetus during the first 4 months of gestation may result in the birth of animals persistently infected with ncBVDV (188). In experimental studies the success rate in producing persistently infected calves may approach 100% (70,188). Only ncBVDV can cause persistent infections (37). It has been estimated that persistently infected animals comprise between 0.4-1.8% of the cattle population (24,80,137,194,214). The virus is able to persist in pestiviraemic animals through immunotolerance. The immunotolerance is a product of the infection of the foetus prior to the development of the immune system. The immunotolerance involves not only the antibody response but also T cell dependent mechanisms (12). It appears to be highly specific for epitopes of the persisting virus (22). The infected animals are able to mount normal humoral immune responses to other pathogens and antigens (59,188,269) and to heterologous BVDV strains (22,26,28,58,59,72,85, 162,266,269,301). The detection of immune complexes containing BVDV antigens in the kidneys of clinically normal, persistently infected animals implies that the immunotolerance is not complete (64). The immune complexes may be formed with non-neutralising BVDV-specific antibodies that are detected in the serum of some persistently infected animals (22,72). Persistently infected calves may appear perfectly normal, though various teratological disorders are also observed (70,188). Common manifestations are intrauterine growth retardation of the whole animal or specific organs, especially the thymus, and defects of the central nervous system (70). In clinically healthy 15 cattle* persistently infected with BVDV microscopic lesions are only detected in the brain and the kidneys (64). BVDV antigen can be detected in epithelial and immune cells in most tissues (12). In the central nervous system BVDV antigen is only observed in neurons (89). In peripheral blood leucocyte (PBL) populations, virus is associated with lymphocytes, monocytes and an uncharacterised population of cells (18,29). Cytofluorimetric analysis showed that the virus is associated with an average of 4.4% of total leucocytes, 5.4% of T lymphocytes and 2.1% of B lymphocytes (29). Significant differences are found between animals in terms of the number of cells associated with virus; however, these differences do not correlate with persistent infection by a specific virus strain. The use of doubleimmunolabelling procedures for phenotyping virus-infected cells showed that 5-36% of PBL are infected, again with a marked variation between animals (18). T lymphocytes represent 40-50% of the infected cells: 60% of these are of the cytotoxic/suppressor phenotype and the remainder are helper cells. Of the remaining infected cells less than 4% are B lymphocytes, 17-24% are monocytes or macrophages and the remaining 24-40% are uncharacterised cells. Infectious virus can be isolated from all the cell populations except B lymphocytes. Total leucocyte numbers are the same as in uninfected control animals; however, there is a significant reduction in the number of T lymphocytes with a concomitant elevation of the other cell populations, especially monocytes. Mucosal Disease The mortality rate of persistently infected cattle is extremely high, often exceeding 50% in the first year (78). Animals die from diverse respiratory and enteric infections (6,188), but the most important clinical cause of death is MD (78). Mucosal disease occurs sporadically, affecting animals 6 months to 2 years 16 of age (5,33,78). Outbreaks of MD often occur concomitantly amongst animals of a similar age in a herd, implying the presence of a common precipitating factor (78). The number of affected animals in a herd is usually low, between 1-5%, but they invariably die (5,38). The first clinical symptom is usually anorexia (38). Other symptoms are pyrexia, depression, profuse watery diarrhoea, rapid respira­ tion and nasal discharges (5,33,78,223). Oral erosions and ulcerations are usually evident, often accompanied by profuse salivation (5,33). Dermatosis is common (5,33). Lameness occurs as a result of erosion and ulceration of the interdigital cleft and coronary band (5,33). Death usually occurs 3 days to 3 weeks after the first clinical symptoms are observed, though some animals die before exhibiting any symptoms (5,33,38,78). The characteristic lesions of MD resemble a graft versus host reaction (11). Severe ulcerative lesions and erosions of the mucosae are found throughout the alimentary tract (5,14,33,78,223). The ulcers are unusual as lymphoid cells initially do not invade the area around the lesion (William Quinn, personal communica­ tion). Severe thymic atrophy is observed (2,10). T lymphocytes are depleted in peripheral lymphoid tissues and lymphocytes are lost from germinal centres (2,10,14). In PBL populations there are increased numbers of B lymphocytes and, occasionally, suppressor T cells; null cells-non-characterised lymphocytes-are I decreased in number (155). Other leucocyte populations are not affected. Both ncBVDV and cBVDV can be isolated from the blood and many tissues of affected animals (6,33,35,36,50,135,166,185,203,265,266,301). The ncBVDV and cBVDV present in an individual animal are antigenically very similar, if not identical (36,58,135,136,265,266). There are indications that the two biotypes may have different tissue tropisms, cBVDV being more readily isolated from the intestines and ncBVDV more prevalent in all other tissues (35,50,166,265,301). 17 Occasionally a more protracted course of disease is observed. This was originally termed chronic BVD but chronic MD is a more apt description (5). Animals so afflicted become progressively more emaciated with persistent or intermittent diarrhoea (5,14). Intractable ocular and nasal discharges are common (5). As with acute MD, the animals invariably die; however, death may not occur. for more than 18 months after the onset of symptoms (5). The pathogenesis of MD is still unclear. It is generally accepted that in utero infection resulting in persistent infection with ncBVDV is a prerequisite for MD and that both virus biotypes are present in animals with MD (6,33,35,36,185). It has been hypothesised that MD results from persistently infected animals becoming superinfected with cBVDV either through mutation of the persistent ncBVDV or superinfection with an antigenically homologous cBVDV (36,38,58,136). In natural outbreaks of MD it appears that both processes occur (122,277). A single animal is often observed to have MD up to a month before other herd members (122). It seems likely that the ncBVDV mutates in this sentinel animal. There is some evidence that the change of biotype may be the result of recombination of the BVDV genomic RNA with cellular RNA (192). Cytopathic BVDV is then transmitted by this animal to the rest of the herd. As persistently infected animals in the herd are probably infected with the same ncBVDV, they will readily contract MD. A similar theory applies to BDV in sheep. Sheep that have recovered from Border disease are persistently infected with BDV. In these animals an MD-Iike syndrome occurs spontaneously, suggesting mutation of the persisting noncytopathie virus (99). The syndrome can also be produced by superinfection with the homologous, persisting virus but not a heterologous virus (99). 18 Experimental induction of MD tends to indicate that the process is more complex (25,26,34,36,58,123,266,301). Challenge of persistently infected animals with homologous cBVDV results in fatal MD after 2-3 weeks (301). However, challenge of persistently infected cattle with a heterologous cBVDV can also result in MD (266,301), but the onset of MD is delayed (301) and the cBVDV isolated from the animals is always antigenically homologous to the persistent ncBVDV not the original challenge virus (266,301). The animals usually produce neutralising antibody against the challenge virus. A similar situation can arise after vaccination of persistently infected animals with a modified live BVDV vaccine (26,28,85,162,269). In the case of heterologous challenge the narrowly defined immunotolerance for the persisting ncBVDV may select for antigenically homologous cBVDV in a generally heterologous cBVDV population. The delay in onset of MD would be due to the selected virus having to replicate to a high enough concentration to induce disease. A homologous cBVDV challenge would require less time to reach such a level. Several other theories have been expounded to explain the conundrum of MD induction. These include: hormonal changes associated with puberty (248), the cooperation of an unrelated defective satellite agent (166) or an immune response to an antigenically different BVDV strain (126). Although the evidence mitigates against them, none of these alternatives can be totally discounted at present. The evidence implies that antigenically homologous cBVDV is required for the development of acute MD. It has been suggested that the degree of homology between the persistent ncBVDV and challenge cBVDV is reflected in the type of clinical response observed (36). There are indications that infection with a heterologous cBVDV induces chronic MD (38). In one case, though, cytopathic 19 virus could not be isolated from the affected animal (28). It was suggested that the animal was superinfected with ncBVDV. At present there is no direct evidence that, superinfection with ncBVDV can cause chronic MD. Experimental challenge of persistently infected animals with ncBVDV produces no untoward reactions (25,203). Epidemiology Persistently infected cattle are the root of the epidemiology of the BVD-MD complex (Figure 2). They are the major mechanism by which the virus is maintained in the cattle population (78). They continually shed a large amount of virus that is readily transmitted to susceptible animals. The most devastating economic effects of BVDV occur when a persistently infected cow or heifer is introduced into a susceptible breeding herd (78,248). The herd is likely to experience any or all of the possible sequelae of acute BVDV infections. All the reproductive losses and abnormalities associated with BVDV infection may occur, including the birth of another generation of persistently infected animals. Persistently infected animals can be bred. In the case of persistently infected heifers, the offspring are also persistently infected (188,272). Such vertical transmission can result in several generations of persistently infected animals (166,249,272). The persistently infected bull is less important in the epidemiology of BVD V. They produce semen that is often of poor quality and that is contami­ nated with BVDV (7,187,239,297). The presence of virus.in the semen may occasionally result.in vertical transmission in cattle and in sheep (98,195). It may also be a problem if the bull is mated with a seronegative cow; the virus seems to prevent conception in seronegative animals (187,302), though not in every case (195,297). 20 P ersistently infected animal Immmunosuppression Seronegative im m unocom petent animal Transplacental infection Acute infection Teratogenic effects Seroconversion Foetal death Stillbirth Figure 2. Epidemiology of BVDV: routes and possible outcomes of infection of cattle It is not clear what role the non-bovine host species of BVDV play in the epidemiology of the virus (78). Control of BVDV It is unlikely that BVDV could be eradicated, as was achieved with HCV in the U.S.A. and Britain, due to its general innocuity and ubiquity in domestic and wild ruminants (249). The aim of any control strategy, therefore, should be to 21 prevent transplacental infections, the means by which the virus is maintained in the cattle population and the major source of economic losses (13,121,249). To this end, it is necessary to address the immune and virus status of animals in a herd (78,249). Table 2 summarises the four possible combinations of immunity and viraemia with respect to BVDV. Table 2. Possible combinations of BVDV and BVDV-specific antibody in cattle and their significance. Virus Antibody - - "+■ - + - + + Significance Fully susceptible to acute infection Immune Persistently infected. Early acute infection, prior to seroconversion. , Persistently infected but with low level of antibody. Acute infection undergoing seroconversion. Persistently infected cattle need to be identified, as do susceptible animals (78). Recently, several enzyme-linked immunosorbent assays (ELISA) have been described for the detection of antibodies to BVDV in serum (20,47,138,140,145, 146,148,300). These can be used to detect animals with low or no immunity to BVDV, that are then screened for persistent viraemia. Viraemic animals can be managed in two ways: they can be culled or they can be retained to act as vaccinator animals until they attain a suitable weight for slaughter (78). Persis­ tently infected cattle should not be bred. Seronegative, nonviraenhc animals should be vaccinated or exposed to a persistently infected animal and not bred until they have seroconverted. Vaccination of animals presents its own problems. The vaccines that are available are so unsafe that none has been licensed for use in Britain (78). 22 Modified live virus vaccines can induce all the clinical manifestations of BVDV infections, including those of the foetus (165,249). They are also immunosup­ pressive (165,249,257). The criterion by which they are termed ‘attenuated’ seems rather dubious as they seem to do little except pre-empt a natural infection (249). Recently, a temperature-sensitive BVDV vaccine strain has been described that appears to obviate these problems (167,168). Killed virus vaccines are also available, though these are also of dubious quality as they are inefficacious in some cases (249). To produce a protective immune response a large antigenic dose has to be administered which makes these vaccines rather expensive to produce. Their advantage over the live vaccines is the lack of adverse clinical reactions but the duration of immunity may be shortlived (249). Recently detergent solubilised BVDV has been shown to be as good an immunogen as a killed virus vaccine (198). It is not clear, at present, whether a single vaccine strain will provide adequate protection against all field viruses. The evidence from the studies with persistently infected animals would argue that a multivalent vaccine will be required. Studies of BDV in sheep Corroborate this (286). Another potential problem is contamination of live virus vaccines with nonattenuated BVDV (249). In the past, vaccine preparations of other bovine viruses have been contaminated with ncBVDV, with serious consequences. The source of the contamination is probably the bovine serum used to supplement the growth medium of the cells the vaccine viruses are propagated in. It has been shown that every lot of commercially produced fetal bovine serum is contaminated with ncBVDV (139). Therefore, great care needs to be exercised in the production of live virus vaccines. In the future recombinant DNA techniques will probably be utilised to produce cheaper, safer and more efficacious vaccines (121). 23 The Virus Morphology The first electron micrographs of negatively stained BVDV virions were prepared by Kniazeff (cited in reference (223)). The micrographs revealed particles 35-55 nm in diameter. Later studies of both negatively stained virus and sections through virus pellets furnished a large range of sizes for BVDV virions (reviewed in references (134,200)). Most estimated the virus particles to be between 40 nm and 60 nm in diameter, though sizes greater than 100 nm were reported. Similar results were obtained in studies of BVDV infected cells, with the majority of virion size estimates ranging from 45-55 nm (16,43,110,174), though sizes in excess of 100 nm were also reported (241,288). The larger virions may result from the packaging of more than one virus nucleocapsid in a single envelope (288). The virus envelope is generally described as smooth, though projections are observed on some particles (46,171,241). This correlates with studies of HCV where glycoprotein spikes are observed on some particles (84). These spikes are lost upon osmotic shock. The envelope of both BVDV and HCV has a character­ istic "rosary" substructure composed of electron-lucent beads about 6 nm in diameter, only visible after removal of surface material (131). Within the virus envelope is the virus core or nucleocapsid, with a diameter of 20-30 nm (43,110,118,131,170). The core exhibits far less size variation than the virus particles, implying that the virus envelope is responsible for the size hetero­ geneity of the virions (131). The core has a central component with a diameter of 13 nm (131). The core appears to be polygonal, suggesting cubic symmetry 24 (131,170). Ringlike subunits are present on the surface of some cores (131,170) and similar structures are seen in disintegrating virus particles (241). The Genome Early studies of BVDV showed that virus replication is not inhibited by halogenated deoxyuridines (inhibitors of DNA synthesis) indicating that the virus has an RNA genome (reviewed in reference (134)). Supporting evidence was provided by later studies that showed that viral replication is not inhibited by actinomycin D (AMD). Diderholm and Dinter (65) showed that the genome is infectious; furthermore, they showed that infectivity is lost after treatment with RNase but not DNase. The infectious nature of the genome was confirmed by Moennig (cited in reference (134)) and Horzinek (132). These results proved that the genome of BVDV is single-stranded, plus-sense RNA. A wide range of sizes have been reported for the genome of BVDV, derived from both sedimentation on sucrose gradients and gel electrophoresis (Table 3). In most cases only one RNA species was detected; however, in two cases several minor RNA species were also detected (31,225). Recent data indicate that the genome is 12.5-13 kilobases (kb) long (54,237). The genomic RNA of HCV appears to be slightly smaller (199,259), approximately 12.3 kb (193). The base composition of the genomic RNA of BVDV is 31.7% A, 22.2% U, 25.7% G and 20.4% C (55). The genomic RNA is not polyadenylated (228,237). The genomic RNA of the cBVDV strains Osloss and NADL has recently been molecularly cloned and sequenced (54,237). The Osloss sequence is 12,490 bases long and has a novel genetic organisation: two open reading frames (ORF) were found, one apparently encoding the structural and the other the nonstructural proteins (236,238). In contrast to this, the NADL sequence is 12,573 25 Table 3. Size and sedimentation coefficient estimates of the genomic RNA of BVDV. Reference Size daltons Moennig (cited in reference (133)) kilobases Sedimentation coefficient 37-40S 3.00xl06 Horzinek (132) Pritchett, Manning and Zee (225) 38S 38S 31S= 24S= 3.22xl06 2.09xl06 1.22xl06 38S Zeegers and Horzinek (306) Felmingham and Brown (88) 40S 3.60xl06 38-39S 35S<,46Sc 27Snc,47Snc Brinton (31) Purchio, Larson and Collett (228) 2.90xl06 Renard et al. (237) Collett et al. (54) 8.2 33S 12.5 12-13 c. Minor RNA species in virions of cBVDV isolates nc. Minor RNA species in virions of ncBVDV isolates nucleotides long and only has one ORF. This encodes 3,988 amino acids, equivalent to 449 kilodaltons (kDa) of protein. Collett et al. (55) compared the sequences of the two viruses. They found that there is 74% homology at the nucleotide level. They also found that insertion of two nucleotides (or the deletion of one) at Osloss nucleotide 4,241 results in a single ORF, with the previously noncoding region now coding for amino acids in register with, and homologous to, those of NADL. This indicates that the double ORF of the Osloss sequence is a cloning or sequencing artifact. The NADL sequence has been intensively analysed (54). At the 5’ end of the genome there is a stretch of 385 nucleotides before the initiation codon. There are two short ORFs present in this sequence but it is not known if they are 26 expressed. At the 3’ end of the genome there are 223 nucleotides present after the termination codon at nucleotide 12,350. The sequence GTATA appears in repeats in both the 5’ and 3’ noncoding regions. The 3’ noncoding region also contains a longer repeated sequence involving two closely spaced 50 nucleotide stretches in which 30 nucleotides are identical. It is not known what functional significance these sequences may have. It is not known if the entire genome of BVDV has been cloned, as the ends of the genomic RNA have not been sequenced for comparison with the cDNA sequence. The genome of the Alfort strain of HCV has also been cloned (193). The sequence of 12,284 nucleotides bares many similarities to that of BVDV- There is a nucleotide homology of 66% but an amino acid homology of 85% with respect to the NADL sequence. The HCV sequence contains an ORF of 3,898 codons. There are 363 nucleotides preceding the initiation codon at the 5’ end of the sequence. Short ORFs are present in this region. At the 3’ end 224 nucleotides are present after the termination codon. The HCV ORF contains 90 codons less than that of NADL. The 90 amino acid difference is accounted for by an insertion at amino acid 1,536 of HCV. Comparison with the Osloss sequence shows that there is an insertion of 76 amino acids at this point (192). Surprisingly, the insertion in Osloss was found to be homologous to the sequence of animal ubiquitin (192), but no homology has been found between the NADL insertion and any other nucleotide sequence. This indicates that the BVDV genome RNA can undergo recombination with cellular RNA. 27 Structural Proteins Frost and Liess (96) were the first to investigate the structural proteins of BVDV, observing three proteins on polyacrylamide gels. Several groups have since studied the structural proteins of the virus. There is little correlation between reports, either in the number of polypeptides or their relative molecular weights (Mr) (Table 4). As the same strain was studied in several cases, the discrepancies are not a reflection of differences between strains. The only common polypeptide detected is a 50-57 kDa glycoprotein. It is referred to as gp53 in the proposed nomenclature for BVDV polypeptides (55). (The proposed nomenclature of Collett et al. (55) (Table 5) will be used, as far as possible, in this manuscript.) This glycoprotein is present in the envelope of the virus. The neutralising monoclonal antibodies to BVDV that have been produced all react with this glycoprotein (21,58,71,172,173,290); however, they do not all bind to the same epitope, as shown by their varying reactivities with different virus strains (21,58,71). It appears that 10 epitopes (55) in at least three domains are involved in virus neutralisation (21). Eight epitopes were clustered in a single domain (55). One domain is highly conserved between BVDV strains and is strongly associated with neutralisation. The other two domains are weakly associated with neutralisation; one appears to be virus-specific and the other appears to be generally conserved among BVDV strains. Similar results are found with HCV (291,292). However, HCV monoclonal antibodies, specific for different domains, can act synergistically to enhance neutralisation (291) but this has not been observed with BVDV monoclonal antibodies (55). A domain of the glyco­ protein of the Singer cBVDV strain containing a neutralisation epitope has been expressed in a vaccinia virus vector (77). Neutralising antibody specific for BVDV is produced in mice immunised with the recombinant virus. 28 Table 4. Ia Reported sizes of the structural polypeptides of BVDV. II Ill IV NADLb NADL OC24Vc NADL V VI VII VIII IX Singer NADL Osloss NADL Singer gp55 gp53 gp52-56 gp48 gp48 140d 138 117 93-110 115 115 80 80 100 • gp75 66 70 50-59 25 gp57 gp54 gp44 gp45 34 35 gp54 gp55 32 26 28 gp25 23 20 16 a. Reference. I Pritchett and Zee (226) II Felmingham and Brown (88) III Matthaeus (182) IV Akkina (I). Strain studied not known but probably NADL V Coria, Schmerr and McClurkin (61) VI Purchio, Larson and Collett (229) VII Renard et al. (238) VTTT Collett, Larson, Belzer and Retzel (53) IX Etchison (unpublished data) b. BVDV strain c. Oregon C24V d. Mr polypeptide gp. glycoprotein 29 Etchison (James Etchison, personal communication) found that gp48, which is often detected in purified virus preparations, is not enriched in peaks of virus infectivity on rate-zonal and isopycnic density gradients. However, four other polypeptides are enriched (Table 4, column IX). This tends to imply that gp48 is not an integral part of the virus structure. The only other structural polypeptide that has been identified is the capsid protein. Matthaeus (182) suggested that the 34 kDa polypeptide he detected served this role. The recent data of Collett et al. (53) and Etchison (James Etchison, personal communication) suggest that it is somewhat smaller, 16-20 kDa. In the proposed nomenclature this is referred to as p20 (55). Matthaeus (184) compared the cross-reactivity of the three structural polypeptides of BVDV he detected (Table 4, column III) with antisera against BVDV or HCV. The most marked difference was that HCV antibodies failed to immunoprecipitate the 34 kDa polypeptide, Matthaeus’s. putative capsid protein. Of the two glycoproteins he detected, gp57 reacted more strongly with BVDV antibodies than did gp44, while the opposite was true with HCV antibodies. He suggested that the 34 kDa polypeptide is species-specific and the glycoproteins are group-specific within the Pestivinis genus. Physicochemical Properties The physicochemical properties of BVDV have been reviewed by Brinton (31) and Horzinek (134). Infectivity is lost after exposure of the virus to ultra­ violet radiation or treatment with ether, chloroform, deoxycholate, saponin, nonidet P40, Tween 80, sodium dodecyl sulphate, formaldehyde, B-propiolactone and trypsin. The virus is relatively stable in a pH range of 5.1-93, with a maximum stability at pH 7.4 (116). The virus also has reasonable thermal 30 stability, losing only 0.2-1.0 Iog10 ID50 units after 24 h at 37°C (134). Tempera­ tures above 50°C result in more rapid inactivation. The reported values for the buoyant density of BVDV, ranging from 1.09-1.17 g/ml, reflect the size heterogeneity of the virus particles (134). Laude and Gelfi (157) observed that the buoyant density of pestiviruses also varies according to the cell line in which they are propagated. Two peaks of infectivity are often observed when BVDV is sedimented to equilibrium on caesium chloride or potassium tartrate gradients but a single peak, at a density of 1.15-1.16 g/ml, is generally observed with sucrose gradients (134). The sedimentation coefficient of the virus has been calculated (157). Analysis by rate zonal centrifugation on 7-35% sucrose gradients yields a value of ISSt IIS 20jwand analysis on isokinetic gradients yields a value of 139+12S20w. Viral Replication and Morphogenesis The Virus Receptor The initial event in virus replication is the binding to cellular receptors. The cell receptor for BVDV has not been identified. However, a monoclonal antibody, raised against a cellular component, has been described that specifically blocks binding of cBVDV to bovine cells (55). The binding of HCV and BDV is not impaired. This implies that there is a specific receptor for BVDV on bovine cells. As the infectivity of BVDV was not totally blocked, the virus may also be taken up by a passive mechanism. Another monoclonal antibody, that completely blocks the binding of bovine enterovirus 3 to its receptor, also interferes with cBVDV infectivity (274), implying that the receptor for BVDV and’bovine enterovirus 3 are closely associated. 31 Growth Phases Early studies of virus growth kinetics in cell culture were reviewed by Horzinek (134). Major differences are found, apparently dependent on the cell lines and virus strains used. Adsorption appears to be a slow process and is maximal after 60-100 min. Different adsorption rates are observed with different cell lines: bovine spleen cells have been shown to adsorb BVDV 13 times faster than bovine kidney cells (260). In general the growth kinetics are as follows: the latent phase lasts for 4-12 h, exponential growth occurs until 12-36 h postinfection (pi) and a plateau phase follows this (134). The duration of each phase appears to be dependent more on the cell line and virus involved than on the multiplicity of infection (moi). Titres of released virus are generally higher than those of cellassociated virus (95,115,205,287). Studies of infected cells by immunofluorescence have shown the presence of viral antigen in the nucleus early in infection (91). Viral antigen is then observed perinuclearly and later becomes dispersed throughout the cytoplasm. The fluorescence disappears late in infection (91,175). Noncytopathic BVDV causes persistent infections of susceptible cells in culture (60). Persistently infected cells continuously produce virus. The cells cannot be freed of the infection by passaging in medium containing antibody to the virus (60) or by treatment with IFN or tumour necrosis factor alpha (15). The persistent infections are not due to the presence of defective interfering particles (60), although these are detected in cBVDV cultures (67,87). BVDV replication is inhibited by proflavine sulphate and acriflavine dihydro­ chloride (68). The nature of the inhibition is not known but it has been suggested that it is a product of the high degree of secondary structure of BVDV genomic RNA (55). Resistant virus strains have been described (69,247). Nuttall (205) 32 observed that cBVDV yield is reduced in the presence of low concentrations of AMD but ncBVDV yield is enhanced under the same conditions. RNA Species and Synthesis Two RNA species are detected in cells infected with BVDV (88,228): one is the same size as the viral genomic RNA and the other is twice the genomic RNA’s mass. The larger species is not degraded by low concentrations of RNase, implying that it is the replicative form (RF) of RNA; the degradation of this RNA at higher concentrations of RNase indicates that it is not a true duplex molecule (228). Denaturation results in only genome-size RNA being detected (228). In no case has a subgenomic messenger RNA been detected. Little is known of the kinetics of RNA synthesis. Nuttall (cited in reference (134)) detected [3Hjuridine incorporation 7.5 h pi; maximum incorporation was observed after 10.5 h. Moorman and Hulst (199) found that the time course of HCV RNA synthesis paralleled that of virus growth. Intracellular Polypeptides As with the viral structural proteins, the numbers and sizes of virus-specific polypeptides detected in infected cells varies between reports (Table 5). There is a marked heterogeneity in the sizes of some polypeptides between both biotypes and strains when directly compared (1,73,217). The most marked variation is found in gp53, the prominent Mr 52-64 kDa glycoprotein that is often resolved as a doublet; this is also the case with HCV (292). This heterogeneity is due to differential glycosylation of a precursor polypeptide (1,53,74,292). The precursor polypeptide is detected in cells labelled in the presence of tunicamycin or after endoglycosidase F digestion of the glycoprotein. It has been estimated to be 51-52 kDa (1,74) or 42 kDa (53) in size. Analysis of the nucleotide sequence Table 5. Molecular weights of BVDV-specific polypeptides detected in cells infected with BVDV. Ia NADLb Singer OC24Vc III • II NY-Id NADL Singer 165e cBVDV 165 ncBVDV IV V VI VII VIII NADL NADL NADL Singer Proposed names 165 gpl65 I 145 135 115 115 115 115 118 118 . 118 120 120 133 135 125 105 pl25 80 85 p80 75 77 gpll6 90 80 80 80 80 80 gP?5 gp62 gp75 87 80 gp75 gp69 gp65 gp62 gp62 58 54 gp54 gp45 gp54 35 35 gp56-58 gp56-58 gp55-64 gp57 gp53-55 gp53 gp52-56 gp45 gp55 45 gp48 gp48 gp48 gp49 gp47 gp48 gp40 35 38 gp37 37 37 37 38 gp54 gp53 .. gp48 37 36 32 33 25 gp23 32 gp26 19 a. Reference I Akkina (I) IV Pocock et al. (217) VII Etchison (unpublished data) b. BVDV strain c. Oregon C24V II Purchio et al. (229) V Magar et al. (172) VIII Collett et al. (55) d. New York-1 e. Mr polypeptide III VI gp25 23 20 16 Donis and Dubovi (73-75) Collett et al. (53) gp. glycoprotein gp25 p20 w w 34 of both BVDV and HCV shows that there are four potential N-glycosylation sites on the precursor (53,292). In cells infected with HCV, gp53 is found connected to a glycoprotein of 31 kDa (292). Monoclonal antibodies have been produced that recognise several BVDV polypeptides. Neutralising monoclonal antibodies recognise only gp53 (21,58,71,173,290). The non-neutralising monoclonal antibodies that have been produced recognise gp48 (58,71), gp53 (58,71), a 20 kDa polypeptide (145), a 79 kDa and 73 kDa polypeptide (213), and a 73 kDa polypeptide with minor specificity for several polypeptides from 28-51 kDa (213). One consistent difference has been detected between cytopathic and noncytopathic strains (1,12,27,73,172,217): a prominent virus-specific polypeptide of Mr 80-87 kDa, that is detected in cells infected with cBVDV strains, is not detected in cells infected with ncBVDV strains. However, in ncBVDV-infected cells a polypeptide of Mr 105-120 kDa, that is also detected in cells infected with cBVDV, is present at an increased level. These two polypeptides are designated p80 and pl25 (55). Peptide analysis has shown that the two polypeptides are related (1,229). This suggests that the lack of p80 in ncBVDV-infected cells is due to a defect in processing of pl25. Collett et al. (53) showed that p80 of the cBVDV strain NADL is derived from a 125 kDa precursor, the other product being a 54 kDa protein. In addition to the above processing difference, a polypeptide, or doublet, of Mr 90 kDa has been detected in cells infected with ncBVDV but not cBVDV (73). Donis and Dubovi (72) studied the antibodies present in animals naturally or experimentally infected with BVDV. They found strong immune responses to the following major polypeptides detected in infected cells: the gp48, gp53, pl25 and p80 in cBVDV-infected animals, pl25 in ncBVDV-infected cells and, occasionally, 35 a 37 kDa polypeptide. Bolin (22) studied the specific immune response of persistently infected cattle to a live cBVDV vaccine. He found a highly strainspecific neutralising antibody response to gp53. He also found a less specific non­ neutralising antibody response to p!25, p80, gp53 and gp48. In normal calves vaccinated with modified live cBVDV vaccine, Bolin and Ridpath (27) detected antibodies to p!25, p80, gp53, gp48, gp25 and a 39 kDa polypeptide. Some calves also produced antibody to gp62 and a 37 kDa polypeptide. Genome Organisation Collett et al. (53) generated a panel of sequence specific antisera raised against short segments along the genome of the NADL cBVDV strain expressed in Escherichia coli. These were used to immunoprecipitate authentic BVDV polypeptides from lysates of infected cells. From this data they were able to identify precursor-product relationships between various polypeptides. This information, coupled with the nucleotide sequence, was used to propose a tentative scheme for the organisation of the BVDV genome (Figure 3). Polypep­ tides were not detected for two hydrophobic regions of the translated genome sequence, representing 30 kDa and 38 kDa of protein. Peptide sequencing of the polypeptides needs to be performed in order to accurately locate the genes on the genome and to substantiate precursor-product relationships. The genetic organisation of the genome is very similar to that of flaviviruses, the structural genes being located at the 5’ end of the genome. There is a striking similarity between the hydrophobicity profiles of BVDV and flavivirus polyproteins (52,53,55). The BVDV ORF is probably translated as a single polyprotein, which is cotranslationally and posttranslationally processed (55). 36 NADL genomic RNA open reading frame (11,964 nucleotides) 5’ 3' p20" gp ll6 pl25b pl33 gp48d gp25 < Structural proteins > < Vegetative functions ggg immunoprecipitated polypeptides I lll regions with no detected intracellular protein product > a. Capsid protein b. Polypeptide that is not cleaved during ncBVDV replication Envelope glycoprotein possessing epitopes recognised by neutralising monoclonal antibodies d. Possibly not a structural polypeptide Figure 3. Putative genomic organisation of the NADL cBVDV isolate. (Adapted from reference (53)). Unfortunately, in vitro translation studies do not illuminate this process (230). Genomic BVDV RN A, after denaturation with methyl mercury, directs the synthesis of a range of polypeptides of Mr 50-150 kDa. Immunoprecipitation of the products with BVDV-specific antiserum does not reveal any products that comigrate with authentic viral proteins. When free polyribosomes are used to program translation, two polypeptides are synthesised that comigrate with 37 authentic BVDV 80 kDa and 115 kDa polypeptides. Membrane-bound polysomes do not direct the synthesis of any BVDV-specific polypeptides. Putative Protein Functions It has been hypothesised that p80 and pl25 may play a role in RNA replica­ tion or translation (73). Some evidence for a role of this polypeptide in viral RNA metabolism comes from earlier studies on a soluble antigen present in cBVDV infected cell cultures (183,184,283,284). This antigen is 82-85 kDa in size (183,284) and is detected both as a monomer and as a dimer (284). It is not a structural protein of BVDV (183) but is antigenically related to a nonstructural polypeptide of HCV (183,284). It is the only BVDV polypeptide that has been shown to have enzymatic properties: it reduces nucleotide tri- and diphosphates to their respective monophosphates and may be a protease (183,284). Recent computer analysis of the amino acid sequence of NADL has identified domains on p!25 that are also present on flavivirus NS3 (8,52,55,106). They contain highly conserved amino acid residues typical, in an N-proximal domain, of chymotrypsin-like serine proteases (8,106) and, in a C-proximal domain, of RNA helicases (106). The amino acids are conserved not only in pestiviruses and flaviviruses but also alphaviruses (106). It is thought that cleavage of pl25 occurs between the two domains (106). Some amino acid residues are also conserved between BVDV p!33/p58:p75 and flavivirus NS5 (52). A conserved Gly-Asp-Asp sequence in this protein is typical of RNA dependent RNA polymerases, implying that p!33/p58:p75 is the BVDV replicase (52,55). 38 Morphogenesis Very little is known about the morphogenesis of BVDV. Chasey and Roedef (43) and Gray and Nettleton (HO) observed modifications of the endoplasmic reticulum (ER) in cBVDV infected cells. The modifications are tubular structures 65-83 nm in diameter and of variable length. One end is sealed and the other terminated in the rough or smooth ER. Mature virions are observed within the tubules and larger vesicles. No virions are observed free within the cytoplasm or budding through intracellular membranes or-plasma membranes of the infected cells. Mahnel (174) found that cells persistently infected with ncBVDV contain smaller and fewer vesicles than cells infected with cBVDV. Again, no virions are observed in the cytoplasm or budding through membranes. Ward and Kaeberle (288) used immunoperoxidase to stain infected cells. Electron micrographs showed the presence of viral antigen around the periphery of vacuoles. Early in infection such vacuoles have well defined limiting membranes and contain intensely staining particles, thought to be viral nucleocapsids. Later in infection, when mature virus is present in the vacuoles, no limiting membranes are detected. Based on their electron microscopic observations of infected cells, Bielefeldt Ohmann and Bloch (16) suggested the following sequence of events occurred in BVDV morphogenesis. Initially virus proteins accumulate in matrices of fibrillar material. These become more condensed and are internalised within membrane vesicles formed from coalescing smooth bi- and multilaminated membranes. Virion maturation takes place within these vesicles through the interaction of virus nucleocapsids and membrane units. Virus release is achieved either through disintegration of the cell or exocytosis. 39 Overview of the Project Although the genomic RNA of two strains of BVDV has been molecularly cloned, there are still major gaps in our knowledge of BVDV RNA. The actual size of the genome is still unclear and it is likely that the size varies between strains. More importantly, no refereed reports concerning the size of the ncBVDV genome have been published. Another major gap involves the synthesis of BVDV RNA in infected cells. The ultimate aim of this project was to accurate­ ly measure the genome of both cBVDV and ncBVDV strains and to define the time course of RNA synthesis in infected cells. Before these questions could be addressed, it was necessary to define a suitable system for studying BVDV. A major problem encountered in working with BVDV is the contamination of cell lines with adventitious virus from bovine serum. Cell lines have to be screened for the presence of the virus and then kept free from contamination. I felt that it would be wise to find a substitute for bovine serum for the growth of the cells. Another important consideration was that, as the virus does not replicate to high titres, a cell line should be chosen that would maximise virus yield. I also deemed it necessary to develop a reliable assay for the titration of ncBVDV. There is a lack of standard procedures for the purification of BVDV. The purification of the virus is complicated by the heterogeneous size and density of the virus, and the low yields of virus from cells. The next part of my research involved a series of experiments to develop a system for the purification of BVDV while limiting the loss of infectious virus. For the study of the size of the BVDV genomic RNA, the main requirement was the use of defined molecular weight markers for the calibration of agarose 40 gels. Initially denatured DNA restriction fragments were used. Later studies involved using VSV mRNAs. The results were checked using northern blots. During the genome sizing studies, I noticed that there were distinct differences between the BVDV strains in terms of their intracellular viral RNA levels. This led to speculation that the genomic RNA of ncBVDV strains was more efficiently packaged into virions. I addressed this question by quantitating the levels of BVDV-specific plus sense and minus sense intracellular RNAs. The results of this study led to a hypothesis that cBVDV strains have lost control of minus sense RNA synthesis, due to a defect in their ability to target plus sense RNA for encapsidation. / 41 MATERIALS AND METHODS Biologicals and Buffers Reagent grade chemicals were obtained from J. T. Baker Chemical Com­ pany, Fisher Scientific, Sigma Chemical Company or Eastman Kodak Company. Radioisotopes were obtained from NEN Research Products, DuPont Company. Cell culture medium was obtained from KC Biologicals, Sigma Chemical Company or Irvine Scientific. Equine serum was obtained from Hyclone Laboratories, Inc. or Sigma Chemical Company. Fluorescein conjugated rabbit anti-bovine gamma­ globulin was purchased from Antibodies Inc. or Sigma Chemical Company. Calf intestinal alkaline phosphatase (CIP), R Q l DNase, RNasin, T4 polynucleotide kinase, Riboprobe Gemini System II kit and restriction endonuclease Sail were purchased from Promega. Bacteriophage X DNA, restriction endonuclease H indlll and vanadyl ribonucleoside complexes (VRC) were purchased from Bethesda Research Laboratories. Proteinase K and restriction endonucleases Bgll, Bglll, Hpal, Kpnl, Pvul and Xbal were obtained from Boehringer Mannheim Biochemicals. Percoll, Sephacryl S-IOOO and dephosphorylated oligodeoxythymidylic acid12.18 were obtained from Pharmacia. The composition of buffers, reaction mixtures and media formulations are listed in Table 6. 42 Table 6. Composition of buffers, reaction mixtures and media. Buffer Composition CaCl2 solution 60 mM CaCl2, 15% vol/vol glycerol, 10 mM PIPES (pH 7.0) CIP buffer 50 mM Tris-HCl (pH 9.0), I mM MgCl2, 0.1 mM ZnCl2, I mM spermidine ChloroformAAA 96% vol/vol chloroform, 4% vol/vol isopentanol Denhardt’s solution 0.02% wt/vol ficoll, 0.02% wt/vol PVP, 0.02% wt/vol BSA DME-% DME supplemented with n% vol/vol equine serum DNA-TAE buffer 40 mM Tris-acetate (pH 7.7), 20 mM sodium acetate, 2 mM EDTA Glyoxal solution I M deionised glyoxal, 50% vol/vol DMSO, 10 mM NaPO4 (pH 6.65) GTE 50 mM glucose, 25 mM Tris-HCl (pH 8.0), 10 mM EDTA Hybridisation solution 50% vol/vol deionised formamide, 5X SSPE, 10 jug/ml sheared salmon sperm DNA, 500 /rg/ml yeast RNA, IX Denhardt’s solution, 10% wt/vol dextran sulphate LB 1% wt/vol tryptone, 0.5% wt/vol yeast extract, 0.5% wt/vol NaCl, I mM NaOH LB agar LB, 1.5% wt/vol agar LB+Amp LB, 100 Mg/ml ampicillin LSB 0.2 M NaCl, 20 mM Tris-HCl (pH 7.4), I mM EDTA Lysis buffer 0.14 M NaCl, 1.5 mM MgCl2, 10 mM Tris-HCl (pH 8.6), 0.5% NP-40, 10 mM VRC MTH 0.5 M MOPS, 0.5 M TES, 0.5 M HEPES (pH 7.2) NET 0.1 M NaCl, 10 mM Tris-HCl (pH 7.4), I mM EDTA 43 Table 6, continued. Buffer Composition NT buffer 50 mM Tris-HCl (pH 7.5), 50 mM NaCl, 10 mM MgCl2, 7 mM 2-mercaptoethanol, 50 jug/ml BSA PBS 0.8% wt/vol NaCl, 0.02% wt/vol KC1, 0.115% wt/vol Na2HPO4, 0.02% wt/vol KH2PO4, 0.01% wt/vol CaCl2, 0.01% wt/vol MgCl2-SH2O Phenol solution 63% vol/vol phenol, 37% vol/vol LSB, 7 mM 8-hydroxyquinoline PK buffer 0.15 M NaCl, 0.1 M Tris-HCl (pH 7.5), 12.5 mM EDTA, 1% wt/vol SDS PNE 20 mM PIPES (pH 6.5), 0.15 M NaCl, I mM EDTA PNK buffer 50 mM Tris-HCl (pH 7.6), 10 mM MgCl2, 5 mM DTT, 0.1 mM spermidine, 0.1 mM EDTA Prehybridisation solution 50% vol/vol deionised formamide, 5X SSPE, 10 jug/ml sheared salmon sperm DNA, 500 //g/ml yeast RN A, 5X Denhardt’s solution RNA-TAE buffer 40 mM Tris-acetate TpH 7.0), 20 mM sodium acetate, 2 mM EDTA, 50% vol/vol deionised formamide SOC medium 0.5% wt/vol yeast extract, 2% wt/vol tryptone, 10 mM NaCl, 2.5 mM KC1, 10 mM MgCl2, 10 mM MgSO4, 20 mM glucose Solution D 4 M guanidinium thiocyanate, 25 mM sodium citrate (pH 7.0), 0.5% wt/vol sarcosyl, 0.1 M 2-mercaptoethanol SSC 0.15 M NaCl, 15 mM sodium citrate (pH 7.0) SSPE 0.18 M NaCl, 10 mM NaPO4 (pH 7.7), I mM EDTA TE 20 mM Tris-HCl (pH 7.4), 2 mM EDTA TE8 10 mM Tris-HCl (pH 8.0), I mM EDTA TE+S 20 mM Tris-HCl (pH 7.4), 2 mM EDTA, 0.1% wt/vol SDS 44 Table 6, continued. Buffer TNES Composition ' 10 mM Tris-HQ (pH 7.4), 0.1 M NaCl, 2 mM EDTA, 0.2% wt/vol SDS Cells. Viruses and Plasmids The Madin-Darby bovine kidney (MDBK) cell line (169) was obtained from the American Type Culture Collection (ATCC, CCL 22), at passage 113, The bovine turbinate (BT) cell line (189), at passage 8, was the kind gift of Dr. Arlan McClurkin of the National Animal Disease Center (NADC), Ames, Iowa. The bovine lung cell line (BLF) was the kind gift of Dr. David Reed of Iowa State University. The cell lines were maintained in DME-10. They were screened for BVDV contamination by direct immunofluorescence, using fluorescent BVDVspecific antiserum obtained from the National Veterinary Services Diagnostic Laboratory, Ames, Iowa. All BVDV strains were the kind gift of Dr. Arlan McClurkin and Dr. Steven Bolin of the NADC. These are listed in Table 7. Virus stocks were prepared by infecting MDBK cells either as monolayers or cells in suspension. After allowing the virus to adsorb for I h a t 37°C, the inoculum was aspirated and replaced with fresh DME-2. The cells were incubated at 37°C until either extensive cell lysis was observed, in the case of most cytopathic strains, or for 48 to 72. h, in the case of noncytopathic strains and Oregon C24V. The tissue culture fluids (tcf) were harvested, clarified by low speed centrifugation and stored at -70°C. The Mudd-Summers strain of the Indiana serotype of vesicular stomatitis virus (VSV) was provided by Dr. James Etchison. 45 Table 7. BVDV strains studied. Strain Biotype New York-1 nc1 Reference Baker et al. (4) Lee and Gillespie (160) Oregon C24V C2 Gillespie et al. (103) NADL C Gutekunst and Malmquist (H 3) Singer C McClurkin et al. (189) TGAC3 - C Bolin et al. (23,25) TGAN nc 1. noncytopathic 2. cytopathic 3. Originally thought to be a cytopathic isolate, TGA (23). Later discovered to be a mixture of two biotypes that were each biologically cloned (25). The plasmid pBV4-p80, provided as plasmid DNA, was the kind gift of Dr. Marc Collett. This is a pGEM-4 plasmid with a 2,306 base insert of BVDV strain NADL cDNA. The sequence is that between nucleotides 5,644 and 7,949 (54), representing the p80 polypeptide gene, inserted in the Smal site of the vector. Preparation of BVDV Antiserum A black angus heifer, seronegative and virus negative for BVDV, was infected with BVDV strain New York-1 by intranasal instillation of 20 ml of infected cell culture fluid, with a titre of LSxlO8 infectious units (IU) of virus per ml. Eight weeks later, the animal was boosted with 4 ml of a 1:1 emulsion of 46 New York-l-infected cell culture fluid and incomplete Freund adjuvant, adminis­ tered intramuscularly. Serum was prepared from blood collected 2 wk later. Infectious Virus Assays End Point Dilution Microtitration Assay CEPDMA') . The assay was adapted from that developed by Robb and Martin for titrating simian virus 40 (SV40) (243) and later adapted for the titration of murine coronaviruses (242). Ten fold serial dilutions of BVDV were prepared in a suspension of IxlO5 MDBK cells per ml in DME-2. The suspension was then dispensed into. Terasaki plates (Nunc), 10 £d of suspension being dispensed into each well. Alternatively, uninfected cells were dispensed into the wells and infected the next day with serial dilutions of virus in DME-2. To prevent dehydration during incubation, 0.5 ml of DME-2 was distributed around the inside edge of the plates. The plates were incubated for 72 h at 37°C. The medium was aspirated, the plates rinsed twice with cold PBS, once with cold absolute methanol and flooded with cold absolute methanol. Five minutes later, the methanol was removed and the plates allowed to dry at room temperature. The plates were used immediately or stored at -70°C. The Terasaki plates were rehydrated by rinsing once with PBS. The primary antiserum was diluted in PBS containing 1% wt/vol porcine gelatin and 10 gl dispensed into each well of the Terasaki plate. The plates were incubated for 2 h at 37°C, in a humid atmosphere. After incubation the plates were rinsed five times with PBS containing 0.05% vol/vol Tween 80. The secondary antiserum, fluorescent rabbit anti-bovine gammaglobulins, was diluted and dispensed, and the plates incubated, as for the primary antiserum. The plates were washed twice u 47 with PBS containing 0.05% vol/vol Tween 80 and twice with distilled water (5 min per wash). Prior to observing the cells for fluorescence, the wells were filled with PBS. An Olympus inverted microscope, with an epifluorescence attachment, was used to observe wells for BVDV-specific fluorescence. The virus litre was calculated from the number of uninfected wells using the Poisson distribution. A Turbo Pascal (Borland) computer program was written to perform these calculations. Plaque Assay Six well plates (Nunc) were seeded with 4x105 MDBK cells per well in DME-IO and incubated overnight. The next day the medium was aspirated and 0.2 ml of 10 fold serial dilutions of virus in DME-2 were dispensed onto the monolayers. Virus was allowed to adsorb for I h, with regular agitation. The monolayers were overlaid with 3 ml 0.7% wt/vol SeaPlaque agarose (FMC) in DME-2. The agarose was allowed to gel at room temperature and the plates incubated inverted at 37°C. Three to 5 days later, the cells were fixed with 0.5 ml 2% vol/vol glutaraldehyde, the agarose removed, the monolayers stained with 0.4% wt/vol crystal violet in 20% vol/vol ethanol and the plaques counted. Alternatively, the cells were stained with 3 ml 0.01% wt/vol neutral red in DME-2 for at least 6 h at 37°C in the dark, fixed with 0.5 ml 2% vol/vol glutaraldehyde, the agarose removed and the plaques counted. . Biological Cloning of BVDV Isolates The BVDV strains New York-1, Singer, NADL and Oregon C24V were all cloned by limiting dilution. Initially, stocks of each were serially diluted in a suspension of SxlO5 MDBK cells per ml in the following ranges: Singer and 48 NADL, IO'3 to IO"6; Oregon C24V, IO'4 to IO"7; and New York-1, IO'2 to IO'5. Thirty, 10 /xl aliquots of each dilution were dispensed into the wells of Terasaki plates. These were incubated for 48 h at 37°C. The tcfs from each well were transfered to the equivalent well of a fresh Terasaki plate, which was stored at 4°C. The original Terasaki plate was processed for immunofluorescence, as for an EPDMA. The tcf from an infected well, selected at random from the lowest dilution exhibiting less than 100% infection, was diluted 10"2 to IO'4, dispensed and incubated as described above. The remaining tcf were stored at -70°C. This process was repeated six times in all, tcf being harvested 30 to 48 h after each infection. After the last cloning, five or six infected tcf were selected at random and amplified in a 96 well plate and then a six well plate. The final tcfs were dispensed as I ml aliquots and stored at -70°C. Virus Growth Curves Twenty, six well plates were seeded with 3x105 MDBK cells per well, five wells per plate. Next day, one well of each plate was infected at a moi of one with BVDV strains Singer, NADL, Oregon C24V or New York-1 in a volume of 0.2 ml. The fifth well of each tray was mock infected with 0.2 ml of DME-2. The virus was allowed to adsorb for I h and 2.8 ml of DME-2 was added to each well. ■: At specific times pi the tcf was harvested from the wells of one plate and 3 ml of fresh DME-2 was dispensed into each well. The tcfs and the plate were placed at -V0°C. The plates were thawed, the cells scraped off and the cell suspension refrozen. The tcfs were also thawed and refrozen. The released and cell-associ­ ated virus titres of Singer, NADL and Oregon C24V were measured by plaque assay and those of New York-1 by EPDMA. 49 Preparation of Cellular RNA Cytoplasmic RNA Cytoplasmic RNA was usually extracted using a method adapted from that described by Maniatis et al. (178). Cell monolayers were rinsed four times with ice-cold PBS and the cells scraped off into I ml of PBS. The cells were pelleted by centrifugation in a microfuge for 3 min and lysed in ice-cold lysis buffer (50 per 35 mm dish or equivalent). The nuclei were removed by centrifugation through a 24% wt/vol sucrose pad. The upper layer was collected, adjusted to IX PK buffer and digested with proteinase K at 37°C for 30 min. The mixture was phenol extracted (mixing with an equal volume of phenol solution, then 0.5 vol­ umes of chloroform/IAA and the aqueous phase was recovered by centrifugation), chloroform/IAA extracted and the RNA precipitated by adding 2.5 volumes ethanol and incubating at -20°C for at least 2 h. The RNA was recovered by centrifugation and the pellet washed with 75% vol/vol ethanol, 0.1 M sodium acetate (pH 5.2) and dried in vacuo. The RNA was dissolved in TE+S. Occasionally, a second method was used for preparing cytoplasmic RNA. Cell monolayers were rinsed once with ice-cold NET. The cells were lysed by adding I ml of NET plus 1% vol/vol NP-40 and incubating the dishes on ice for 5 min. The lysate was placed in a 15 ml Corex tube, the volume adjusted to 2 ml with NET and 0.2 ml of 10% wt/vol SDS, 0.2 ml of 4 M NaCl and 0.1 ml of 0.2 M EDTA were added. Proteinase K was added to approximately 40 jiig/ml and the mixture was incubated at 50°C for 15 min. It was phenol extracted twice and chloroform/IAA extracted twice. Sodium chloride was added to a final concentration of 1.4 M and the RNA was ethanol precipitated. The RNA was 50 recovered by centrifugation, the pellet washed with 70% vol/vol ethanol, dried in vacuo and dissolved in TE+S. Nuclear Fraction RNA Nuclear fraction RNA was prepared by resuspending the pelleted nuclei, from the cytoplasmic RNA extraction procedure above, in lysis buffer. The DNA was sheared by forcing the suspension through a 20-gauge needle. The nucleic acids were extracted as described above for cytoplasmic RN A. The DNA was removed by digestion with R Q l DNase. Total Cell RNA The acid guanidinium thiocyanate-phenol-chloroform extraction method of Chomczynski and Sacchi (45) was used. Cells were solubilised in 2 ml solution D per 10 cm diameter dish. To this was added 0.2 ml 2 M sodium acetate (pH 4.0), 2 ml phenol and 0.4 ml chloroform/IAA, with thorough mixing between additions. The mixture was vortexed vigorously for 10 s and incubated on ice for 15 min. The aqueous and organic phases were separated by centrifugation at 10,000 g for 20 min at 4°C. An equal volume of isopropanol was added to the aqueous phase and the RNA precipitated at -20°C for at least I h. The RNA was recovered by centrifugation, redissolved in 400 /ri solution D and reprecipitated in an equal volume of isopropanol at -20°C. The RNA was recovered by centrifugation, washed with 75% vol/vol ethanol, 0.1 M sodium acetate (pH 5.2), dried in vacuo and dissolved in TE+S. 51 Virus Purification and RNA Extraction Tissue culture fluids from infected cells were initially clarified by centrifuga­ tion at 4000 g and 4°C for 20 min. The tcf was adjusted to pH 6.5 by addition of 0.1 volumes of 10X PNE. Large volumes were concentrated by tangential flow ultrafiltration, using a Minitan unit (Millipore) with 100,000 molecular weight limit filters. Virus was recovered from the Minitan retentate or unconcentrated tcf by one of three methods. 1) Virus was pelleted by centrifugation at 115,000 gmax for 3 h at 4°C. If the virus was to be further purified, the pellets were resuspended in PNE. If the viral RNA was to be extracted directly, the pellets were either resuspended in lysis buffer, and processed as described for cytoplasmic RN A, or solubilised in solution D, and processed as described for total cell RNA. 2) For rough virus purification, retentate or tcf was underlaid with 20% wt/wt glycerol in PNE, which was underlaid with 80% wt/wt glycerol in PNE. These discontinuous gradients were centrifuged at 275,000 gmax for 1.5 h. For extraction of viral RNA, the interface between the glycerol layers was collected, diluted with one volume of 2X PK buffer and processed as described for cytoplasmic RNA. 3) For very pure virus, retentate or tcf was layered on top of a 20-0% wt/wt glycerol, 0-40% wt/wt potassium tartrate isopycnic gradient (108). Both compo­ nents were prepared in PNE. The gradients were centrifuged at 90,000 gav for 16 h at 4°C. The gradients were fractionated and fractions with a density between 1.11 and 1.19 g/ml pooled, diluted with PNE and virus recovered by centrifugation as described under I) above. If desired, viral RNA was purified by sedimentation on 10-30% wt/wt sucrose gradients, prepared in TNES. The viral RNA was denatured by incubation in 52 50% vol/vol DMSO at 70°C for 5 min and diluted to 25% vol/vol DMSO with TNES before layering on the gradient. Gradients were centrifuged for 4.5 h at 275,000 gmax and 20°C and fractionated. For detecting BVDV vRNA an aliquot of each fraction was either TCA precipitated, if the RNA was radiolabelled, or dot blotted and hybridised with a BVDV-specific probe. Radiolabelling of Viral RNA [32Pj-Iabelled RNA was prepared essentially as described previously (246). Briefly, monolayers of MDBK or BT cells were infected with virus in DME-2 or mock infected with DME-2 alone. Virus was allowed to adsorb for I h at 37°C. The inoculum was aspirated and fresh DME-2 added. This was replaced 4 h prior to the addition of label with phosphate free DME-2, supplemented with dialysed equine serum and buffered with MTH. Immediately before adding label the medium was replaced with fresh phosphate free DME-2, to which was added [32P]-orthophosphate to a final concentration of 150 /xCi/ml. When desired, AMD was added to the DME-2 and the cells incubated for 10 min before adding label. Cytoplasmic and virion RNA were extracted, after the desired labelling period had elapsed, as described above. Labelling of RNA with [3Hj-Uridine was performed basically as for [32Pjlabelling except deficient medium was not used. After infection, the cells were maintained in DME-2. Labelling was carried out in fresh DME-2 to which [5,6-3Hj-uridine was added to a final concentration of 50 juCi/ml. When required, AMD was added to the medium as described above. 53 Preparation of A DNA Molecular Weight Markers Lambda DNA was digested with the restriction endonucleases LTmdIII or Sail, according to the. suppliers instructions. To label the 5’ termini of the fragments the forward reaction was used (178). Five picomoles of 5’ restriction fragment ends (10 of H indlll digested DNA; 26.7 ng of Sail digested DNA) were dephosphorylated using CIP in 50 /d of CIP buffer. The DNA was phenol extracted, purified by Sephadex G-50 chromatography and ethanol precipitated. The DNA was recovered by centrifugation, the pellet dried and dissolved in water. The 5’ ends were labelled using 20 units of T4 polynucleotide kinase in 50 jul of PNK buffer containing 150 ^Ci [y-32P]dATP (GammaPrep-A, Promega). The DNA was purified as described above. Agarose Gel Electrophoresis of RNA Formamide Gels These were prepared as previously described (246). Twenty centimetre, 1% wt/vol agarose gels were prepared in RNA-TAE buffer. Prior to electro­ phoresis, 8 jjA of RNA dissolved in TE+S was mixed with 12 jul of deionised formamide buffered with 2 mM phosphate (pH 7.0) and incubated at 65°C for I min. Twenty microlitres of a 1:1 mixture of glycerol and deionised formamide, containing 0.2% wt/vol agarose beads and bromophenol blue, were added and the sample was loaded on the gel. Electrophoresis was overnight at 50 Vi If desired, gels were stained with ethidium bromide (10 jUg/ml in RNA-TAE buffer minus formamide) for 30 min, destained for I h i n water and photographed. For autoradiography, gels were fixed in 10% vol/vol formaldehyde for 30 min, washed in water for 30 min, blotted onto Whatman DE81 paper and autoradiographed. 54 Glyoxal Gels These were prepared according to the protocol of McMaster and Carmichael (191). In general, 0.7% wt/vol agarose gels (20 cm x 22 cm) were prepared in 10 mM sodium phosphate (pH 6.65). The RNA samples were denatured at 50°C for I h i n glyoxal solution. The samples were chilled on ice and 0.1 volumes of 50% vol/vol glycerol, saturated with bromophenol blue, were added. Electro­ phoresis was at 100 V with buffer recirculation. Gels were stained either for 10 min with acridine orange (33 Mg/ml in 10 mM phosphate buffer) and destained in three changes of 10 mM phosphate buffer in an enameled metal pan (191) or for 20 min in 50 mM NaOH, 0.5 jug/ml ethidium bromide then for 40 min in 0.1 M tris-HCl (pH 7.4), 0.5 Aig/ml ethidium bromide. The gels stained with acridine orange were photographed with the aid of a red 25A filter (Kodak). For autoradiography, gels were either fixed and blotted as described above for formamide gels or fixed in 20% vol/vol methanol in 10 mM sodium phosphate and dried down onto 3MM paper (Whatman). For fluorography the gels were soaked for 10 min in Fluoro-Hance (Research Products International Corp.), blotted or dried down as described above and exposed to preflashed X-ray film (156). Northern Transfers RNA was transfered from glyoxal gels to nitrocellulose (Schleicher and Schuell, BA85) essentially as described by Thomas (275). Transfers were performed in IOX SSPE overnight. Filters were dried and baked for 2 h at SO0C in vacuo. The filters were treated with 20 mM Tris-HCl (pH 8.0) for 5 min at 65°C to reverse glyoxylation. Filters were stored dry at room temperature. 55 RNA Dot Blots Samples for RNA dot blots were either denatured with glyoxal solution, as described above for glyoxal gels, or with 7.5% vol/vol formaldehyde (63). The RNA was adjusted to a final concentration of 15X SSPE and, if desired, serially diluted in 15X SSPE. Nitrocellulose was prewet in water, equilibrated in IOX SSPE and placed on top of two sheets of 3MM paper soaked in IOX SSPE ,. in a Biorad manifold. Wells were rinsed with 0.5 ml IOX SSPE, the samples applied to the filter and the wells rinsed with a further 0.5 ml of 10X SSPE. The filters were dried and baked, as described above for northern transfers. Agarose Gel Electrophoresis of DNA Electrophoresis of DNA was performed in minigels, either after denaturation with glyoxal as described above or as previously described (204). In the latter case, 0.8% wt/vol agarose minigels were prepared in DNA-TAE buffer. One microgram of DNA dissolved in 15 /d of TE8 was mixed with 5 /d of 0.2% wt/vol . agarose beads in 10% glycerol, 10 mM Tris-HCl (pH 7.5), 10 mM EDTA satu­ rated with bromophenol blue. To this was added 2.5 //I of 80% vol/vol glycerol saturated with bromophenol blue. Electrophoresis was at 50 V for 2 h. The gels were stained with ethidium bromide and photographed. Preparation of Plasmid DNA Transformation of Escherichia coli DH5 Transformation of Escherichia coli (E. coli) DH5 cells was performed using the basic calcium chloride protocol (263). To prepare competent cells, 200 ml culture of E. coli DH5 in LB was incubated at 37°C, until the culture reached an 56 OD590 of 0.375. The culture was chilled on ice and the cells collected by centrifu­ gation at 1,000 g for 7 min. They were gently washed with ice cold CaCl2 solution, resuspended in 40 ml of CaCl2 solution and incubated on ice for 30 min. The cells were collected as above, resuspended in 8 ml CaCl2 solution and left on ice overnight. The pBV4-p80 plasmid DNA preparation provided by Mark Collett was diluted in CaCl2 solution to give a final concentration of I Mg/^l- To 10 /il of this DNA solution was added 100 /d of the competent E. coli DH5 suspension. After incubation on ice for 10 min, the cells were heat-shocked at 42°C for 2 min, I ml of LB added and the suspension incubated for I h at 37°C. The cells were diluted in SOC medium and spread on LB+Amp agar plates, which were incubated overnight at 37°C. Screening of Transformants Transformants were screened by alkaline lysis plasmid minipreps (159). Individual colonies from the LB+Amp plates were inoculated into 10 ml of LB+Amp and incubated overnight at 37°C. A 1.5 ml aliquot of each culture was pelleted by spinning in a microfuge for 20 s. The cells were resuspended in 100 ^l TE8 and incubated at room temperature for 5 min. Cells were lysed by adding 200 ii\ 0.2 M NaOH, 1% wt/vol SDS and incubating on ice for 5 min. After adding 150 ^l 3 M potassium acetate the lysate was vortexed, incubated on ice for 5 min and debris removed by centrifugation. Two volumes of 95% ethanol were added, followed by incubation at room temperature for 2 min. Nucleic acids were pelleted, washed with 70% vol/vol ethanol and dried. After redissolving in 20 /rl TE8, RNA was removed by digestion with 10 ng DNase-free RNase. Plasmid DNA was analysed by electrophoresis in agarose gels. 57 Large Scale Plasmid Purification Large scale plasmid purification involved the use of the alkaline lysis method for producing crude cell lysates, followed by purification on CsCl gradients (128). One litre of LB+Amp was inoculated with a 10 ml overnight culture of E. coli DH5 transformed with pBV4-p80 and incubated overnight at 37°C. Cells were recovered by centrifugation at 5,000 g for 10 min, resuspended in 40 ml GTE and lysed by mixing gently with 80 ml 0.2 M NaOH, 1% wt/vol SDS, followed by incubation for 10 min at room temperature. Potassium acetate (pH 4.8) was gently mixed in to a final concentration of I M. After incubating at room tem per­ ature for a further 5 min, debris was removed by centrifugation at 5,000 g for 10 min. The supernatant was carefully decanted off, I volume of isopropanol added and the nucleic acids allowed to precipitate at room temperature for 45 min. Nucleic acids were pelleted, lyophilised until nearly dry and redissolved in 8 ml TE8. To purify pBV4-p80 plasmid DNA, the nucleic acids were sedimented on CsCl isopycnic gradients (128). The nucleic acid solution was adjusted to a final volume of 10 ml in TE8, 0.4 mg/ml ethidium bromide, in which was dissolved 9.5 g CsCl. This solution was dispensed into two 12 ml centrifuge tubes and overlaid with mineral oil. The tubes were centrifuged for 45 h in an SW 41 Ti rotor at 210,000 g and 20°C. Plasmid bands were visualised by U.V. light and the bottom band collected by side-puncture of the tube. Ethidium bromide was removed by repeated extractions with water-saturated n-butanol. The DNA was desalted by Sephadex G-50 chromatography. The plasmid DNA was characterised by digestion with restriction endo­ nucleases. 58 Purification of the pBV4-p80 BVDV cDNA segment The BVDV cDNA segment was excised from pBV4-p80 by digestion with restriction endonucleases Kpnl, Xbal and Bgll (Figure 4). For purifying the BVDV cDNA insert, lorn melting temperature agarose was used (264). The fragments were electrophoresed on a 1% wt/vol low melting agarose gel, prepared in DNA-TAE buffer. The largest DNA band was excised, the agarose melted at 65°C, diluted in LSB and the DNA recovered using an Elutip-d column (Schleicher and Schuell). Preparation of BVDV-specific Probes Nick Translation of cDNA This was performed as previously described (204). Briefly, 0.5 /zg cDNA was incubated with I ng DNase I, 6 U DNA polymerase I, 125 pCi a-32P-dCTP and 2 pM dNTPs in 50 pi of NT buffer at 15°C for 2 h. Unincorporated nucleotides were removed by Sephadex G-50 chromatography. In vitro Transcription of pBV4-p80 For SP6 RNA polymerase transcription, to produce BVDV-specific plus sense RNA probes, pBV4-p80 plasmid DNA was linearised by digestion with Xbal. For TV RNA polymerase transcription, to produce BVDV-specific minus' sense RNA probes, the plasmid DNA was digested either with Kpnl, followed by digestion with 5 U//zg Klenow fragment of DNA polymerase I to remove 3’ overhanging ends, or with Hpal. In vitro transcription was performed using a Riboprobe Gemini System II kit. Reactions were performed with 0.5 /zg linearised pBV4-p80 DNA and 50 pCi O--32P-CTP, in a volume of 20 /zl, according to the manufacturer’s instructions. 59 KPn ' Wpa I BVDV cDNA Figure 4. Plasmid pBV4-p80. The map shows the restriction sites used in extrac­ tion of the BVDV cDNA and in in vitro transcription. SP6 transcrip­ tion gives viral-sense RNA; T l transcription, viral complementary RNA. Template DNA was removed by digesting with I U RQ l DNase at 37°C for 15 min. Hybridisations Northern and dot blot hybridisations with cDNA probes were performed as described previously (204). Nitrocellulose filters were prehybridised in prehybridi­ sation buffer (0.05 ml per cm2 of filter area) for 3 to 6 h at 42°C, in heat-sealed 60 polythene bags. The prehybridisation solution, was replaced with hybridisation solution (0.01 ml per cm2 of filter area) containing IxlO6 cpm/ml of 32P-Iabelled cDNA probe. Hybridisations were incubated at 42°C overnight. The next day, the filters were washed twice in 2X SSPE, 0.1% wt/vol SDS for 10 min at room temperature; twice in 0.1X SSPE, 0.1% wt/vol SDS at room temperature; and in 0.1X SSPE, 0.1% wt/vol SDS at 65°C for I h. The damp filters were wrapped in plastic film and autoradiographed. Hybridisations with RNA probes were performed as described for cDNA probes with the following exceptions. Prehybridisations and hybridisations were performed at 55 to 60°C. The filters were washed twice with IX SSPE for I h at 65°C and with 0.1X SSPE for I h at 60°C. If filters were to be reprobed, the old probe was stripped off by rinsing them with boiling water. Detection of 3’ Polvadenosine Sequences Half a microgram of dephosphorylated oligo (dT)12„18.was end labelled using T4 polynucleotide kinase as described above. Labelled oligonucleotides were recovered by affinity chromatography on DEAE Trisacryl (LKB). The oligonuc­ leotides were bound in TE8 and eluted in TE8, I M NaCl. Filters to be probed were prehybridised at 25°C for I h in 20 ml of 6X SSPE, 0.1% SDS, 5X Denhardt’s solution. The filters were hybridised overnight at 25°C with IO6 cpm/ml of [32P]-labelled oligo (dT) in 10 ml of 6X SSPE, 0.1% SDS, IX Denhardfs solution, 200 ng/ml tRNA. The filters were washed three times with 3X SSPE, 0.1% SDS at room temperature and then for 10 min in IX SSPE, 0.1% SDS at 25°C. The filters were blotted dry, wrapped in plastic film and autoradiographed. 61 Quantitation of BVDV-specific RNA Plus and minus sense RNA was transcribed from pBV4-p80, as described for preparation of RNA probes, except reactions were carried out in a volume of 100 /il with 2 fig of linearised DNA and 0.5 mM CTP for 2 h. The RNA was glyoxalated, as described above, and diluted to give a range of concentrations of 0.316 to 1000 pg in 50 /d 12X SSPE. Total cell RNA was glyoxalated and diluted to a final concentration of 5, 0.5 and 0.05 Hg in 50 ^l of 12X SSPE. Duplicate dot blots were then prepared, as described above. Filters were hybridised at SB0C overnight with either IxlO6 cpm/ml of minus sense RNA probe or 2xl06 cpm/ml plus sense RNA probe. After washing, the filters were dried and both filters autoradiographed on the same sheet of preflashed X-ray film. For calculating the RNA concentration, the autoradiographs were scanned with a Hoefer GS-300 scanning densitometer and the peaks integrated using the Hoefer GS-350 Data System software supplied with the instrument. Linear regression analysis was performed on the integrated values for the in vitro tran­ scribed, serially diluted RNA samples, using Sigmaplot 4.0 (Jandel Scientific) to provide calibration curves for BVDV-specific RN A. Only curves with an R value greater than 0.98 were used. The BVDV-specific RNA content in the cellular RNA samples was calculated by the standard equation, y - mx + b These were corrected for nonspecific binding by subtracting the value for mockinfected cell RNA. Values which did not exceed the maximum value for the calibration curve were corrected to picograms of BVDV RNA per microgram of total cell RNA using the following equation, 62 pg BVDV-specific RNA i Atg RNA in sample N length of BVDV genome (12.6 kb) :------------ length of BVDV sequences in probe The mean BVDV-specific RNA concentration was then calculated for each time point from the values for individual dilutions and. exposures. 63 RESULTS Selection of Cell Lines A major complication of studying BVDV in cell culture is that ncBVDV contaminates many, if not all, lots of commercially prepared bovine serum (139,206,252). This can result in infection of susceptible cells, grown in medium containing contaminated serum. Any cell line, to be used in the study of BVDV, has to be free of the virus and maintained in this pristine state. Another problem, encountered in the use of bovine serum, is the presence of BVDV neutralising antibody. Three cell lines were considered for use in these studies: MDBK, BT and BLF cells. All three cell lines were shown to be free of BVDV by direct immuno­ fluorescence. All three cell lines supported the growth of BVDV. To obviate the potential problems of maintaining the cells in bovine serum supplemented medium, the growth of the different cell lines in KClOO serum-free medium (the kind gift of Paul Baker) and in medium supplemented with equine serum was studied. Only MDBK cells grew in KClOO medium and they grew very slowly. None of the cell lines were adversely affected by growth in equine serum (Figure 5). I decided to use MDBK cells for my research, as they grew much faster than the other two cell lines and, being a permanent cell line, were not likely to undergo crisis at some point in the future. Some experiments were also per­ formed using BT cells. 64 96 108 120 Tim e (h) Figure 5. Growth of MDBK cells in KClOO serum-free medium (■) or DME supplemented with either 10% equine ( • ) or 10% foetal bovine ( A) serum. Cvtopathic Effect of the BVDV Strains The project initially involved the comparison of four strains of BVDV. These were the noncytopathic strain New York-1 and the cytopathic strains Singer, NADL and Oregon C24V. Due to the different cytopathology of Oregon C24V, it is classified as being a different biotype from the other two cBVDV 65 strains (93). The Singer and NADL strains were highly cytopathic. They produced extensive vacuolation in infected cells by 24 h pi. Total destruction of the cell monolayers occured by 36-48 h pi. Oregon C24V was less cytopathic. Vacuolation of infected cells was observed, though this occurred later than with Singer or NADL. The cell monolayer was rarely extensively damaged, lysis of cells appearing to be due to coalition of the vacuoles, rather than the cellular degeneration observed with the other cytopathic strains. In the case of cells infected with the noncytopathic strain, New York-1, no cellular damage was observed. The nature of the cytopathologies of the four strains seemed to influence the amount of viral antigen present in the cells. When infected cells were studied by immunofluorescence, Singer and NADL-infected. cells fluoresced brightly; Oregon C24V-infected cells fluoresced less brightly; and New York-l-infected cells fluoresced poorly (data not shown). Titration of Infectious Virus The variation in cpe between the strains influenced the method by which they were titrated. Singer and NADL were titrated using standard plaque assays, in which the cell monolayers were stained either with neutral red or with crystal violet. Oregon C24V was also titrated by plaque assay but plaques were only readily detected by neutral red staining (Figure 6). Plaques were occasionally observed after prolonged staining of New York-l-infected cell monolayers with neutral red (data not shown) but this was not reproducible, so a different assay method was sought. Several alternative assays have been developed to titrate ncBVDV strains but these were indirect, time consuming, statistically inaccurate or not comparable with plaque assays (90,95,105,140,141,149,176,210,231,271). To 66 Figure 6. Plaques of BVDV strain Oregon C24V: comparison of staining with (A) neutral red and (B) crystal violet. The monolayers were stained 4 days after infection. obviate these drawbacks, an end point dilution assay, originally developed for titrating SV40 (243), was adapted for the titration of BVDV (245). As this assay involved indirect immunofluorescence for detecting BVDV-infected cells, BVDVspecific antiserum had to be produced. Raising of BVDV-specific Antiserum When initially setting up the EPDMA, bovine hyperimmune serum raised against the NADL strain of BVDV (the kind gift of Steven Bolin), was used. This worked well for assaying cBVDV strains but the assay of New York-1 proved more difficult. The level of virus-specific fluorescence observed was often barely above background, increasing the chance of scoring false positives or negatives. Fernelius (90), showed that fluorescent antiserum raised against ncBVDV is better for detecting both biotypes than that raised against cBVDV. I decided that antiserum raised against ncBVDV strain New York-1 should be used in the assay. 67 • The stock animals of the Veterinary Research Laboratory were screened to identify cattle seronegative for BVDV by a plaque reduction assay. These animals were then screened for persistent BVDV infection, by co-cultivation of PBL with MDBK cells. The MDBK cells were subsequently assayed for the presence of BVDV by indirect immunofluorescence. Three seronegative and BVDV-free animals were identified. One was selected at random and immunised with tcf containing live New York-1 virus. The specificity of the antiserum for BVDV was titrated by comparing the immunofluorescence observed on BVDV-infected and uninfected MDBK cells. With uninfected cells, a 1:12 dilution of the antiserum resulted in the elimination of nonspecific fluorescence, while BVDV-infected cells were readily detected. The cells infected with BVDV had a distinctive fluorescence that was confined to their cytoplasm (Figure 7); uninfected cells either did not fluoresce or had a low level of fluorescence over the entire cell. End Point Dilution Microtitration Assay BVDV-specific fluorescence could be detected as early as 12 h pi in MDBK cells (data not shown). With care, plates could be scored after 24 h but the accuracy of the microtitration assay was enhanced by incubating the cells either 48 h, for titration of cBVDV strains, or 72 h, for titration of ncBVDV strains. This allowed the virus to propagate, amplifying the viral antigens in infected wells, resulting in more rapid and accurate scoring of the plates. The wells infected with higher virus dilutions had discrete foci of fluorescence that were easy to score. With lower virus dilutions the fluorescence was less distinct, due, presumably, to the adsorption of the primary BVDV-specific antiserum over the entire monolayer. This meant that the secondary antiserum was also widely distributed, 68 Figure 7. Focus of infection of the ILLC strain of BVDV on MDBK cells. The cells were fixed 72 h pi. Virus-infected cells were detected by indirect immunofluorescence. X150. resulting in an apparent reduction in fluorescence, causing the wells to appear uninfected. For this reason, the plates infected with higher virus dilutions were scored first. The virus titre was calculated from the Poisson distribution using the following equation; P(O) = e m where P(0) is the number of uninfected wells and m is the multiplicity of infection per well (IU per 10 /d). For an example of how the virus titre is calculated, consider-a Terasaki plate where 24 of 36 wells were uninfected at a dilution of 10‘6. This gives a value of P(O) of 0.67 (24/36) so m equals 0.41 (-In P(O)). To 69 find the titre per millilitre of the original virus stock, m is divided by the product of the volume of the well (0.01 ml) and the dilution (IO"6). The result is a titre of 4. IxlO7 lU/ml. . Large statistical errors occur when litres are calculated from plates where either very few or most of the wells are infected (243). The accuracy of the assay is also dependent on the number of wells used per dilution. In general, half the wells of a Terasaki plate, 30 or 36 wells depending on whether it was a 60 dr 72 well plate, were used per dilution. Comparison With Plaque Assay To assess the accuracy of the microtitration assay as compared with a plaque assay, ten strains of BVDV (6 cBVDV and 4 ncBVDV) were titrated using the two methods. Four of the strains compared were the standard laboratory strains studied throughout this project, the other six were paired strains isolated from animals with naturally acquired MD (Steven Bolin, personal communication). Plaque assays were carried out in duplicate plates. After 4 days, one plate was stained with neutral red and the other with crystal violet. The titre was taken as the average of the duplicate plates, except for Oregon C24V where only the plate stained with neutral red was considered. The litres determined by the individual assays were in very close agreement (Table 8). Although the data presented were from a single experiment, similar results were consistently produced. The detection of plaques of the ncBVDV SSDN strain, indicated that this putatively noncytopathic strain was contaminated with cBVDV. This was not surprising as this strain, and the cBVDV SSDC strain, were recovered from an animal suffering from chronic BVD (Steven Bolin, 70 personal communication); the two BVDV biotypes present had obviously not been totally separated from each other in the ncBVDV stock. Table 8. Comparison of BVDV titres determined by plaque assay and EPDMA. Strain Virus titre Plaque assay EPDMA (PFU/ml) (lU/ml) Singer NADL LQxlO7 7. IxlO6 LlxlO7 6.9xl06 Oregon C24V 5.3x107 5.4xl07 a 2.5xl07 TGAC 5.6x10s 4.9xl05 TGAN a 3.6xl06 ILLC 2.2xl06 3.6xl06 ILLN a 4.9x106 SSDC 7.9xl06 9.4xl06 SSDN 6.0xl05 l.OxlO6 New York-1 a. Plaques not detected; noncytopathic. Biological Cloning of BVDV Strains For consistency, I decided to biologically clone the four strains by limiting dilution. The viruses were subjected to six rounds of selection, two wells of the Terasaki plate being selected at each round for subcloning. At one point, subcloned NADL failed to yield any virus. MDBK cells were infected with the remaining seven infected tcf s from the previous round of NADL cloning. Two of these yielded virus, which was further subcloned. 71 , Of the final five or six clones of each strain, one was selected at random for further study. These were Singer E6, NADL B7, Oregon C24V D9 and New York-1 CS. Virus Growth Kinetics The first step, in developing a protocol for the purification of BVDV, was to ascertain the time postinfection that maximum amounts of virus were present. The growth curves of the four strains, in terms of both released and cell-associated virus, are shown in Figure 8. The high levels of free virus initially observed were probably residual virus from the inoculum. The level of free virus was always greater than that of cell-associated virus. The litres of Oregon. C24V were somewhat lower, probably because the virus samples were frozen and thawed three times, whereas the other virus samples were only frozen and thawed twice. Each strain had a latent phase for cell-associated virus of approximately 9 h. In the case of strain New York-Ii the latent phase for released virus and for cellassociated virus appeared to be the same. However, the three cBVDV strains all had a longer latent phase for released virus of 12-15 h. The rate of cBVDV production appeared to be higher than that of ncBVDV during the logarithmic phase. The peak litre of Singer and NADL occurred at approximately 30 h, that of Oregon C24V at 36 h and that of New York-1 at 42 h. Virus was collected at these times for subsequent virus purifications. Purification of BVDV Concentration of BVDV from large volumes of tcf was readily achieved by the use of a Minitan Ultrafiltration System, with 100,000 molecular weight limit filters. The retentate from the Minitan could be further concentrated using a 72 S in g er O regon C24V 0 12 2 4 36 48 60 72 8 4 0 NADL New Y o r k - 1 12 2 4 36 4 8 60 72 8 4 Time p o s ti n f e c ti o n (h) Figure 8. One step multiplication curves of four BVDV strains. Titres are PFU/ml except New York-1 which is lU/ml. ( • ) free virus, (O) cellassociated virus. 73 CX-30 filter unit (Millipore). This resulted in the rapid concentration of virus, with negligible loss of viability. Table 9 shows the recovery of BVDV strain Oregon C24V during concentration by ultrafiltration. Table 9. Recovery of BVDV during concentration. Sample Volume (ml) Virus titre (RJ/ml) Total virus in sample (IU) % virus recovery Medium 1,100 2.3x10? 2.5xl010 100 Minitan fil­ trate 1,120 I.'2xl04 IJxlO 7 0.05 Minitan retentate 41 6.3xl08 2.6xl010 104 CX-30 retentate 9 3.4xl09 3.IxlO10 124 The purification of the virus was complicated by its heterogeneous size and density. Several approaches were assessed: sedimentation on Percoll, potassium tartrate or glycerol-tartrate density gradients; and Sephacryl S-1000 column chromatography. Percoll gradients and Sephacryl S-1000 column chromatography failed to resolve the virus as a discrete peak (data not .shown). Figure 9 shows a comparison of BVDV sedimentation on a 10-40% wt/wt potassium tartrate gradient and a 30-0% wt/wt glycerol, 0-50% wt/wt potassium tartrate gradient. The glycerol-tartrate gradient resolved the virus as a single broad peak. The potassium tartrate gradient resolved the virus as an initial peak in the same density range as the peak on the glycerol-tartrate gradient, but most of the virus was recovered from the bottom of the gradient. Potassium tartrate isopycnic density gradients generally resolved the virus as two peaks; a broad peak at a density of 1.13-1.15 g/ml and a sharp peak at a density of 1.22 g/ml (data not shown). Glycerol-tartrate gradients resolved the virus as one broad peak at a 74 D ensity (g /m l) Figure 9. Comparison of sedimentation of NADL strain of BVDV on potassium tartrate (O) and glycerol-tartrate (#)density gradients. density of 1.13-1.17 g/ml. A gradient of 20-0% wt/wt glycerol, 0-40% wt/wt potassium tartrate gave the best resolution of virus (data not shown). These conditions were used in all subsequent virus purifications. The virus appeared as a diffuse band at a density of 1.11-1.19 g/ml. This band contained 90 to 95% of the virus loaded on the gradient (data not shown). For recovery of virus, gradient fractions in this density range were pooled, diluted and centrifuged. The virus pellets were often quite loose, so the supernatant had to be carefully decanted. 75 Sizing of the BVDV Genomic RNA A time course study of BVDV-specific RNA synthesis in Singer-infected MDBK cells, indicated that BVDV-specific cytoplasmic RNA concentration peaked at approximately 18 h pi (data not shown). Cytoplasmic RNA was labelled with 32P-Orthophosphate in the presence of AMD. Surprisingly, in addition to BVDV-specific RNA, two lower molecular weight bands were seen. These were not subgenomic BVDV mRNAs, as they were also present in the mock-infected control RNA samples. The two bands disappeared after RNase treatment, as did the Singer mRNA band (data not shown). They were not present in intracellular RNA from BT cells, indicating that they were cell-specific. Recently, the same bands were described in MDBK cells from the same source (259). As I felt that these bands might interfere with the identification of BVDVspecific RNAs, subsequent studies were carried out in BT cells. For comparing intracellular BVDV-specific RNAs, BT cells were pulsed for 6 h with 32P-orthophosphate, in the presence and absence of AMD, prior to RNA extraction. Cytoplasmic RNA was extracted at the assumed peak of RNA concentration and peak of virus production for each strain: Singer and NADL 18 h and 30 h pi; Oregon C24V 22 h and 36 h pi; and New York-1 24 h and 42 h pi, respectively. The RNA was electrophoresed on an agarose-formamide gel. The autoradiograph of the gel showed that a high molecular weight RNA species was present in cells infected with Singer, NADL and Oregon C24V but not in the mock-infected control or New York-l-infected cell RNA samples (data not shown). This RNA was more prominent at the time of peak RNA synthesis than at the time of peak virus production. It was assumed that this was cBVDV genomic RNA. There was no evidence of any BVDV subgenomic mRNA species. 76 To find out how large the cBVDV genomic RNA band was, the RNA samples were glyoxalated and electrophoresed on a 0.8% wt/vol agarose gel with glyoxalated lambda H indlll fragments as molecular weight markers. The molecu­ lar weight markers were visualised by acridine orange staining. Comparison of the migration of the markers and the genomic RNA band, using the Gel program of Fristensky, indicated, that the BVDV genome was 16.3 kb in size. There was no detectable difference in migration of the genomic RNA band of the three cBVDV strains (data not shown). The inability to detect BVDV genomic RNA in cells infected with New York-1 was puzzling. A series of experiments were performed to address this question. Genome size RNA was only found in New York-1 virions. It was not detected in the cytoplasm or nucleus of New York-1 infected MDBK or BT cells, at the level of sensitivity of in vivo 32P-Iabelling. However, genomic RNA could be detected in cytoplasmic RN A, by probing northern transfers of infected cell RNA with BVDV-specific probes (see below). Comparison with X H indlll fragments indicated that the New York-1 virus genome was 14.8 kb in size, somewhat smaller than that of the cBVDV strains (data not shown). To see if the genomic RNA of the cBVDV strains and New York-1 were actually different sizes, the cytoplasmic and .virion RNAs of the four strains were again compared. Bovine turbinate cells were labelled for 12 h prior to RNA extraction. Cytoplasmic RNA was extracted from Singer and NADL-infected cells at 18 h pi, from Oregon C24V-infected cells at 21 h pi and from New York-Iinfected cells at 25 h pi. Virions were pelleted directly from tcf and RNA was extracted from the pellets. The RNA was electrophoresed on a Wo wt/vol agarose-formamide gel. The autoradiograph of the gel showed that the different cytoplasmic RNA preparations were not equivalent in terms of the quantity of 77 label present in ribosomal RNA (rRNA), implying that the total amount of RNA was different. As far as possible, the different cytoplasmic RNA preparations were balanced on the basis of 18S rRNA labelling and the virion RNAs on the basis of 32P content. The RNA samples were glyoxalated and electrophoresed on a 0.7% wt/vol agarose gel, with end labelled, glyoxalated A HindlW and A Sail fragments as molecular weight markers. Lambda Sail fragments were included, as one fragment is 15,258 bases, approximately the same size as the BVDV genomic RNA. Although some minor differences were noted between the migration of genomic RNA bands of the four strains, these were not deemed to be significant (Figure 10). The genomic RNA was estimated to be 13.4 in size. As the molecular weight standards were DNA fragments, the estimate had to be correct­ ed for the difference in mass of nucleotides in RNA and DNA. This resulted in an adjusted size estimate of 12.8 kb. Major differences were seen in the ratio of label incorporation into the cytoplasmic BVDV RNA of the different strains, as compared with rRNA. In decreasing order of intracellular BVDV RNA content were Singer, NADL, Oregon C24V and New York-1. As before, no BVDV RNA was detectable in the cytoplasmic RNA sample from New York-l-infected cells. As a final check of the size estimate for the BVDV genome, VSV mRNAs were used as molecular weight standards. Cytoplasmic RNA, labelled with 32P in the presence of AMD for 3 h, was extracted from VSV-infected MDBK cells 5 h pi. Cytoplasmic RNA was extracted from Singer-infected BT cells 18 h pi, after a 4 h 32P pulse in the presence of AMD- Singer virion RNA was also prepared. The RNAs were glyoxalated and electrophoresed on a 0.7% .wt/volume agarose gel. The resultant autoradiograph yielded a size estimate of 12.4 kb for the BVDV genome (Figure 11). 78 > T S Figure 10. Sizing of the genome RNA of BVDV: comparison of viral intracellular (C) and virion (V) RNA with denatured X HindlW (H) and 5fl/I (S) restriction fragments. MI, mock-infected cell RNA. 12.4 kb 11162 .... # W 1672 1333 830 Figure 11. Sizing of BVDV genomic RNA: comparison of BVDV strain Singer mRNA with VSV mRNA. RNA was labelled in the presence of AMD. MI, mock-infected cell RNA. RNA size is expressed in terms of nucleotides. 80 The final size estimate of the BVDV genomic RNA was the average of the estimates from the DNA and RNA markers: 12.6 kb. Northern Blot Comparisons The difficulty encountered in comparing the intracellular RNA species of the four strains, demanded that a more sensitive approach be used. Northern transfers of RNA from glyoxal gels to nitrocellulose were performed. The RNA samples included cytoplasmic and total cell RNA, from BVDV infected and uninfected BT and MDBK cells, and virion RNA. All 10 of the BVDV strains available were represented. Initially, the filters were probed with random primed cDNAs, reverse transcribed from Oregon C24V and Singer vRNAs. These gave rather unsatisfac­ tory results. The next probe used was the BVDV cDNA from the plasmid pBV4-p80. Computer-assisted analysis of the nucleotide sequence of the BVDV cDNA insert, showed that it did not contain restriction sites for the restriction endonucleases Kpnl and Xbal. Restriction sites for these two enzymes flank the restriction site of Sm al in the multiple cloning site of pGEM-4, where the cDNA had been inserted. Digestion of pBV4-p80 with Kpnl and Xbal yielded a fragment containing the whole BVDV sequence plus only 11 nucleotides from the vector. However, the remaining vector was also a single fragment, of a size that could cause problems in gel purification of the BVDV cDNA fragment. I decided that the vector fragment ’ should be digested further. The restriction endonuclease Bgll was chosen, as this would cut the pGEM-4 fragment approximately in half and the restriction site was not present in the BVDV cDNA. Triple digestions of pBV4-p80 were followed by purification of the Kpnl-Xbal fragment by low melting agarose gel electrophoresis. 81 The fragment was nick translated and used to probe northern transfers. The genomic RNA of all the strains was detected on one or more filters (data not shown). The results obtained supported the conclusions from the in vivo 32P-Iabelling studies: no significant difference in the size of the genomic RNAs of the strains and no subgenomic mRNAs were detected. The final probe used was a 32P end labelled oligo (dT)12.18, to see if a 3’ polyadenosine sequence was present on any of the genomic RNAs. With low stringency hybridisations, at 19°C to detect sequences longer than 12 bases, there appeared to be specific hybridisation to Oregon C24V virion RNA and possibly to TGAC virion RNA (data not shown). However, this was accompanied with high nonspecific background. Increasing the stringency of hybridisation, so that only 15-mers or longer sequences would be detected, resulted in no specific hybridisa­ tion to BVDV RN A, though nonspecific binding was markedly reduced. At both high and low stringency, there was considerable hybridisation to cellular mRNAs. This shows that the genomic RNA of all the strains was not 3’ polyadenylated but that Oregon C24V and TGAC may have short stretches of adenosine residues. Time Course of BVDV RNA Synthesis The differences in cytoplasmic BVDV RNA levels observed between strains, that did not appear to be associated with lower virus yields, indicated that there might be differences between the strains in terms of the control of RNA synthesis or RNA packaging. To address this question, six BVDV strains were compared on the basis of the time course of plus and minus sense RNA synthesis, in infected MDBK cells. For quantifying the RN A, in vitro transcribed BVDV-specific plus and minus sense RNA was prepared, using pBV4-p80 as a template. For SP6 RNA 82 polymerase transcription, to produce plus sense BVDV-specific RNA, the plasmid was digested with restriction endonuclease Xbal. Gel electrophoresis of the RNA transcripts showed that they were homogeneous; a single RNA species, that migrated to the same position as denatured pBV4-p80 Kpnl-Xbal fragment DNA (data not shown). Transcription with T7 RNA polymerase proved to be more of a problem. Using pBV4-p80 digested with Kpnl as the template resulted in a heterogeneous RNA product: a smear of RNA, the same size as or larger than the SP6 transcript (data not shown). This was due to the 3’ protruding ends produced by digestion with Kpnl (227). To overcome this problem, the 3’-5’ exonuclease activity of the Klenow DNA polymerase was used to blunt end the template prior to transcription. This greatly reduced the heterogeneity of the RNA product, resulting in a major band the same size as the SP6 transcript (data not shown). The smear was still evident after gel electrophoresis though. For the preparation of total infected cell RNA, two sets of 10 cm diameter dishes were seeded with, MDBK cells 10 h apart. They were also infected 10 h apart, at an moi of 4. Virus was allowed to adsorb for I h. The inoculum was rinsed off, the monolayer washed twice with DME-O and 5 ml of DME-2 placed in each dish. One hour after the second set of dishes had been infected, and every 2 h thereafter, total cell RNA was extracted from one dish of each set. This gave a range of samples from I h to 24 h pi. An initial attempt at quantifying the BVDV-specific RNA in total cell RNA with dot blots was somewhat flawed. A basic problem was that the samples were on two filters. Even though both filters were calibrated in the same way, they did not seem to match. This may have been due to differences in hybridisation conditions. Low stringency hybridisation conditions were used, which resulted in high levels of hybridisation to mock-infected cell RNA. Formaldehyde was used 83 to denature the RNA prior to blotting, which appeared to inhibit the degree of hybridisation. To quantify the hybridisations, the filters were cut up and the individual dots were scintillation counted. This was inherently inaccurate, as distortion of the filter during the hybridisation process made it difficult to accu­ rately locate the dots. The calibration curves appeared to lose linearity above I ng of target RNA. I was also concerned by the heterogeneity of the RNA used for the minus sense RNA calibrations and probes, derived from Kpnl digested pBV4-p80 templates. I decided that it would be better to linearise pBV4-p80 with a different restriction endonuclease, that did not produce 3’ overhanging ends. No suitable restriction site was available in the multiple cloning site ,of pGEM-4. Analysis of the BVDV cDNA sequence showed that a SgZII site was located at the very end of the cDNA sequence. Attempted digestion of pBV4-p80 with SgZII showed that this restriction site was not present. A Hpal restriction site, 149 nucleotides into the BVDV cDNA sequence, was present, so this enzyme was used for linearising pBV4-p80. This template was used for providing the minus sense RNA for calibrations and probes for plus sense RNA. The dot blot and hybrid detection protocols were modified in the light of the problems encountered in the initial study. The concentration of RNA in the total cell RNA preparations was remeasured. The RNA samples were glyoxalated to denature them. The range of RNA concentrations were reduced for both the cellular RNA and the in vitro transcribed RNA. All samples were applied to a single pair of duplicate filters. Hybridisation conditions were more stringent and carefully controlled and plus and minus sense RNA hybridisations were performed at the same time to match conditions for each. The filters were autoradiographed on the same sheet of preflashed X-ray film and the hybrids were quantitated by 84 3# < z DC O 3) SP6 ♦ Ml I Time pi Ihl 3 5 7 9 11 # # 5 12 # 1 0 0 0 pg 0.5 0.0 5 0.05 IOOOpg t 0 05 # 5 # 13 14 Ml # 0.5 • 0 .05 1 * 0 # # # # # 16 18 2 0 22 2 4 3 7 5 9 0.316p g 11 12 O 0 05 0.5 5 0.316 pg • # » # # # # 13 14 16 18 2 0 2 2 24 Figure 12. Quantitation of BVDV intracellular RNA. The illustration shows an autoradiograph of dot blots probed for BVDV NADL intracellular RNA. SP6 is the plus sense RNA calibration, T7 is the minus sense RNA calibration. 85 scanning the autoradiographs with a Hoefer GS-300 densitometer. These modifi­ cations resulted in the production of much cleaner data. A typical autoradiograph is shown in Figure 12, with the calibration curves for plus and minus sense RNA derived from it shown in Figure 13. The system was able to detect as little as I pg of BVDV RNA. 15000 12500 10000 7500 5000 2500 B VD V R N A (p g ) Figure 13. Calibration curves for BVDV NADL plus ( • ) and minus (O) sense RNA, derived from Figure 12 as described in the text. Above 100 pg of RNA the curves were no longer linear. As a further check, 5 jug of each RNA preparation was electrophoresed on a 0.8% wt/vol agarose gel. The ribosomal RNAs were visualised by UV shadowing to check the integrity of the RNA. In no case was any degradation observed and the concentrations were consistent between samples. The RNA was then transfered to nitrocellulose by northern transfer. 86 A cursory analysis of the RNA synthesis curves, generated from the dot blot autoradiographs, showed that there was a marked difference in the levels of intracellular BVDV RN A, of both senses, between strains (Figures 14,16-20). BVDV NADL RNA Synthesis As NADL was the BVDV strain the cDNA was cloned from, I shall describe the results with this strain first. Both plus and minus sense RNA were first detectable, at very low levels at 7.2 h pi and 9 h pi, respectively, but were not really evident until 12.25 h pi (Figure 14). There appeared to be an initial peak of RNA synthesis at 14 h pi but synthesis did not really increase markedly until after .16 h pi. Plus sense RNA synthesis increased rapidly, reaching a peak of 7 ng per jug of total cell RNA at 22 h pi. Minus sense RNA increased rapidly between 16 h and 18 h pi, remained fairly constant until 22 h pi, at a concentration of 1.5-1.75 ng per jitg of total cell RN A, after which time it decreased. Probing of the northern blot with plus sense RNA supported the conclusions from the dot blots. Hybridisation to minus sense RNA was visible at 9 h pi (Figure 15). This hybridisation was not to genome size RNA but to an apparently higher molecular weight smear of RNA, which, at some time points, appeared to be a discrete band. Hybridisation to genome size minus sense RNA was detected later in infection but not to the same extent as to the high molecular weight species. Probing with minus sense RNA revealed a similar picture, except a greater proportion of hybridisation was to genome size RNA (Figure 15). Plus sense RNA was detectable at 7.2 h pi. 87 log virus litr e (lU /m l) Time post infection (h) Figure 14. Time course of RNA synthesis of BVDV NADL The RNA concentra­ tion and virus titres were measured as described in M aterials an d M eth ods. 88 Time pi |h) Ml 1 3 5 7 + Figure 15. Northern blots of total cell RNA from MDBK cells infected with BVDV NADL The filter was first probed for minus sense RNA (-) with an SP6 polymerase transcript of pBV4-p80. After autoradio­ graphy, the probe was stripped off with boiling water and the filter reprobed for plus sense RNA (+ ) with a T7 polymerase transcript of pBV4-p80. The 12.6 kb bands represent BVDV genome size RN A. RI and RF represent putative replicative intermediate and replicative form RNA respectively. MI is mock-infected cell RNA. 89 BVDV Singer RNA Synthesis Plus sense RNA was first detected at 7 h pi and the concentration increased rapidly until it exceeded the calibration range, at approximately 16 h pi (Figure 16) Although not shown, the RNA concentration peaked at between 18 h and 20 h pi, at a concentration of approximately HO ng per ng of total cell RNA i.e. 11% of total cellular RN A. Minus sense RNA followed a similar time course to that of NADL minus sense R N A It was first detected at 12 h pi and increased to a plateau, of approximately 10 ng per /xg of total cell RN A, at 18-24 h pi. The northern blot, probed with plus sense RNA, showed a similar pattern of hybridisation to that seen with NADL (data not shown). Most probe hybridised to the high molecular weight RNA species. This hybridisation was first evident at 5 h pi, indicating a shorter lag phase than was evident from the graph. Very little minus sense RNA was present in the genome size band at any time point com­ pared to the high molecular weight smear. Probing with minus sense RNA, showed that plus sense BVDV RNA was detectable at 1.7 h pi. This was prob­ ably the infecting virus genome. Plus sense RNA began to increase at 7 h pi. As with NADL, the probe hybridised not only to a discrete band, the Singer genome, but also to higher molecular weight RNAs and to a smear of RNA beneath the genome RNA band. BVDV Oregon C24V RNA Synthesis . Plus sense RNA was first detected at 11 h pi (Figure 17). Like NADL, there was an initial small peak. The amount of plus sense RNA rapidly increased after 13 h pi, peaking at 22 h pi, at a concentration of approximately 2.25 ng per jug of total cell RNA. Minus sense RNA followed a very similar time course. It was first detectable at 11 h pi. It increased steadily from there, peaking at 22 h pi, at 90 • +RNA D -RNA I Virus li t r e log viru s litr e (lU /m l) ng BVDV RNA per /xg cell RNA I O 4 8 12 16 20 24 Time post infection (h) Figure 16. Time course of RNA synthesis of BVDV Singer. The RNA concentra­ tion and virus litres were measured as described in M aterials an d M eth ods. 91 • +RNA O -RNA I Virus l i t r e log virus litr e (lU /m l) ng BVDV RNA per yu-g ce ll RNA I O 4 8 12 16 20 24 Time post infection (h) Figure 17. Time course of RNA synthesis of BVDV Oregon C24V. The RNA concentrations and virus litres were measured as described in M aterials a n d M eth ods. 92 a concentration of approximately 0.5 ng per ng of total cell RN A. The northern blot exhibited a similar distribution of target RNA to that of NAJDL and Singer. Minus sense RNA was detected mainly in a high molecular weight smear. It was first detectable at 7.25 h pi. Only at 22 h pi was any detected in a discrete genome size band (data not shown). As with Singer, plus sense BVDV RNA was detectable in a genome size band at 1.4 h pi but only started to increase after 7.25 h pi. BVDV New York-1 RNA Synthesis The time course of New York-1 RNA synthesis was rather different to that of the cBVDV strains (Figure 18). Plus sense RNA was detectable at 1.6 h pi. It increased rapidly between 9 h and 14 h pi, at which time it became relatively constant, at a concentration of 1.7 ng per of total cell RN A. There was another rapid increase between 20 h and 22 h pi, to a peak of 2.4 ng per /ig of total cell RNA. Minus sense RNA synthesis was also somewhat different. It was first detectable at 11 h pi. As with the plus sense RNA, there was a biphasic time course with peaks at 14 h and 20 h pi, of 0.09 ng and 0.15 ng per fig of total cell RNA, respectively. Minus sense RNA was detected on the northern blot at 5 h pi (data not shown). As with the other strains, this was in a high molecular weight RNA smear. However, compared with the other strains, more was present in a discrete genomic size band later in infection. Surprisingly, plus sense RNA was detected at 1.6 h pi but was not detectable again until 9 h pi. This is also reflected in the plus sense RNA plot from the dot blots. It is unclear what caused this. Most of the plus sense RNA on the northern blot was detected in a discrete genome size band. 93 • +RNA D -RNA I Virus lit r e log virus litr e (lU /m l) ng BVDV RNA per yu-g cell RNA I O 4 8 12 16 20 24 Time post infection (h) Figure 18. Time course of RNA synthesis of BVDV New York-1. The RNA concentrations and virus litres were measured as described under M aterials a n d M eth ods. 94 BVDV TGAC RNA Synthesis Plus sense RNA was not detectable until 12.75 h pi (Figure 19). After this it increased rapidly to a biphasic peak between 18 h and 24 h pi, of 1.1-1.2 ng per Hg total cell RN A. Minus sense RNA was detectable at 9.8 h pi. It increased steadily after this until 22 h pi and increased sharply between then and 24 h pi. The northern blot supported the findings of the dot blots (data not shown). Minus sense RNA was detected at 9.8 h pi and was detected mainly in a high molecular weight smear. Plus sense RNA was detectable at 12.2 h pi and was mainly present in a discrete genome size band. BVDV TGAN RNA Synthesis TGAN had a similar biphasic time course to New York-1 (Figure 20). Plus sense RNA was first detected at 12 h pi, after which it increased rapidly to an initial peak of 1.15 ng per [Ag total cell RNA at 14 h pi. There was a second peak of approximately 1.4 ng per [Ag of total cell RNA at 22 h pi. Though not evident from the graph, minus sense RNA was detected at 12 h pi, increased to an initial peak at 12.7 h pi, with a second peak at 18 h pi and was rapidly increasing at the termination of the experiment. The two peaks were at a concentration of 0.012 ng per [Ag of total cell RN A. The northern blot showed that minus sense RNA was present at 8 h pi, in a high molecular weight RNA smear (data not shown). Probing with minus sense RNA showed that plus sense RNA was detectable at 12 h pi, in a discrete genome size band. The concentration of the plus sense RNA was very low. • +RNA a -RNA I I Virus lit r e log virus litr e (lU /m l) 0 .7 5 ng BVDV RNA per Hg cell RNA 95 0 4 8 12 16 20 24 Time post infection (h) Figure 19. Time course of RNA synthesis of BVDV TGAC. The RNA concentra­ tions and virus litres were measured as described in M aterials an d M eth ods. 96 • +RNA D -RNA I Virus lit r e log virus litr e (lU /m l) ng BVDV RNA per /Ltg ce ll RNA I O 4 8 12 16 20 24 Time post infection (h) Figure 20. Time course of RNA synthesis of BVDV TGAN. The RNA concentra­ tions and virus titres were measured as described in M aterials an d M eth ods. 97 Comparison of Plus Sense RNA Levels With Levels of Infectious Virus The level of virus production was measured at approximately 6 h intervals during the course of the experiment. The virus titres compared to plus sense RNA synthesis can be seen in Figures 14,16-20. No relationship was observed between the production of plus sense RNA and production of infectious virus. In fact, the strains that produced the lowest cellular levels of plus sense RNA generally had the highest virus titres. Comparison of the Ratio of Plus to Minus Sense RNA Though the ratio of minus sense to plus sense RNA of each strain was rather variable, general trends were observed (data not shown). The ratio observed for cBVDV strains was generally less varied throughout the course of infection. The ratios of NADL and Oregon C24V remained quite constant, while those of Singer and TGAC started off quite high but fell steadily throughout the course of infection. With the ncBVDV strains, there was a much greater degree of variation. Two major peaks were observed in each case, which occurred immediately after the peaks in minus sense RNA levels. The average ratios of the two ncBVDV strains were also higher than those of the cBVDV strains. Although not as consistent as the the data from the second set of quantita­ tions, the general trends evident in the quantitations from the original study supported the conclusions concerning the RNA ratios. 98 DISCUSSION Virus Strains The four BVDV strains I studied in the bulk of this work-Singer, NADL, Oregon C24V and New York-1—could be considered the standard BVDV strains. They represent all the important traits of BVDV cytopathology. Most of the important studies of BVDV at the molecular level have involved one or more of these strains. The strains TGAC and TGAN were included in later studies because of the emerging data on the pathogenesis of MD. The two strains were originally isolated from a cow with MD and have since been used to reproduce the disease experimentally. Due to their known ability to cause severe disease, they act as a useful yardstick for the relevance of the findings with the four original strains, which had been maintained in cell culture for many years. The six virus strains were all obtained from the same source. This is an important point in light of the differences found in ostensibly identical strains from different laboratories (42). I have used the term strain to represent virus from established laboratory stocks. I would term BVDV recovered from a field case an isolate. Some workers in pestivirology refer to all BVD viruses as isolates, as the viruses cannot be truly differentiated on a serological basis, so they cannot be truly called strains. 99 Development of the Experimental System When I started this research project, three things were evident: there was no standard system for growing the virus; there was no standard system for titrating the virus; and there was no standard system for purifying the virus. To quote Horzinek (134): ...the dilemma encountered by all workers in this field: the lack of suit­ able systems for in vitro virus growth and titration. Without exaggera­ tion it can be stated that this is the reason why non-arbo togaviruses rank among the least known animal viruses, especially with regard to their molecular structure and biology. Most studies of BVDV are carried out in primary bovine cell lines. The inherent variation between cell preparations and likely problems in maintaining a system free of BVDV, rendered the use of primary cells impractical. An estab­ lished cell line was required. Routine isolations of BVDV, for diagnostic pur­ poses, are carried out with BT cells (William Quinn, personal communication). As this is a secondary cell line, that undergoes crisis at approximately passage 30 (Arlan McClurkin, personal communication), an early passage of the cell line was procured. These cells grew rather slowly and did not achieve very high cell densities. The BLF cell was also a secondary cell line. It grew faster than BT cells and to higher cell densities. The cell line had only recently been established, so its long term stability in culture was unknown. The MDBK cell line was a permanent cell line of known growth characteristics. It grew faster than the other two cell lines and to a higher cell density. In this cell system the litres of the BVDV strains Singer, Oregon C24V and New York-1 were consistently between 3x107 and IxlO8 lU/ml or PFU/ml. This is an unusually high titre for BVDV (134,237). Strain NADL was consistently of lower titre, approximately 3x10* PFU/ml. Although this cell and virus combination had been used in the past 100 (179), it had probably fallen from favour as the MDBK cell line available from the ATCC for many years was persistently infected with ncBVDV. Fortunately, a stock of the cells had been discovered that was free of BVDV and this was the cell line that I used, MDBK (NBL-1). The evidence of a MDBK cell contaminant that I found during the 32P-Iabelling studies was puzzling. The two prominent RNA bands observed migrated at the same rate as 16S and 23S RN A, the size of prokaryotic rRNA. This suggested a contaminant but no evidence for the presence of mycoplasma or bacteria was found. As the putative contaminant did not appear to affect BVDV replication ! and BVDV-specific probes did not hybridise to the RNA its presence was ignored. When first obtained, the cell lines were maintained in medium supplemented with foetal bovine serum. The serum was treated with /3-propiolactone, which destroyed any virus in the serum; however, it would not remove BVDV neutral­ ising antibody from the serum. An alternative to bovine serum was equine serum. Horses are not infected by BVDV '(124), so there was no possibility that equine serum would contain BVDV or neutralising antibody to it. The cell lines all grew well in medium supplemented with equine serum, so it was used for all subsequent cell maintenance. The use of serum-free medium was also studied but it impaired the growth of the cells. The development of the EPDMA was an important aspect of my initial studies of BVDV. As I previously stated, several different assays were available for the titration of ncBVDV but these were indirect, time consuming, relatively inaccurate or not comparable with plaque assays (90,95,105,141,176,210,271). Several of the assays are dependent on the indirect effects of ncBVDV on the replication of challenge viruses to indicate dilution end points. Two, the interfer­ ence assay of Gillespie et al (105), and the reverse plaque assay of Itoh et al. 101 (141), rely on the ability of ncBVDV to inhibit cBVDV cpe. The interference assay suffers from ill-defined end points, leading to statistical inaccuracies. The END assay depends on the enhancement of Newcastle disease virus cytopathogenicity (210) and has been shown to be less sensitive than the other assays for ncBVDV (141). The PINBA assay of Maisonnave and Rossi (176) relies on challenge with VSV. The accuracy of this assay varies between cell lines, some cells infected with ncBVDV being resistant to challenge with VSV (254). Compared to the direct assays described for titrating ncBVDV, EPDMA is generally more accurate statistically, due to the larger number of replicates involved (90,95,140,149). Two plaque assays have been described for the direct titration of ncBVDV. Straver (271) described the visualisation of plaques by prolonged staining of monolayers with neutral red. I have seen this phenomenon but it is not very reproducible and the staining period is extremely long. Rae et al. (231) recently described a fluorescent focus forming assay for ncBVDV. This is a plaque assay in which plaques are visualised by indirect immunofluorescence. It is likely that this assay is as accurate as EPDMA. In addition to the advantages of EPDMA for titrating, ncBVDV, there were also certain advantages to using it over plaque assays for cBVDV. It took less time to set up, required less materials and could be read in two or three days as compared to four or five days for a plaque assay. The ability to titrate both biotypes of BVDV with the same assay was a distinct advantage. As plaque assays and EPDMA gave equivalent titres for cBVDV strains, IU and PFU could be assumed to be equivalent. This agreed with the findings of Robb and Martin (243). I felt that it was reasonable to assume that titration of ncBVDV strains by EPDMA was equally accurate. The protocol also proved extremely useful in the biological cloning of the different 102 BVDV strains. The only disadvantages of the assay were due to low specificity or titre of the antiserum used. This made it difficult to identify ncBVDV infected wells. The use of the antiserum raised against ncBVDV strain New York-1 alleviated these problems. The use of a BVDV-specific monoclonal antibody would probably enhance the assay considerably. The growth curves of BVDV have been observed to be cell line and virus strain specific (134). The latent phase of 9 h was comparable to the 4-10 h that has been generally observed. The growth curves after this are rather complicated. There are indications that they do not represent a single growth cycle. There is a distinct shoulder on most of the curves at 18-24 h pi. This may represent the end of the logarithmic phase of growth of the inoculum. The curve then increases again in all cases. This may represent a second cycle of virus growth, due to virus released in the initial growth phase infecting cells not infected by the inoculum. As an moi of I was used for the initial infection, 36.8% of the cells were not infected, according to the Poisson distribution. If one assumes that the cellassociated virus was more indicative of actual virus growth and the free virus was more a measure of virus release, then the logarithmic phase of Singer and NADL lasted from 9-18 h pi and of Oregon C24V and New York-1 from 9-21 h pi. The peak of virus release occurred after this, between 30 h and 42 h pi; again, compa­ rable to previous reports (134). The plateau that was reached at this point bore indications that cycles of virus growth were still occurring. However, monolayers infected with Singer and NADL were destroyed by 48 h pi. This implies that there is little virus growth occurring during this phase. Overall, there appears to be little difference between the strains. The highly cytopathic strains appear to have a shorter logarithmic phase. As is generally found with BVDV (134), the four strains had 1-1.5 Iog10 more released virus than cell-associated virus. 103 The concentration and purification protocol that was defined gave very high yields of virus. Ultrafiltration has been used by several groups for concentration of BVDV (61,171,237). The use of glycerol-tartrate gradients has been reported independently for the purification of BVDV (229). The two methods have not been used in concert before. Although some loss of virus occurred during ultrafiltration, this was a negligible amount. Other groups have reported much greater losses, from 50% (171) to 85% (61), compared with 0.05% in the present study. Further losses also occurred from sedimentation on glycerol-tartrate gradients but these were much less than those incurred from the other purification methods studied. Why glycerol-tartrate gradients resolve BVDV as a single peak, while potassium tartrate gradients resolve the virus in two peaks, is not clear. The glycerol may help maintain the integrity of the viral envelope, the major viral component responsible for its buoyant density. The major drawbacks to glyceroltartrate gradients were that the virus was resolved in a broad peak and had to be separated from the potassium tartrate after sedimentation. This was achieved by diluting fractions containing virus and collecting the virus by centrifugation. However, this resulted in large losses in the yield of infectious virus (data not shown). A better approach might have been to desalt the virus by gel filtration, followed by concentration using a CX-30 filter unit. Though the degree of purity of the virus was not studied in terms of the ratio of infectious virus to total protein content, the recovery of infectious virus was far better than achieved by other methods (61,134,171,229,237). Overall, the system developed for the growth, titration and purification of BVDV would appear to be equal or superior to those previously described. 104 BVDV RN A: Size and Synthesis The size estimate for the genomic RNA of BVDV was comparable to the actual length of the BVDV NADL cDNA sequence of Collett et al. (54): 12.6 kb compared with 12,573 bases. In the light of the difference of 42 nucleotides between the ORF of BVDV strains Osloss and NADL (55), the autoradiograph of the size comparison of the four BVDV strains was reanalysed. Some difference in size had previously been observed but this was dismissed as being due to distortion of the gel or the difference in the width of the band, due to differences in radio­ activity. The reanalysis results are shown in Table 10. The comparison shows that NADL has the longest genome and, as expected, New York-1 has the shortest. Interestingly the estimate for the size of the genome of New York-1 is very similar to the length of the molecularly cloned cDNA of HCV. The genomes of Singer and Oregon C24V are approximately the same size. The mean size of the genomes is the same as the original size estimate, showing the accuracy of the original estimate. Due to the degree of error incurred in measuring the distance of migration of bands on autoradiographs, the actual differences in genome size are probably not as great as estimated. The time course of BVDV RNA synthesis appears to follow the same time course as virus growth, as has also been observed for HCV (199). This implies that the time course of RNA synthesis will be cell line and virus strain dependent. The former was not investigated but the latter certainly proved to be the case. Great variation was found not .only in the time course but also the quantity of RNA synthesis between the different strains. The early events in RNA synthesis were best defined by the time course of BVDV Singer. A lag phase of 5 h occurred before minus sense RNA was detected. Plus sense RNA production was 105 Table 10. Reanalysis of the size of the genomic RNA of four strains of BVDV Strain NADL Singer Oregon C24V New York-1 Mean size Genomic RNA (kb) . 13.2 12.9 12.8 12.5 12.8 net size of genome (kb)a 13.0 12.6 12.5 12.2 12.6 a. net genome size was calculated in each case from the difference between the two Singer strain estimates detected 2 h later. Both plus and minus sense RNA synthesis increased exponen­ tially after this. A similar time course for protein synthesis has been described in Singer-infected cells (75). Though not evident from the data in most cases, probably due to the lower BVDV RNA concentrations, a similar time course probably applied to the other strains. However, the NADL strain had a distinct 4 h lag compared to the other strains. The latent phase before RNA synthesis was detected has also been observed in cells infected with flaviviruses (298). The two biotypes seemed to differ in the time course of RNA synthesis during the eclipse phase. In the case of cytopathic strains, plus and minus sense RNA production increases to a peak at 20-22 h pi. The increased minus sense RNA synthesis, observed between 22 h and 24 h pi in cells infected with the Singer and TGAC strains, may indicate the start of a second round of virus production, as was observed in the virus growth curves. The most remarkable difference between the four cytopathic strains is the level of RNA synthesis. The Singer strain produced 1.5 times more plus sense RNA than NADL, 40 times more than Oregon C24V and 80 times more than TGAC. Surprisingly, this was not reflected in the production of infectious virus, TGAC-infected cells producing 106 as much as Singer infected cells. However, the total number of virus particles produced was not assessed. The noncytopathic strains appeared to exert more control over RNA synthesis. The initial exponential increase in RNA levels only lasted until 14 h pi. Minus sense RNA synthesis increased again from 16-20 h pi with a concomitant increase in plus sense RNA levels until 22 h pi. As with the cytopathic strains, there was no relationship between plus sense RNA levels and the yield of infec­ tious virus. Apart from the difference in the time course of RNA synthesis, ncBVDV strains differed from cBVDV strains in the ratio of plus to minus sense RN A. In cells infected with ncBVDV only 1-3% of total BVDV RNA was minus sense RNA; whereas, in cBVDV infected cells minus sense RNA represented 5-20% of total BVDV RNA. The ratio fluctuated markedly during ncBVDV infection but remained relatively constant during cBVDV infection. What could cause this major difference in ratios? Minus sense RNA has a single role in virus replica­ tion; the template for plus sense RNA transcription. Conversely, plus sense RNA has three roles: mRNA, template for minus sense RNA transcription and genome of progeny virus. These roles are not necessarily mutually exclusive. In flavivirus replication, encapsidation is thought to be controlled by binding of the C protein, equivalent to the BVDV p20 capsid protein, to the plus sense RNA (298). This would also prevent its use as a template for minus sense RNA transcription. It is likely that a similar process occurs in BVDV replication. The homology in functional domains of flavivirus NS3 and BVDV p!25 may hold the key. In flavivirus infected cells, NS3 is thought to be part of the replication complex with NS5, the viral RNA dependent RNA polymerase (298). In cBVDV infected cells, p!25 is cleaved. This cleavage apparently occurs between a protease and a RNA 107 helicase domain (106). The RNA helicase is thought to be involved in the dissociation of daughter plus sense RNA strands from the minus sense RNA template in the replication intermediates of RNA viruses (107). The disassociation of the two domains may render newly synthesised plus sense RNA less accessible to p20, resulting in the use of the RNA as a template for minus sense RNA transcription. This would have two results, an increase in minus sense RNA levels, with a subsequent increase in plus sense RNA synthesis, and a reduction in the amount of progeny virus. This was indicated by the lower ratio of plus to minus sense RNA in cBVDV infected cells I detected. The increased plus sense RNA synthesis would increase the amount of BVDV mRNA, reflected in the higher level of BVDV antigen detected in infected cells by immunofluorescence. As p 125 is also detected in cBVDV infected cells, albeit at much lower levels than, the cleavage products, encapsidation of plus sense RNA would occur in replication complexes containing the uncleaved protein. The increased levels of BVDV proteins would offset the lower net encapsidation rate, by increasing the number of replication complexes and the concentration of structural proteins required for virion assembly. The amount of virus produced would depend on the ratio of cleaved pl25 to native pl25 in the replication complexes. In ncBVDV infected cells all the replication complexes would contain p!25. The degree of minus sense RNA transcription would be controlled by the availability of p20. Initially, most plus sense RNA would be mRNA or template for minus sense RNA, as the levels of p20 would be low. As the level of BVDV mRNA increased, so would the level of BVDV proteins, due to the translation of the mRNA. This would increase the level of p20, leading to more encapsidation, with a reduction in minus sense RNA synthesis. The p20 levels would then fall due to removal by encapsidation and a 108 fall in BVDV mRNA levels. A dynamic equilibrium would then be set up, as I observed in the case of ncBVDV infected cells. One intriguing implication of the low level of pl25 in cBVDV-infected cells, compared to the level of p80, is that cBVDV is replication defective. As with other replication defective viruses, it requires a helper virus for its replication, the helper virus being ncBVDV. A low level of ncBVDV is required to allow encapsidation of the cBVDV genome. It has been hypothesised that MD is actually the result of a mixed infection, in which cBVDV is present the whole time. Mucosal disease results when the cBVDV is able to replicate to a higher level than ncBVDV in certain tissues (12). The low ratio of plus to minus sense RNA in cells infected with cBVDV may be responsible for the cpe observed. The low ratio implies the presence of higher levels of double stranded RNA (dsRNA) in cBVDV-infected cells than in ncBVDV-infected cells. It is known that dsRNA is cytotoxic (144). It is also known that dsRNA is a potent inducer of IFN-a and IFN-/? (144). These two cytokines can also induce cytotoxic effects in cells (144). The reaction of cBVDVinfected cells to the direct and indirect cytotoxic effects of dsRNA could result in the cpe observed. The low ratio of plus to minus sense RNA observed in Oregon C24V-infected cells, without appreciable cpe in most of the cells, implied that the virus-cell interactions resulting in cpe were not solely due to the presence of dsRNA. The northern blots probed for plus sense and minus sense RNA produced some interesting results. The most obvious observation was that denaturation of the BVDV RNA was not complete. The RNA of BVDV has a high degree of very stable secondary structure ((55,134), personal observation). The distribution of BVDV RNA on the autoradiographs bares a striking similarity to the 109 distribution of flavivirus 44S genomic RNA, RI and RF (48,49,51). The RI and RF of flaviviruses contain both plus sense and minus sense RNA, as found on the northern blots in this case. The incomplete denaturation observed on the northern blots raises a question of how quantitative the dot blot assay was. I feel that denaturation was not a problem as the baking of the filters will have denatured the RNA and the high temperature of hybridisation in the presence of formamide will also cause the relaxation of double stranded regions. There is also the question of probe specificity. Again, I don’t feel that this is a problem as the probe used was from the most highly conserved region of the genome (55), showing 96-99% homology between NADL and Osloss. The proof of my hypothesis, that the cleavage of pl25 leads to a loss of control of minus sense RNA synthesis due to a failure to target plus sense RNA for encapsidation, can be approached in several ways. The involvement of p20 in control of RNA synthesis could be easily assessed by measuring intracellular levels of the protein during virus replication. An antigen capture assay, using monospecific antisera or a monoclonal antibody specific for p20, could be used. If p20 were involved in controlling minus sense RNA synthesis, then the intracellular concentration of the protein should fluctuate out of phase with minus sense RNA concentration i.e. p20 levels should be high when minus sense RNA levels are low and vice versa. For assessing how cleavage of p!25 affects RNA synthesis directly, some very complex tools will be required that are not presently available in pestivirology. One approach would be the in vitro assembly of BVDV replication complexes into which native and cleaved p!25 could be selectively incorporated. Although flavivirus replication complexes have been studied in vitro (49,112), I feel that it is unlikely that such studies can be performed on BVDV in the near future, no due to the dearth of information on the components of BVDV replication complexes, assuming they exist at all. Another approach would be to produce an infectious cDNA clone of a ncBVDV strain. Site directed mutagenesis could then be performed on the p!25 gene and the effects on RNA synthesis assessed. The p20 gene could also be mutagenised to reduce its binding efficiency to genomic RNA to see if this would result in the same pattern of RNA synthesis as I observed in cBVDV-infected cells. It will probably be extremely difficult to produce an infectious cDNA clone of a ncBVDV strain, so such experiments are not likely to be attempted in the near future. I ll LITERATURE CITED 1. Akkina, R.K. (1982). Analysis o f immunogenic determinants o f bovine viral diarrhea virus, PhD. Thesis. University, of Minnesota. 2. Akkina, R.K., Muscoplat, C.C., Atluru, D. and Johnson, D.W. (1980). Immunosuppression of bovine lymphocytes, plasma cells and monocytes by bovine viral diarrhea virus. In: Proceedings o f the International Congress on Diseases o f Cattle, XL Tel Aviv, 1980, edited by Mayer, E. Bregman Press, Haifa, p. 327-332. 3. Atluru, D., Notowidjojo, W., Johnson, D.W. and Muscoplat, C.C. (1979). Suppression of in vitro immunoglobulin biosynthesis in bovine spleen cells by bovine viral diarrhea virus. Clinical Immunology and Immunopathology, 13: 254-260. . 4. Baker, J.A., York, C.J., Gillespie, J.H. and Mitchell, G.B. (1954). Virus diarrhea in cattle. American Journal o f Veterinary Research, 15: 525-531. 5. Baker, J.C. (1987). Bovine viral diarrhea virus: A review. Journal o f the American Veterinary Medical Association, 190: 1449-1458. 6. Barber, D.M.L., Nettleton, P.F. and Herring, J.A. (1985). Disease in a dairy herd associated with the introduction and spread of bovine virus diarrhoea > virus. Veterinary Record, 117: 459-464. 7. Barlow, R.M., Nettleton, P.F., Gardiner, A.C., Greig, A., Campbell, J.R. and Bonn, J.M. (1986). Persistent bovine virus diarrhoea virus infection in a bull. Veterinary Record, 118: 321-324. 8. Bazan, J.F. and Fletterick, R.J. (1989). Detection of a trypsin-like protease domain in flaviviruses and pestiviruses. Virology, 171: 637-639. 9. Bielanski, A. and Hare, W.C.D. (1988). Effect in vitro of bovine viral diarrhea virus on bovine embryos with the zona pellucida intact, damaged and removed. Veterinary Research Communications, 12: 19-24. 10. Bielefeldt Ohmann, H. (1983). Pathogenesis of bovine viral diarrhoeamucosal disease: Distribution and significance of BVDV antigen in diseased calves. Research in Veterinary Science, 34: 5-10. 11. Bielefeldt Ohmann, H. (1987). Double-immunolabeling systems for phenotyping of immune cells harboring bovine viral diarrhea virus. Journal o f Histochemistry and Cytochemistry, 35: 627-633. 112 12. Bielefeldt Ohmann, H. (1988). BVD virus antigens in tissues of persistently viraemic, clinically normal cattle: Implications for the pathogenesis of clinically fatal disease. Acta Veterinaria Scandinavica, 29: 77-84. 13. Bielefeldt Ohmann, H. (1989). Bovine virus diarrhea (BVD). VIDO Fact Sheet, 11. 14. Bielefeldt Ohmann, H. and Babiuk, L A . (1986). Viral infections in domestic animals as models for studies of viral immunology and pathogene­ sis. Journal o f General Virology, 66: 1-25. 15. Bielefeldt Ohmann, H. and Babiuk, L A . (1988). Influence of interferons Ct1I and T and of tumour necrosis factor on persistent infection with bovine viral diarrhoea virus in vitro. Journal o f General Virology, 69: 1399-1403. 16. Bielefeldt Ohmann, H. and Bloch, B. (1982). Electron microscopic studies of bovine viral diarrhea virus in tissues of diseased calves and in cell cultures. Archives o f Virology, 71: 57-74. 17. Bielefeldt Ohmann, H., Jensen, M.H., Serensen, K.J. and Dalsgaard, K. (1982) . Experimental fetal infection with bovine viral diarrhea virus: I. Virological and serological studies. Canadian Journal o f Comparative Medicine, 46: 357-362. 18. Bielefeldt Ohmann, H., Rensholt, L. and Bloch, B. (1987). Demonstration of bovine viral diarrhea virus in peripheral blood mononuclear cells of persistently infected, clinically normal cattle. Journal o f General Virology, 68: 1971-1982. 19. Binkhorst, G.J., Journee, D.L.H., Wouda, W., Straver, P.J. and Vos, J.H. (1983) . Neurological disorders, virus persistence and hypomyelination in calves due to intrauterine infections with bovine virus diarrhoea virus. I. Clinical symptoms and morphological lesions. Veterinary Quarterly, 5: 145-155. 20. Bock, R.E., Burgess, G.W. and Douglas, I.C. (1986). Development of an enzyme linked immunosorbent assay (ELISA) for the detection of bovine serum antibody to bovine viral diarrhoea virus. Australian Veterinary Journal, 63: 406-408. 21. ' Bolin, S., Moennig, V., Kelso Gourley, N.E. and Ridpath, J. (1988). Monoclonal antibodies with neutralizing activity segregate isolates of bovine viral diarrhea virus into groups. Archives o f Virology, 99: 117-123. 22. Bolin, S.R. (1988). Viral and viral protein specificity of antibodies induced in cows persistently infected with noncytopathic bovine viral diarrhea virus after vaccination with cytopathic bovine viral diarrhea virus. American Journal o f Veterinary Research, 49: 1040-1044. 23. Bolin, S.R., McClurkin, A.W. and Coria, M.F. (1985). Effects of bovine viral diarrhea virus on the percentages and absolute numbers of circulating 113 B a n d T lymphocytes in cattle. American Journal o f Veterinary Research, 46: 24. Bolin, S.R., McClurkin, A.W. and Coria, M.F. (1985). Frequency of persistent bovine viral diarrhea virus infection in selected cattle herds. American Journal o f Veterinary Research, 46: 2385-2387. 25. Bolin, S.R., McClurkin, A.W., Cutlip, R.C. and Coria, M.F. (1985). Severe clinical disease induced in cattle persistently infected with noncytopathic bovine viral diarrhea virus by superinfection with cytopathic bovine viral diarrhea virus. American Journal o f Veterinary Research, 46: 573-576. 26. Bolin, S.R., McClurkin, A.W., Cutlip, R.C. and Coria, M.F. (1985). Response of cattle persistently infected with noncytopathic bovine viral diarrhea virus to vaccination for bovine viral diarrhea and to subsequent challenge exposure with cytopathic bovine viral diarrhea virus. American Journal o f Veterinary Research, 46: 2467-2470. 27. Bolin, S.R. and Ridpath, J.F. (1989). Specificity of neutralizing and precipi­ tating antibodies induced in healthy calves by monovalent modified-live bovine viral diarrhea virus vaccines. American Journal o f Veterinary Research, 50: 817-821. 28. Bolin, S.R., Roth, J.A., Uhlenhopp, E.K. and Pohlenz, J.F. (1987). Immunologic and virologic findings in a bull chronically infected with noncytopathic bovine viral diarrhea virus. Journal o f the American Veterinary Medical Association, 190: 1015-1017, 29. Bolin, S.R., Sacks, J.M. and Crowder, S.V. (1987). Frequency of association of noncytopathic bovine viral diarrhea virus with mononuclear leukocytes from persistently infected cattle. American Journal o f Veterinary Research, 48: 1441-1445. 30. Braun, R.K., Osburn, B.I. and Kendrick, J.W. (1973). Immunologic response of bovine fetus to bovine viral diarrhea virus. American Journal of Veterinary Research, 34: 1127-1132. 31. Brinton, M.A. (1980). Non-arbo togaviruses. In: The Togaviruses. Biology, structure,replication, edited by Schlesinger, R.W. Academic Press, New York. p. 623-666. 32. Brown, T.T., Schultz, R.D., Duncan, J.R. and Bistner, S.I. (1979). Serologi­ cal response of the bovine fetus to bovine viral diarrhea virus. Infection and Immunity, 25: 93-97. 33. Brownlie, J. (1985). Clinical aspects of the bovine virus diarrhoea/mucosal disease complex in cattle. In Practice, 7: 195-202. 34. Brownlie, J., Clarke, M.C. and Howard, C.J. (1984). Emerimental produc­ tion of fatal mucosal disease in cattle. Veterinary Record, 114: 535-536, 114 35. Brownlie, J., Clarke, M.C. and Howard, C.J. (1985). Aetiology and patho­ genesis of mucosal disease: current concepts, observations and speculation. Australian Veterinary Journal, 62: 142-143. 36. Brownlie, J., Clarke, M.C. and Howard, C.J. (1987). Clinical and experi­ mental mucosal disease - defining a hypothesis for pathogenesis. In: Pestivirus infections o f ruminants, edited by Harkness, J.W. Commission of the European Communities, EUR 10238, Luxembourg, p. 147-156. 37. Brownlie, J., Clarke, M.C. and Howard, C.J. (1989). Experimental infection of cattle in early pregnancy with a cytopathic strain of bovine virus diarrhoea virus. Research in Veterinary Science, 46: 307-311. 38. Brownlie, J., Clarke, M.C., Howard, C.J. and Pocock, D.H. (1987). Patho­ genesis and epidemiology of bovine virus diarrhoea virus infection of cattle. Annales de Recherches Veterinaires, 18: 157-166. 39. Brownlie, J., Nuttall, PA ., Stott, E.J., Taylor, G. and Thomas, L.H. (1980). Experimental infection of calves with two strains of bovine virus diarrhoea virus: Certain immunological reactions. Veterinary Immunology and Immunopathology, I: 371-378. 40. Castrucci, G., Avellini, G., Cilli, V., Pedini, B., McKercher, D.G. and Valente, C. (1975). A study of immunologic relationships among serologi­ cally heterologous strains of bovine viral diarrhea virus by cross immunity tests. Cornell Veterinarian, 65: 65-72. 41. Castrucci, G., Cilli, V. and Gagliardi, G. (1968). Bovine virus diarrhea in Italy. I. Isolation and characterization of the virus. Archiv fur die gesamte Vimsforschung, 24: 48-64. 42. Cay, B., Chappuis, G., Coulibaly, C., Dinter, Z., Edwards, S., Greiser-Wilke, I., Gunn, M., Have, P., Hess, G., Juntti, N., Liess, B., Mateo, A., McHugh, P., Moennig, V., Nettleton, P. and Wensvoort, G. (1989). Comparative analysis of monoclonal antibodies against pestiviruses: Report of an interna­ tional workshop. Veterinary Microbiology, 20: 123-129. 43. Chasey, D. and Roeder, P.L. (1981). Virus-like particles in bovine turbinate cells infected with bovine virus diarrhoea/mucosal disease virus. Archives o f Virology, 67: 325-332. 44. Childs, T. (1946). X disease of cattle - Saskatchewan. Canadian Journal o f Comparative Medicine, 10: 316-319. 45. Chomczynski, P. and Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry, 162: 156-159. 46. Chu, H.-J. and Zee, Y.C. (1984). Morphology of bovine viral diarrhea virus. American Journal o f Veterinary Research, 45: 845-850. 115 47. Chu, H.-J., Zee, Y.C., Ardans, A.A. and Dai, K. (1985)..Enzyme-linked immunosorbent assay for the detection of antibodies to bovine viral diarrhea virus in bovine sera. Veterinary Microbiology, 10: 325-333. 48. Chu, P.W.G. and Westaway, E.G. (1985). Replication strategy of Kunjin virus: Evidence for recycling role of replicative form RNA as template in semiconservative and asymmetric replication. Virology, 140: 68-79. 49. Chu, P.W.G. and Westaway, E.G. (1987). Characterization of Kunjin virus RNA-dependent RNA polymerase: Reinitiation of synthesis in vitro. Virology, 157: 330-337. 50. Clarke, M.C., Brownlie, J. and Howard, C.J. (1987). Isolation of cytopathic and non-cytopathic bovine viral diarrhoea virus from tissues of infected animals. In: Pestivirus infections o f mmmants, edited by Harkriess, J.W. Commission of the European Communities, EUR 10238, Luxembourg. p. 3-12. 51. Cleaves, G.R., Ryan, T.E. and Schlesinger, R.W. (1981). Identification and characterization of type 2 Dengue virus replicative intermediate and replicative form RN As. Virology, 111: 73-83. 52. Collett, M.S., Anderson, D.K. and Retzel, E. (1988). Comparisons of the pestivirus bovine viral diarrhoea virus with members of the Flaviviridae. Journal o f General Virology, 69: 2637-2643. 53. Collett, M.S., Larson, R., Belzer, S.K. and Retzel, E. (1988). Proteins encoded by bovine viral diarrhea virus: the genomic organization of a pestivirus. Virology, 165: 200-208. 54. Collett, M.S., Larson, R., Gold, C., Strick, D., Anderson, D.K. and Purchio, A.F. (1988). Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology, 165: 191-199. 55. Collett, M.S., Moennig, V. and Horzinek, M.C. (1989). Recent advances in pestivirus research. Journal o f General Virology, 70: 253-266. 56. Corapi, W.V., Elliott, R.D., French, T.W., Arthur, D.G., Bezek, D.M. and Dubovi, E.J. (1990). Thrombocytopenia and hemorrhages in veal calves infected with bovine viral diarrhea virus. Journal of the American Veterinary Medical Association, 196: 590-596. 57. Corapi, W.V., French, T.W. and Dubovi, E.J. (1989). Severe thrombocyto­ penia in young calves experimentally infected with noncytopathic bovine viral diarrhea virus. Journal of Virology, 63: 3934-3943. 58. Cordell, B., Donis, R.O. and Dubovi, E.J. (1988). Monoclonal antibody analyses of cytopathic and noncytopathic viruses from fatal bovine viral diarrhea virus infections. Journal o f Virology, 62: 2823-2827. 116 59. Coria, M.F. and McClurkin, A.W. (1978). Specific immune tolerance in an apparently healthy bull persistently infected with bovine viral diarrhea virus. Journal o f the American Veterinary Medical Association, 172: 449-451. 60. Coria, M.F. and McClurkin, A.W. (1981). Effects of incubation tempera­ tures and bovine viral diarrhea virus immune serum on bovine turbinate cells persistently infected with a noncytopathogenic isolate of bovine viral diarrhea virus. American Journal o f Veterinary Research, 42: 647-649. 61. Coria, M.F., Schmerr, M.J.F. and McClurkin, A.W. (1983). Characterization of the major structural proteins of purified bovine viral diarrhea virus. Archives o f Virology, 76: 335-339. 62. Corthier, G. and Aynaud, J.-M. (1973). Maladie des musqueuses: raise en vidence de variations antigniques entre diverses souches a 1’aide de la technique de sroneutralisation en culture cellulaire. Annales de Recherches Veterinaires, 4: 79-86. 63. Costanzi, C. and Gillespie, D. (1987). Fast blots: immobilization of DNA and RNA from cells. Methods In Enzymology, 152: 582-587. 64. Cutlip, R.C., McClurkin, A.W. and Coria, M.F. (1980). Lesions in clinically healthy cattle persistently infected with the virus of bovine viral diarrhea glomerulonephritis and encephalitis. American Journal o f Veterinary Research, 41: 1938-1941. 65. Diderholm, H. and Dinter, Z. (1966). Infectious RNA derived from bovine virus diarrhoea virus. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene, Abteilung I, 201: 270-272. 66. Diderholm, H. and Dinter, Z. (1966). Interference between strains of bovine virus diarrhea virus and their capacity to suppress interferon of a heterologous virus. Proceedings of the Society for Experimental Biology and Medicine, 121: 976-980. 67. Diderholm, H., Klingeborn, B. and Dinter, Z. (1974). A hazy (‘bull’s eye’) plaque variant of bovine viral diarrhea virus. Archiv fiir die gesamte Virusforschung, 45: 169-172. 68. Dinter, Z. and Diderholm, H. (1971). Bovine virus diarrhoea virus: Inhibition of growth by proflavine sulphate. Archiv fiir die gesamte Virusfor­ schung, 34: 388-390. 69. Dinter, Z. and Diderholm, H. (1972). Bovine virus diarrhoea virus: Acquired resistance to acriflavine. Archiv fiir die gesamte Virusforschung, 38: 70. Done, J.T., Terlecki, S., Richardson, C., Harkness, J.W., Sands, J.J., Patterson, D.S.P., Sweasey, D., Shaw, I.G., Winkler, G E . and Duffell, S.J. (1980). Bovine virus diarrhoea-mucosal disease virus: Pathogenicity for the fetal calf following maternal infection. Veterinary Record, 106: 473-479. 117 71. Donis, R.O., Corapi, W. and Dubovi, E.J. (1988). Neutralizing monoclonal antibodies to bovine viral diarrhoea virus bind to the 56K to 58K glycopro­ tein. Journal o f General Virology, 69: 77-86. 72. Donis, R.O. and Dubovi, E.J. (1987). Molecular specificity of the antibody responses of cattle naturally and experimentally infected with cytopathic and noncytopathic bovine viral diarrhea virus biotypes. American Journal of Veterinary Research, 48: 1549-1554. 73. Donis, R.O. and Dubovi, E.J. (1987). Differences in virus-induced polypep­ tides in cells infected by cytopathic and noncytopathic biotypes of bovine virus diarrhea-mucosal disease virus. Virology, 158: 168-173. 74. Donis, R.O. and Dubovi, E.J. (1987). Glycoproteins of bovine viral diarrhoea-mucosal disease virus in infected bovine cells. Journal o f General Virology, 68: 1607-1616. 75. Donis, R.O. and Dubovi, E.J. (1987). Characterization of bovine viral diarrhoea-mucosal disease virus-specific proteins in bovine cells. Journal of General Virology, 68: 1597-1605. 76. Doyle, L.G. and Heuschele, W.P. (1983). Bovine viral diarrhea virus infection in captive exotic ruminants. Journal o f the American Veterinary Medical Association, 183: 1257-1259. 77. Dubovi, E.J., Donis, R., White, R.T., Cordell, B. and Dale, B. (1987). Expression of a glycoprotein antigen of bovine viral diarrhea virus that elicits neutralizing antibody production in mice. In: Vaccines87. Modem Approaches to New Vaccines: Prevention o f AIDS and Other Viral, Bacterial, and Parasitic Diseases, edited by Chanock, R.M., Lerner, R A ., Brown, F. and Ginsberg, H. Cold Spring Harbor Laboratory, New York. p. 435-439. 78. Duffell, S.J. and Harkness, J.W. (1985). Bovine virus diarrhoea-mucosal disease infection in cattle. Veterinary Record, 117: 240-245. 79. Duffell, S.J., Sharp, M.W. and Bates, D. (1986). Financial loss resulting from BVD-MD virus infection in a dairy herd. Veterinary Record, 118: 38-39. 80. Edwards, S., Drew, T.W. and Bushnell, S.E. (1987). Prevalence of bovine virus diarrhoea, virus viraemia. Veterinary Record, 120: 71-71. 81. Edwards, S., Sands, J.J. and Harkness, J.W. (1988). The application of monoclonal antibody" panels to characterize pestivirus isolates from ruminants in Great Britain. Archives o f Virology, 102: 197-206. 82. Edwards, S., Wood, L., Hewitt-Taylor, C. and Drew, T.W. (1986). Evidence for an immunocompromising effect of bovine pestivirus on bovid herpes­ virus I vaccination. Veterinary Research Communications, 10: 297-302. 118 83. Ellis, J.A., Davis, W.C., Belden, E.L. and Pratt, D.L. (1988). Flow cytofluorimetric analysis of lymphocyte subset alterations in cattle infected with bovine viral diarrhea virus. Veterinary Pathology, 25: 231-236. 84. Enzmann, P.J. and Hartner, D. (1977). Studies on the structure of swine fever virus. In: Hog Cholera/Classical Swine Fever and African Swine Fever, Commission of the European Communities (EUR 5904 EN), Brussels. 85. Ernst, P.B. and Butler, D.G. (1983). A bovine virus diarrhea calfhood vaccination trial in a persistently infected herd: effects on titres, health and growth. Canadiati Journal o f Comparative Medicine, 47: 118-123. 86. Espinasse, J., Parodi, A.L., Constantin, A., Viso, M. and Laval, A. (1986). Hyena disease in cattle: a review. Veterinary Record, 118: 328-330. 87. Etchison, J.R., Riesselman, M. and Roberts, P.C. (1988). Generation of defective interfering virus particles of bovine viral diarrhea virus. (UnPub) 88. Felmingham, D. and Brown, F. (1977). Purification and characterisation of bovine viral diarrhoea virus. In: Hog Cholera/Classical Swine Fever and African Swine Fever, Commission of the European Communities (EUR 5904 EN), Brussels', p. 58-69. 89. Fernandez, A., Hewicker, M., Trautwein, G., Pohlenz, J. and Li ess, B. (1989). Viral antigen distribution in the central nervous system of cattle persistently infected with bovine viral diarrhea virus. Veterinary Pathology, 26: 26-32. 90. Fernelius, A L . (1964). Noncytopathogenic bovine viral diarrhea viruses detected and titrated by immunofluorescence. Canadian Journal of Comparative Medicine and Veterinary Science, 28: 121-126. 91. Fernelius, A L . (1969). Characterization of bovine viral diarrhea viruses. IV. Sequential development of BVD viral antigen in cell cultures studied by immunofluorescence. Archiv fiir die gesamte Vimsforschung, 27: 1-12. 92. Fernelius, AL., Lambert, G. and Booth, G.D. (1971). Bovine viral diarrhea virus-host cell interactions: Serotypes and their relation to biotypes by cross neutralization. American Journal of Veterinary Research, 32: 229-236. 93. Fernelius, A.L., Lambert, G. and Hemness, G.J. (1969). Bovine viral diarrhea virus-host cell interactions: Adaptation and growth of virus in cell lines. American Journal of Veterinary Research, 30: 1561-1572. 94. Fernelius, AL., Lambert, G., Packer, R A . and (1969). Bovine viral diarrhea virus-host cell interactions: adaption, propagation, modification and detection of virus in rabbits. American Journal o f Veterinary Research, 30: 1541-1550. 119 95. Frey, H.-R. and Li ess, B. (1971). Vermehrungskinetik und Verwendbarkeit eines stark zytopathogenen VD-MD-Virusstammes fiir diagnostische Untersuchungen mit der Mikrotiter-Methode. Zentralblatt fiir Veterindrmedizin, Reihe B, 18: 61-71. 96. Frost, J.W. and Liess, B. (1973). Separation of structural proteins of bovine viral diarrhea virus, after degradation by different splitting techniques. Archiv fiir die gesamte Vinisforschung, 42: 297-299. 97. Fulton, R.W., Downing, M.M. and Cummins, J.M. (1984). Antiviral effects of bovine interferons on bovine respiratory tract viruses. Journal o f Clinical Microbiology, 19: 492-497. 98. Gardiner, A.C. and Barlow, R.M. (1981). Vertical transmission of Border disease infection. Journal o f Comparative Pathology, 91: 467-470. 99. Gardiner, AC., Nettleton, P.F. and Barlow, R.M. (1983). Virology and immunology of a spontaneous and experimental mucosal disease-like syndrome in sheep recovered from clinical Border disease. Journal o f Comparative Pathology, 93: 463-469. 100. Ghram, A , Reddy, P.G., Blecha, F. and Minocha, H.C. (1989). Effects of bovine respiratory disease viruses and isoprinosine on bovine leukocyte function in vitro. Veterinary Microbiology, 20: 307-314. 101. Giangaspero, M., Wellemans, G., Vanopdenbosch, E., Belloli, A. and Verhulst, A. (1988). Bovine viral diarrhoea. Lancet, 8602: HO. 102. Gillespie, J.H. and Baker, J.A. (1959). Studies on virus diarrhea. Cornell Veterinarian, 49: 439-443. 103. Gillespie, J.H., Baker, J.A. and McEntee, K. (1960). A cytopathogenic strain of virus diarrhea virus. Cornell Veterinarian, 50: 73-79. 104. Gillespie, J.H., Coggins, L., Thompson, J. and Baker, J.A. (1961). Compari­ son by neutralization tests of strains of virus isolated from virus diarrhea and mucosal disease. Cornell Veterinarian, 51: 155-159. 105. Gillespie, J.H., Madin, S.H. and Darby, N. (1962). Cellular resistance in tissue culture induced by non-cytopathogenic strains to a cytopathogenic strain of virus diarrhea virus of cattle. Proceedings of the Society for Experi­ mental Biology and Medicine, HO: 248-250. 106. Gorbalenya, A E ., Donchenko, A.P., Koonin, E.V. and Blinov, V.M. (1989). N-terminal domains of putative helicases of flavi- and pestiviruses may be serine proteases. Nucleic Acids Research, 17: 3889-3897. 107. Gorbalenya, A.E., Koonin, E.V., Donchenko, A P. and Blinov, V.M. (1988). A novel superfamily of nucleoside triphosphate-binding motif containing proteins which are probably involved in duplex unwinding in DNA and RNA replication and recombination. FEBS Letters, 235: 16-24. 120 108. Gould, E A and Clegg, J.C.S. (1985). Growth, titration and purification of togaviruses. In: Virology: a practical approach, edited by Mahy, B.W.J. IRL Press, Oxford, p. 43-78. 109. Grahn, T.C., Fahning, M.L. and Zemjanis, R. (1984). Nature of early reproductive failure caused by bovine viral diarrhea virus. Journal o f the American Veterinary Medical Association, 185: 429-432. HO. Gray, E.W. and Nettleton, P.F. (1987). The ultrastructure of cell cultures infected with Border disease and bovine virus diarrhoea viruses. Journal of General Virology, 68: 2339-2346. 111. Greiser-Wilke, I., ization of various clonal antibodies. J.W. Commission p. 29-41. 112. Grun, J.B. and Brinton, M.A. (1986). Characterization of West Nile virus RNA-dependent RNA polymerase and cellular terminal adenylyl and uridylyl transferases in cell-free extracts. Journal o f Virology, 60: 1113-1124. 113. Gutekunst, D.E. and Malmquist, W.A. (1963). Separation of a soluble antigen and infectious particles of bovine viral diarrhea viruses and their relationship to hog cholera. Canadian Journal o f Comparative Medicine and Veterinary Science, 27: 121-123. 114. Gutekunst, D.E. and Malmquist, W.A. (1964). Complement-fixing and neutralizing antibody response to bovine viral diarrhea and hog cholera antigens. Canadian Journal o f Comparative Medicine and Veterinary Science, 28: 19-23. . 115. Hafez, S.M. and Liess, B. (1972). Studies on bovine viral diarrhea-mucosal disease virus. I. Cultural behaviour and antigenic relationship of some strains. Acta Virologica, 16: 388-398. 116. Hafez, S.M. and Liess, B. (1972). Studies on bovine viral diarrhea-mucosal disease virus. II. Stability and some physico-chemical properties. Acta Virologica, 16: 399-408. 117. Hafez, S.M., Liess, B. and Frey, H.-R. (1976). Studies on the natural occur­ rence of neutralizing antibodies against six strains of bovine viral diarrhea virus in field sera of cattle. Zentralblatt fiir Veterinarmedizin, Reihe B, 23: 669-677. 118. Hafez, S.M., Petzoldt, K. and Reczko, E. (1968). Morphology of bovine viral diarrhoea virus. Acta Virologica, 12: 471-473. 119. Hamblin, C. and Hedger, R.S. (1979). The prevalence of antibodies to bovine viral diarrhoea/mucosal disease virus in African wildlife. Comparative Immunology, Microbiology and infectious Diseases, 2: 295-303. Peters, W., Moennig, V. and Liess, B. (1987). Character­ strains of bovine viral diarrhea virus by means of mono­ In: Pestivirus infections o f ruminants, edited by Harkness, of the European Communities, EUR 10238, Luxembourg, 121 120. Harkness, J.W. (1985). Classical swine fever and its diagnosis: A current view. Veterinary Record, 116: 288-293. 121. Harkness, J.W. (1987). The control of bovine viral diarrhoea virus infection. Annales de Recherches Veterinaires, 18: 167-174. 122. Harkness, J.W. and Roeder, P.L. (1988). The comparative biology of classical, swine fever. In: Classical swine fever and related viral infections, edited by Liess, B. Martinus Nijhoff Publishing, Boston, p. 233-288. 123. Harkness, J.W., Roeder, P.L. and Wood, L. (1984). Mucosal disease in cattle. Veterinary Record, 115: 186-186. 124. Harkness, J.W., Sands, J.J. and Richards, M.S. (1978). Serological studies of mucosal disease virus in England and Wales. Research in Veterinary Science, 24: 98-103. 125. Harkness, J.W. and Vantsis, J.T. (1982). Virology. In: Border Disease of Sheep: a virus-induced teratogenic disorder (Advances in Veterinary Medicine, 36), edited by Barlow, R.M. and Patterson, D.S.P. Verlag Paul Parey, Berlin and Hamburg, p. 56-64. 126. Harkness, J.W., Wood, L. and Drew, T. (1984). Mucosal disease in cattle. Veterinary Record, 115: 283-283. 127. Hedstrom, H. and Isaksson, A. (1951). Epizootic enteritis in cattle in Sweden. Cornell Veterinarian, 41: 251-253. 128. Heilig, J.S. (1990). Large-scale preparation of plasmid DNA. In: Current Protocols in Molecular Biology, edited by Ausubel, F.M„ Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A. and Struhl, K. John Wiley & Sons, New York. p. 1.7.1-1.7.11. 129. Hess, R.G., Coulibaly, C.O.Z., Greiser-Wilke, I., Moennig, V. and Liess, B. (1988). Identification of hog cholera viral isolates by use of monoclonal antibodies to pestiviruses. Veterinary Microbiology, 16: 315-321. 130. Heuschele, W.P. (1978). New perspectives on the epidemiology of bovine virus diarrhea- mucosal disease (BVD). Bovine Practitioner, 13: 51-53. 131. Horzinek, M., Maess, J. and Laufs, R. (1971). Studies on the substructure of togaviruses. II. Analysis of equine arteritis, rubella, bovine viral diarrhea, and hog cholera viruses. Archivfur die gesamte Virusforschung, 33: 306-318. 132. Horzinek, M.C. (1972). . In: International Virology 2. Proceedings of the second International Congress for virology, Budapest 1971, edited by Melnick, J.L. S. Karger, New York. p. 274-275. 133. Horzinek, M.C. (1976). Synthesis of togavirus RNA. In: Studies on Virus Replication, Commission of the European Communities (EUR 5451), Brussels, p. 9-29. 122 134. Horzinek, M.C. (1981). Non-arthropod-bome Togaviruses, Academic Press, New York. 135. Howard, C.J., Brownlie, J. and Clarke, M.C. (1987). Immunoenzyme techniques for bovine viral diarrhoea virus. In: Pestivirus infections of ruminants, edited by Harkness, J.W. Commission of the European Cnmmnnities, EUR 10238, Luxembourg, p. 69-78. 136. Howard, C.J., Brownlie, J. and Clarke, M.C. (1987). Comparison by the neutralisation assay of pairs of non- cytopathogenic and cytopathogenic strains of bovine virus diarrhoea virus isolated from cases of mucosal disease. Veterinary Microbiology, 13: 361-369. 137. Howard, C.J., Brownlie, J. and Thomas, L.H. (1986). Prevalence of bovine virus diarrhoea virus viraemia in cattle in the UK. Veterinary Record, 119: 628-629. 138. Howard, C.J., Clarke, M.C. and Brownlie, J. (1985). An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to bovine viral diarrhoea virus (BVDV) in cattle sera. Veterinary Microbiology, 10: 359-369. 139. Hyclone Laboratories Inc. (1986). BVD virus: Contamination of fetal bovine serum and infection of cell cultures. Art to Science, 5(3): 1-2,6. 140. Hyera, J.M.K., Liess, B. and Frey, H.-R. (1987). A direct neutralizing peroxidase-linked antibody assay for detection and titration of antibodies to bovine viral diarrhoea virus. Journal o f Veterinary Medicine, B, 34: 227-239. 141. . Itoh, O., Sasaki, H. and Hanaki, T. (1983). Reverse plaque formation method for titration of non-cytopathogenic bovine viral diarrhea-mucosal disease virus. National Institute for Animal Health Quarterly, 23: 27-31. 142. . Itoh, O., Sasaki, H. and Hanaki, T. (1984). A study of serologic relation­ ships among non-cytopathogenic strains of bovine viral diarrhea-mucosal disease virus by reverse plaque technique. Japanese Journal o f Veterinary Science, 46: 669-675. 143. Johnson, D.W. and Muscoplat, C.C. (1973). Immunologic abnormalities in calves with chronic bovine viral diarrhea. American Journal o f Veterinary Research, 34: 1139-1141. 144. Joklik, W.K. (1990). Interferons. In: Virology, Ed. 2, edited by Fields, B.N., Knipe, D.M., Chanock, R.M., Hirsch, M.S., Melnick, J.L. and Monath, T.P. Raven Press, New York. p. 383-410. 145. Juntti, N., Larsson, B. and Fossum, .C. (1987). The use of monoclonal anti­ bodies in enzyme linked immunosorbent assays for detection of antibodies to bovine viral diarrhoea virus. Journal o f Veterinary Medicine, B, 34: 356-363. 123 146. Justewicz, D.M., Magar, R., Marsolais, G. and Lecomte, J. (1987). Bovine viral diarrhea virus-infected MDBK monolayer as antigen in enzyme-linked immunosorbent assay (ELISA) for the measurement of antibodies in bovine sera. Veterinary Immunology and Immunopathology, 14: 377-384. 147. Kahrs, R.F. (1981). Bovine viral diarrhea. In: Viral Diseases o f Cattle, Iowa State University Press, p. 89-106. 148. Katz, J.B. and Hanson, S.K. (1987). Competitve and blocking enzymelinked immunoassay for detection of fetal bovine serum antibodies to bovine viral diarrhea virus. Journal o f Virological Methods, 15: 167-175. 149. Katz, J.B., Ludemann, L., Pemberton, J. and Schmerr, M.J. (1987). Detection of bovine virus diarrhea virus in cell culture using an immunoperoxidase technique. Veterinary Microbiology, 13: 153-157. 150. Kendrick, J.W. (1976). Bovine viral diarrhea virus-induced abortion. Theriogenology, 5: 91-93. 151. Ketelsen, A T., Johnson, D.W. and Muscoplat, C.C. (1979). Depression of bovine monocyte chemotactic responses by bovine viral diarrhea virus. Infection and Immunity, 25: 565-568. 152. Kniazeff, A.J., Huck, R A ., Jarrett, W.F.H., Pritchard, W.R., Ramsey, F.K., Schipper, LA., Stober, M. and Liess, B. (1961). Antigenic relationship of some bovine viral diarrhoea-mucosal disease viruses from the United States, Great Britain, and West Germany. Veterinary Record, 73: 768-769. 153. Lambert, G., McClurkin, A.W. and Fernelius, A.L. (1974). Bovine viral diarrhea in the neonatal calf. Journal o f the American Veterinary Medical Association, 164: 287-289. 154. Lamontagne, L., Lafortune, P. and Fournel, M. (1989). Modulation of the cellular immune responses to T-cell-dependent and T cell-independent antigens in lambs with induced bovine viral diarrhea virus infection. Ameri­ can Journal of Veterinary Research, 50: 1604-1608. 155. Larsson, B., Possum, C. and Aehius, S. (1988). A cellular analysis of immu­ nosuppression in cattle with mucosal disease. Research in Veterinary Science, 44: 71-75. 156. Laskey, R A . and Mills, A.D. (1975). Quantitative detection of 3H and 14C in polyacrylamide gels by tluorography. European Journal of Biochemistry, 56: 335-341. 157. Laude, H. (1979). Nonarbo-togaviridae: comparative hydrodynamic properties of the Pestivirus genus. Archives of Virology, 62: 347-352. 158. Laude, H. and Gelfi, J. (1979). Properties of border disease virus as studied in a sheep cell line. Archives o f Virology, 62: 341-346. 124 159. Lech, K. and Brent, R. (1990). Minipreps of plasmid DNA. In: Current Protocols in Molecular Biology, edited by Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, I.A. and Struhl, K. John Wiley & Sons, New York. p. 1.6.1-1.6.6. 160. Lee, K.M. and Gillespie, J.H. (1957). Propagation of virus diarrhea virus of cattle in tissue culture. American Journal o f Veterinary Research, 18: 952-953. 161. Liess, B., Frey, H.-R., Kittsteiner, H., Baumann, F. and Neumann, W. (1974). Beobachtungen und Untersuchungen tiber die "Mucosal Disease" des Rindes-einer immunobiologisch erklarbaren Spatform der BVD-MDVirusinfektion mit Kriterien einer "slow virus infection"?). Deutsche Tierdrztliche Wochenschrift, 81: 481-487. 162. Liess, B., Frey, H.-R., Orban, S. and Hafez, S.M. (1983). Bovine Virusdiarrhoe (BVD)-"Mucosal Disease": Persistente BVD-Feldvirusinfektionen bei serologisch selektierten Rindern. Deutsche Tierdrztliche Wochenschrift, 90: 261-266. 163. Liess, B., Frey, H.-R. and Prager, D. (1977). Antibody response of pigs following experimental infections with strains of hog cholera, bovine viral diarrhoea virus. In: Hog Cholera/Classical Swine Fever and African Swine ■Fever, Commission of the European Communities EUR 5904 EN, Brussels, p. 200-213. 164. Liess, B., Orban, S., Frey, H.-R. and Trautwein, G. (1987). Experimental transplacental transmission of BVD virus to the bovine fetus. In: Pestivims infections o f mminants, edited by Harkness, J.W. Commission of the European Communities, Luxembourg, p. 159-166. 165. Liess, B., Orban, S., Frey, H.-R., Trautwein, G., Wiefel, W. and Blindow, H. (1984). Studies on transplacental transmissibility of a bovine virus diarrhoea (BVD) vaccine virus in cattle: II. Inoculation of pregnant cows without detectable neutralizing antibodies to BVD virus 90-229 days before parturi­ tion (51st to 190th day of gestation). Zentralblatt fiir Veterindrmedizin. Reihe B, 31: 669-681. 166. Littlejohns, LR. and Walker, K.H. (1985). Aetiology and pathogenesis of mucosal disease of cattle: current concepts, observations and speculation. Australian Veterinary Journal, 62: 101-103. 167. Lobmann, M., Charlier, P., Florent, G. and Zygraich, N. (1984). Clinical evaluation of a temperature-sensitive bovine viral diarrhea vaccine strain. American Journal o f Veterinary Research, 45: 2498-2503. 168. Lobmann, M., Charlier, P., Klaassen, C.L. and Zygraich, N. (1986). Safety of a temperature-sensitive vaccine strain of bovine viral diarrhea virus in pregnant cows. American Journal o f Veterinary Research, 47: 557-560. 125 169. Madin, S.H. and Darby, N.B. (1958). Established kidney cell lines of normal adult bovine and ovine origin. Proceedings o f the Society for Experimental Biology and Medicine, 98: 574-576. 170. Maess, J. and Reczko, E. (1970). Electron optical studies of bovine viral diarrhea-mucosal disease virus (BVDV). Archiv fiir die gesamte Vimsforschung, 30: 39-46. 171. Magar, R. and Lecomte, J. (1987). Comparison of methods for concentra­ tion and purification of bovine viral diarrhea virus. Journal o f Virological Methods, 16: 271-279. 172. Magar, R., Minocha, H.C. and Lecomte, J. (1988). Bovine viral diarrhea virus proteins: heterogeneity of cytopathogenic and nomcytopathogenic strains and evidence of a 53K glycoprotein neutralization epitope. Veterinaiy Microbiology, 16: 303-314. 173. Magar, R., Minocha, H.C., Montpetit, C., Carman, P.S. and Lecomte, J. (1988). Typing of cytopathic and noncytopathic bovine viral diarrhea virus reference and Canadian field strains using a neutralizing monoclonal antibody. Canadian Journal of Veterinary Research, 52: 42-45. 174. Mahnel, H. (1984). Untersuchungen tiber die Vermehrung von cytopathogenem und nichtcytopathogenem BVD-Kulturvirus. Zentralblatt fiir Veterindrmedizin, Reihe B, 31: 261-273. 175. Mahnel, H. and Moreau, H. (1984). Untersuchungen fiber die Vermehrung von cytopathogenem und nichtcytopathogenem BVD-Kulturvirus. I. Quantitative und fluoreszenzserologische Vergleiche. Zentralblatt fur Veterindrmedizin, Reihe B, 31: 131-140. 176. Maisonnave, J. and Rossi, C.R. (1982). A microtiter test for detecting and titrating non-cytopathogenic bovine viral diarrhea virus. Archives o f Virology, 72: 279-287. 177. Malmquist, W.A. (1968). Bovine viral diarrhea-mucosal disease: Etiology, pathogenesis and applied immunity. Journal o f the American Veterinary Medical Association, 152: 763-770. 178. Maniatis, T., Fritsch, E.F. and Sambrook, J. (1982). Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, New York. 179. Marcus, S.J. and Moll, T. (1968). Adaption of bovine viral diarrhea virus to the Madin-Darby bovine kidney cell line. American Journal o f Veterinary Research, 29: .817-819. 180. Markham, R.J.F. and Ramnaraine, M.L. (1985). Release of immuno­ suppressive substances from tissue culture cells infected with bovine viral diarrhea virus. American Journal o f Veterinary Research, 46: 879-883. 126 181. Martin, S.W., Bateman, K.G., Shewen, P.E., Rosendal, S. and Bohac, J.E. (1989). The frequency, distribution and effects of antibodies, to seven putative respiratory pathogens, on respiratory disease and weight gain in feedlot calves in Ontario. Canadian Journal o f Veterinary Research, 53: 182. Matthaeus, W. (1979). Detection of three polypeptides in preparations of bovine viral diarrhoea virus. Archives o f Virology, 59: 299-305. 183. Matthaeus, W. (1980). Analysis of soluble bovine viral diarrhoea virus antigens and serological relationship to virus structural glycoproteins. Zentralblatt fiir Veterindrmedizin, Reihe B, 27: 734-741. 184. Matthaeus, W. (1981). Differences in reaction behaviour of structural polypeptides of bovine viral diarrhoea virus (BVDV) with antisera against BVDV and hog cholera virus (HCV). Zentralblatt fiir Veterindrmedizin, Reihe B, 28: 126-132. 185. McClurkin, A.W., Bolin, S.R. and Coria, M.F. (1985). Isolation of cytopathic and noncytopathic bovine viral diarrhea virus from the spleen of cattle acutely and chronically affected with bovine viral diarrhea. Journal of the American Veterinary Medical Association, 186: 568-569. 186. McClurkin, A.W. and Coria, M.F. (1978). Selected isolates of bovine viral diarrhea (BVD) virus propagated on bovine turbinate cells: Virus titer and soluble antigen production as factors in immunogenicity of killed BVD virus. Archives o f Virology, 58: 119-125. 187. McClurkin, A.W., Coria, M.F. and Cutlip, R.C. (1979). Reproductive performance of apparently healthy cattle persistently infected with bovine viral diarrhea virus. Journal o f the American Veterinaiy Medical Association, 174: 1116-1119. 188. McClurkin, A.W., Littledike, E.T., Cutlip, R.C., Frank, G.H., Coria, M .F .. and Bolin, S.R. (1984). Production of cattle immunotolerant to bovine viral diarrhea virus. Canadian Journal of Comparative Medicine, 48: 156-161. 189. McClurkin, A.W., Pirtle, E.C., Coria, M.F. and Smith, R.L. (1974). Compar­ ison of low- and high-passage bovine turbinate cells for assay of bovine viral diarrhea virus. Archiv fiir die gesamte Virusforschung, 45: 285-289. 190. McHugh, P.H., Mackie, D.P., McNulty, M.S. and McFerran, J.B. (1988). Production of monoclonal antibodies to bovine virus diarrhoea virus and their reactivity with selected pestivirus. isolates. Journal o f Veterinary Medicine, B, 35: 207-213. 191. McMaster, G.K. and Carmichael, G.G. (1977). Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proceedings o f the National Academy of Sciences, USA, 74: 4835-4838. 127 192. Meyers, G., Rumenapf, T. and Thiel, H-J. (1989). Ubiquitin in a togavirus. Nature, 341: 491. 193. Meyers, G., Rumenapf, T. and Thiel, H-J. (1989). Molecular cloning and nucleotide sequence of the genome of hog cholera virus. Virology, 171: 194. Meyling, A. (1983). An immunoperoxidase (PO) technique for detection of BVD virus in serum of clinically and subclinically infected cattle. Proceedings International Symposium World Association o f Veterinary.Labora­ tory Diagnosticians, 3: 179-184. 195. Meyling, A. and Jensen, AM . (1988). Transmission of bovine virus diarrhoea virus (BVDV) by artificial insemination (Al) with semen from a persistently-infected bull. Veterinary Microbiology, 17: 97-105. 196. Meyling, A., Ronsholt, L., Dalsgaard, K. and Jensen, A.M. (1987). Experi­ mental exposure of vaccinated and non-vaccinated pregnant cattle to isolates of bovine viral diarrhoea virus (BVDV). In: Pestivirus infections of ruminants, edited by Harkness, J.W. Commission of the European Communities, EUR 10238, Luxembourg, p. 225-231. 197. Mills, J.H.L., Nielsen, S.W. and Luginbuhl, R.E. (1965). Current status of bovine mucosal disease. Journal o f the American Veterinary Medical Associa­ tion, 146: 691-696. 198. Mohanty, J.G., Elazhary, Y. and Talbot, B. (1989). Detergent solubilised bovine viral diarrhea virus elicits a similar immune response as the inactivated virus. Comparative Immunology, Microbiology and Infectious Diseases, 12: 129-137. 199. Moorman, R.J.M. and Hulst, M.M. (1988). Hog cholera virus: identification and characterization of the viral RNA and the virus-specific RNA synthesized in infected swine kidney cells. Virus Research, 11: 281-291. 200. Murphy, F.A. (1980). Togavirus morphology and morphogenesis. In: The Togaviruses. Biology, structure, replication, edited by Schlesinger, R.W. Academic Press, New York. p. 241-316. 201. Muscoplat, C.C., Johnson, D.W. and Stevens, J.B. (1973). Abnormalities of in vitro lymphocyte responses during bovine viral diarrhea virus infection. American Journal of Veterinary Research, 34: 753-755. 202. Muscoplat, C.C., Johnson, D.W. and Teuscher, E. (1973). Surface immuno­ globulin of circulating lymphocytes in chronic bovine diarrhea: Abnormal­ ities in cell populations and cell function. American Journal o f Veterinary Research, 34: 1101-1104. 203. Nettleton, P.F., Gardiner, AC., Gilmour, J.S. and Barber, D.M.L. (1987). Cytopathic and non-cytopathic isolates of BVDV in a closed dairy herd. In: •128 Pestivirus infections o f ruminants, edited by Harkness, J.W. Commission of tbje European Communities, EUR 10238, Luxembourg, p. 13-27. 204. Norton, J.D., Cook, F., Roberts, P.C., Clewley, J.P. and Avery, R.J. (1984). Expression of Kirsten murine sarcoma virus in transformed nonproducer and revertant NIH/3T3 cells: Evidence for cell-mediated resistance to a viral oncogene in phenotypic reversion. Journal o f Virology, 50: 439-444. 205. Nuttall, PA . (1980). Growth characteristics of two strains of bovine virus diarrhoea virus. Archives o f Virology, 66: 365-369. 206. Nuttall, PA ., Luther, P.D. and Stott, E.J. (1977). Viral contamination of bovine foetal serum and cell cultures. Nature, 266: 835-837. 207. Nuttall, PA ., Stott, E.J. and Thomas, L.H. (1980). Experimental infection of calves with two strains of bovine virus diarrhoea virus: virus recovery and clinical reactions. Research in Veterinary Science, 28: 91-95. 208. Olafson, P., MacCallum, A.D. and Fox, F.H. (1946). An apparently new transmissible disease of cattle. Cornell Veterinarian, 36: 205-213. 209. Olafson, P. and Rickard, C.G. (1947). Further observations on the virus diarrhea (new transmissible disease) of cattle. Cornell Veterinarian, 37: 104-106. 210. Omori, T., Inaba, Y., Morimoto, T., Tanaka, T., Kurogi, H. and Matumoto, M. (1967). Bovine diarrhea virus: I. Isolation of non-cytopathogenic strains detectable by END method. Japanese Journal o f Microbiology, 11: 133-142. 211. Osburn, B.I., Clarke, G.L., Stewart, W.C. and Sawyer, M. (1973). Border disease-like syndrome in lambs: antibodies to hog cholera and bovine viral diarrhea viruses. Journal of the American Veterinary Medical Association, ■ 163: 1165-1167. 212. Perdrizet, JA ., Rebhun, W.C., Dubovi, E.J. and Donis, R.O. (1987). Bovine virus diarrhea - clinical syndromes in dairy herds. Cornell Veterinarian, 77: 46-74. 213. Peters, W., Greiser-Wilke, I., Moennig, V. and Liess, B. (1986). Preliminary serological characterization of bovine viral diarrhoea virus strains using monoclonal antibodies. Veterinary Microbiology, 12: 195-200. 214. Peters, W., Liess, B., Frey, H.-R. and Trautwein, G. (1987). Incidence and impact of persistent infections with BVD virus in the field. In: Pestivirus infections o f ruminants, edited by Harkness, J.W. Commission of the European Communities, EUR 10238, Luxembourg, p. 133-143. 215. Plant, J.W., Acland, H.M., Card, G.P. and Walker, K.H. (1983). Clinical variation of Border disease in sheep according to the source of the inoculum. Veterinary Record, 113: 58-60. 129 216. Plant, J.W., Littlejohns, LR., Gardiner, A G , Vantsis, J.T. and Hack, R A . (1973). Immunological relationship between, border disease, mucosal disease and swine fever. Veterinary Record, 92: 455. 217. Pocock, D.H., Howard, C.J., Clarke, M.C. and Brownlie, J. (1987). Varia­ tion in the intracellular polypeptide profiles from different isolates of bovine virus diarrhoea virus. Archives o f Virology, 94: 43-53. 218. Potgieter, L.N.D., McCracken, M.D., Hopkins, F.M. and Guy, J.S. (1985). Comparison of the pneumopathogenicity of two strains of bovine viral diarrhea virus. American Journal o f Veterinary Research, 46: 151-153. 219. Potgieter, L.N.D., McCracken, M.D., Hopkins, F.M. and Walker, R.D. (1984). Effect of bovine viral diarrhea virus infection on the distribution of infectious bovine rhinotracheitis virus in calves. American Journal of Veterinary Research, 45: 687-690. 220. Potgieter, L.N.D., McCracken, M.D., Hopkins, F.M., Walker, R.D. and Guy, J.S. (1984). Experimental production of bovine respiratory tract disease with bovine viral diarrhea virus. American Journal o f Veterinary Research, 45: 1582-1585. 221. Potts, B.J., Sever, J.L., Tzan, N.R., Huddleston, D. and Elder, GA . (1987). Possible role of pestiviruses in microcephaly. Lancet, 8539: 972-973. 222. Pritchard, G.C., Borland, E.D., Wood, L. and Pritchard, D.G. (1989). Severe disease in a dairy herd associated with acute infection with bovine virus diarrhoea virus, Leptospira hardjo and Coxiella burnetii. Veterinary Record, 124: 625-629. 223. Pritchard, W.R. (1963). The bovine viral diarrhea-mucosal disease complex. Advances in Veterinary Science, 8: 1-47. 224. Pritchard, W.R., Carlson, R.G., Moses, H.E. and Taylor, D.E.B. (1955). Virus diarrhea and mucosal disease. Proceedings o f the U.S.Livestock Sanitation Association, 59: 173-188. 225. Pritchett, R., Manning, J.S. and Zee, Y.C. (1975). Characterization of bovine viral diarrhea virus RN A. Journal o f Virology, 15: 1342-1347. 226. Pritchett, R.F. and Zee, Y.C. (1975). Structural proteins of bovine viral diarrhea virus. American Journal o f Veterinary Research, 36: 1731-1734. 227. Promega Biotec (1985). Optimizing transcription performance of Riboprobe SP6 and T7 RNA polymerases. Promega Notes, I: 2-6. 228. Purchio, A.F., Larson, R. and Collett, M.S. (1983). Characterization of virus-specific RNA synthesized in bovine cells infected with bovine viral diarrhea virus. Journal o f Virology, 48: 320-324. 130 229. Purchio, A.F., Larson, R. and Collett, M.S. (1984). Characterization of bovine viral diarrhea virus proteins. Journal of Virology, 50: 666-669. 230. Purchio, A.F., Larson, R., Torborg, L.L. and Collett, M.S. (1984). Cell-free translation of bovine viral diarrhea virus RN A. Journal o f Virology, 52: 231. Rae, A G ., Sinclair, J.A. and Nettleton, P.F. (1987). Survival of bovine virus diarrhoea virus in blood from persistently infected cattle. Veterinary Record, 120: 504. 232. Ramsey, F.K. and Chivers, W.H. (1953). Mucosal disease of cattle. North American Veterinarian, 34: 629-633. 233. Rebhun, W.C., French, T.W., Perdrizet, LA., Dubovi, E.J., Dill, S.G. and Karcher, L.F. (1989). Thrombocytopenia associated with acute bovine virus diarrhea infection in cattle. Journal of Veterinary Internal Medicine, 3: 42-46. 234. Reggiardo, C. (1975). Cell-mediated immune responses in cattle, Ph.D.Thesis, Iowa State University. 235. Reggiardo, C. and Kaeberle, M.L. (1981). Detection of bacteremia in cattle inoculated with bovine viral diarrhea virus. American Journal o f Veterinary Research, 42: 218-221. 236. Renard, A., Brown-Shimmer, S., Schmetz, D., Guiot, C., Dagenais, L., Pastoret, P.-P., Dina, D. and Martial, J. (1987). Molecular cloning, sequenc­ ing and expression of BVDV RN A. In: Pestivirus infections o f ruminants, edited by Harkness, J.W. Commission of the European Communities, EUR 10238, Luxembourg, p. 53-64. 237. Renard, A., Guiot, C., Schmetz, D., Dagenais, L., Pastoret, P.-P., Dina, D. and Martial, J.A. (1985). Molecular cloning of bovine viral diarrhea viral sequences. D NA, 4: 429-438. 238. Renard, A., Schmetz, D., Guiot, C., Brown-Shimmer, S., Dagenais, L., Pastoret, P.-P., Dina, D. and Martial, J.A. (1987). Molecular cloning of the bovine viral diarrhea virus genomic RN A. Annales de Recherches Veterinaires, 18: 121-125. 239. Revell, S.G., Chasey, D., Drew, T.W. and Edwards, S. (1988). Some observ­ ations on the semen of bulls persistently infected with bovine virus diarrhoea virus. Veterinary Record, 123: 122-125. 240. Rinaldo, C R ., Isackson, D.W., Overall, J.C., Glasgow, LA., Brown, T.T., Bistner, S.I., Gillespie, J.H. and Scott, F.W. (1976). Fetal and adult bovine interferon production during bovine viral diarrhea virus infection. Infection and Immunity, 14: 660-666. 131 241. Ritchie, A.E. and Fernelius, A.L. (1969). Characterization of bovine viral diarrhea viruses. V. Morphology of characteristic particles studied by electron microscopy. Archiv filr die gesamte Vmisforschung, 28: 369-389. 242. Robb, J.A. and Bond, C.W. (1979). Pathogenic murine coronaviruses: I. Characterization of biological behaviour in vitro and virus-specific intracell­ ular RNA of strongly neurotropic JHMV and weakly neurotropic AS9V viruses. Virology, 94: 352-370. 243. Robb, J.A. and Martin, R.G. (1970). Genetic analysis of simian virus 40: I. Description of microtitration and replica-plating techniques for virus. Virology, 41: 751-760. 244. Roberts, D.H., Lucas, M.H., Wibberley, G. and Westcott, D. (1988). Response of cattle persistently infected with bovine virus diarrhoea virus to bovine leukosis virus. Veterinary Record, 122: 293-296. 245. Roberts, P.C., Etchison, J.R. and Bond, C.W. (1988). A rapid, quantitative assay for titration of bovine virus diarrhoea-mucosal disease virus. Veterinary Microbiology, 18: 209-217. 246. Roberts, P.C., Norton, J.D. and Avery, R.J. (1984). Virus-like 30S RNA is selectively packaged by Kirsten leukemia virus in virions of smaller size class. Archives o f Virology, 81: 353-357. 247. Rockborn, G., Diderholm, H. and Dinter, Z. (1974). Bovine viral diarrhea virus: Acquired resistance to acriflavine as marker of an attenuated strain. Archiv fiir die gesamte Vmisforschung, 45: 128-134. 248. Roeder, P.L. and Drew, T.W. (1984). Mucosal disease of cattle: A late sequel to fetal infection. Veterinary Record, 114: 309-313. 249. Roeder, P.L. and Harkness, J.W. (1986). BVD virus infection: Prospects for control. Veterinary Record, 118: 143-147. 250. Roeder, P.L., Jeffrey, M. and Cranwell, M.P. (1986). Pestivirus fetopathogenicity in cattle: Changing sequelae with fetal maturation. Veterinary Record, 118: 44-48. 251. Roeder, P.L., Jeffrey, M. and Drew, T.W. (1987). Variable nature of Border disease on a single farm: the infection status of affected sheep. Research in Veterinary Science, 43: 28-33. 252. Rossi, C.R., Bridgman, C.R. and Kiesel, G.K. (1980). Viral contamination of bovine fetal lung cultures and bovine fetal serum. American Journal o f Veterinary Research, 41: 1680-1681. 253. Rossi, C.R. and Kiesel, G.K. (1980). Factors affecting the production of bovine type I interferon on bovine embryonic lung cells by polyriboinosinicpolyribocytidylic acid. American Journal o f Veterinary Research, 41: 557-560. 132 254. Rossi, C.R. and Kiesel, G.K. (1983). Characteristics of the polyriboinosinic acidrpolyribocytidylic acid assay for noncytopathogenic bovine viral diarrhea virus. American Journal o f Veterinary. Research, 44: 1916-1919. 255. Rossi, C.R., Kiesel, G.K. and Hoff, E.J. (1980). Factors affecting the assay of bovine type I interferon on bovine embryonic lung cells. American Journal o f Veterinary Research, 41: 552-556. 256. Roth, J.A., Bolin, S.R. and Frank, D.E. (1986). Lymphocyte blastogenesis and neutrophil function in cattle persistently infected with bovine viral diarrhea virus. Americati Journal o f Veterinary Research, 47: 1139-1141. 257. Roth, J.A. and Kaeberle, M.L. (1983). Suppression of neutrophil and lymphocyte function induced by a vaccinal strain of bovine viral diarrhea virus with and without the administration of ACTH. American Journal of Veterinary Research, 44: 2366-2372. 258. Roth, J.A., Kaeberle, M.L. and Griffith, R.W. (1981). Effects of bovine viral diarrhea virus infection on bovine polymorphonuclear leukocyte function. American Journal of Veterinary Research, 42: 244-250; 259. Rumenapf, T., Meyers, G., Stark, R. and Thiel, H.-J. (1989). Hog cholera virus-characterization of specific antiserum and identification of cDNA clones. Virology, 171: 18-27. 260. . Schiff, L.J., Storz, J. and Collier, J.R. (1973). Kinetics of viral adsorption in singly and multiply infected bovine cells. American Journal o f Veterinary Research, 34: 1453-1455. 261. Schipper, LA., Eveleth, D.F., Shumard, R.F. and Richards, S.H. (1955). Mucosal disease of cattle. Veterinary Medicine, 50: 431-434,450. 262. Schultz, R.D. (1973). Developmental aspects of the fetal bovine immune response: a review. Cornell Veterinarian, 63: 507-535. 263. Seidman, C.E. (1990). Introduction of plasmid DNA into cells: transforma­ tion using calcium chloride. In: Current Protocols in Molecular Biology, edited by Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A. and Struhl, K. John Wiley & Sons, Inc., New York. p. 1.8.1-1.8.3. 264. Selden, R.F. and Chory, J. (1990). Purification of large DNA restriction fragments. In: Current Protocols in Molecular Biology, edited by Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A. and Struhl, K. John Wiley & Sons, New York. p. 2.6.1-2.6.8. 265. Shimizu, M., Murakami, S. and Satou, K. (1989). Serological comparison of cytopathogenic and non-cytopathogenic bovine viral diarrhea-mucosal disease viruses.isolated from cattle with mucosal disease. Japanese Journal o f Veterinary Science, 51: 157-162. 133 266. Shimizu, M., Satou, K., Nishioka, N., Yoshino, T., Momotani, E. and Ishikawa, Y. (1989). Serological characterization of viruses isolated from experimental mucosal disease. Veterinary Microbiology, 19: 13-21. 267. Shope, R.E., Muscoplat, C.C., Chen, A.W. and Johnson, D.W. (1976). Mechanism of protection from primary bovine viral diarrhea virus infection: I. The effects of dexamethasone. Canadian Journal o f Comparative Medicine, 40: 355-359. 268. Simonds, J.B. and Brown, G.T. (1871). Acorn-poisoning. The Veterinarian, 44: 125-142. 269. Steck, F., Lazary, S., Fey, H., Wandeler, A., Huggler, C., Oppliger, G., Baumberger, H., Kaderli, R., Martig, J. and (1980). Immune responsive­ ness in cattle fatally affected by bovine virus diarrhea-mucosal disease. Zentralblatt fiir Veterindrmedizin, Reihe B, 27: 429-445. 270. Stott, E.J., Thomas, L.H., Collins, A.P., Crouch, S., Jebbett, J., Smith, G.S., Luther, P.D. and Caswell, R. (1980). A survey of virus infections of the respiratory tract of cattle and their association with disease. Journal of Hygiene, Cambridge, 85: 257-270. 271. Straver, P.J. (1971). Plaque formation by non-cytopathogenic bovine virus diarrhea strains. Archiv fiir die gesamte Virusforschung, 34: 131-135. 272. Straver, P.J., Journe, D.L.H. and Binkhorst, G.J. (1983). Neurological disorders, virus persistence and hypomyelination in calves due to intrauter­ ine infections with bovine virus diarrhoea virus. II. Virology and epizootiology. Veterinary Quarterly, 5: 156-164. 273. Terpstra, C. and Wensvoort, G. (1988). Natural infections of pigs with bovine viral diarrhoea virus associated with signs resembling swine fever. Research in Veterinary Science, 45: 137-142. 274. Teyssedou, E., Magar, R., Justewicz, D.M. and Lecomte, J. (1987). Cellprotective monoclonal antibodies to bovine enterovirus-3 and partial or no activity against other serotypes. Journal o f Virology, 61: 2050-2053. 275. Thomas, P.S. (1980). Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proceedings o f the National Academy o f Sciences, USA, 77: 5201-5205. 276. Thomson, R.G. and Savan, M. (1963). Studies on virus diarrhea and mucosal disease of cattle. Canadian Journal of Comparative Medicine and Veterinary Science, 27: 207-214. 277. Torgerson, P.R., Dalgleish, R. and Gibbs, H.A. (1989). Mucosal disease in a cow and her suckled calf. Veterinary Record, 125: 530-531. 134 278. Toth, T. and Hesse, R A . (1983). Replication of five bovine respiratory viruses in cultured bovine alveolar macrophages. Archives o f Virology, 75: 279. Truitt, R.L. and Shechmeister, I.L. (1973). The replication of bovine viral diarrhea-mucosal disease virus in bovine leukocytes in vitro. Archiv fur die gesamte Virusforschung, 42: 78-87. 280. Turk, J.R., Corstvet, R.E., McClure, J.R., Gossett, K.A., Enright, F.M. and Pace, L.W. (1985). Synergism of bovine virus diarrhea virus and Pasteurella haemolytica serotype I in bovine respiratory disease complex: I. Leukocyte alterations and pulmonary lesion volumes. Proceedings o f the American Association o f Veterinary Laboratory Diagnosticians, 28: 67-80. 281. Tyler, D.E. and Ramsey, F.K. (1965). Comparative pathologic, immuno­ logic, and clinical responses produced by selected agents of the bovine mucosal disease-virus diarrhea complex, American Journal o f Veterinary Research, 26: 903-913. 282. Underdahl, N.R., Grace, O.D. and Hoerlein, A.B. (1957). Cultivation in tissue-culture of cytopathogenic agent from bovine mucosal disease. Proceedings o f the Society for Experimental Biology and Medicine, 94: 795-797. 283. Van Aert, A. (1975). The immunologically related swine fever and mucosal disease precipitinogens and nucleotide metabolism. Vlaams Diergeneeskundig Tijdschrift, 44: 349-364. 284. Van Aert, A., Degraef, R. and Wellemans, G. (1975). Purification and some properties of swine fever related mucosal disease precipitinogen. Vlaams Diergeneeskundig Tijdschrift, 44: 339-348. 285. Van Oirschot, J.T. (1983). Congenital infections with nonarbo togaviruses. Veterinary Microbiology, 8: 321-361. 286. Vantsis, J.T., Barlow, R.M. and Gardiner, AC. (1980). The effects of challenge with homologous and heterologous strains of Border disease virus on ewes with previous experience of the disease. Journal o f Comparative Pathology, 90: 39-45. 287. Wafula, J.S. (1986). Some antigenic and cultural properties of Border disease and bovine viral diarrhoea viruses. Veterinary Research Communica­ tions, 10: 189-194. 288. Ward, A.C.S. and Kaeberle, M.L. (1984). Use of an immunoperoxidase stain for the demonstration of bovine viral diarrhea virus by light and electron microscopies. American Journal o f Veterinary Research, 45: 165-170. 289. Ward, G.M., Roberts, S.J., McEntee, K. and Gillespie, J.H. (1969). A study of experimentally induced bovine viral diarrhea- mucosal disease in pregnant cows and their progeny. Cornell Veterinarian, 59: 525-538. 135 290. Weiland, E., Thiel, H-J., Hess, G. and Welland, F. (1989). Development of monoclonal neutralizing antibodies against bovine viral diarrhea virus after pretreatment of mice with normal bovine cells and cyclophosphamide. Journal o f Virological Methods, 24: 237-244. 291. Wensvoort, G. (1989). Topographical and functional mapping of epitopes on hog cholera virus with monoclonal antibodies. Journal o f General Virology, 70: 2865-2876. 292. Wensvoort, G., Boonstra, J. and Bodzinga, B.G. (1990). Immunoaffinity purification and characterization of the envelope protein E l of hog cholera virus. Journal o f General Virology, 71: 531-540. 293. Wensvoort, G. and Terpstra, C. (1988). Bovine viral diarrhoea virus infections in piglets born to sows vaccinated against swine fever with contaminated vaccine. Research in Veterinary Science, 45: 143-148. 294. Wensvoort, G., Terpstra, C., Boonstra, J., Bloemraad, M. and Van Zaane, D. (1986). Production of monoclonal antibodies against swine fever virus and their use in laboratory diagnosis. Veterinaiy Microbiology, 12: 101-108. 295. Wensvoort, G., Terpstra, C., De Kluijver, E.P., Kragten, C. and Warnaar, J.G. (1989). Antigenic differentiation of pestivirus strains with monoclonal antibodies against hog cholera virus. Veterinary Microbiology, 21: 9-20. 296. Wensvoort, G., Terpstra, C. and De Kluyver, E.P. (1989). Characterization of porcine and some ruminant pestiviruses by cross-neutralization. Veterinary Microbiology, 20: 291-306. 297. Wentink, G.H., Remmen, J.L.A.M. and van Exsel, A.C.A. (1989). Pregnancy rate of heifers bred by an immunotolerant bull persistently infected with bovine viral diarrhoea virus. Veterinary Quarterly, 11: 171-174. 298. Westaway, E.G. (1987). Flavivirus replication strategy. Advances in Virus Research, 33: 45-90. 299. Westaway, E.G., Brinton, M.A., Gaidamovich, S.Ya., Horzinek, M.C., Igarashi, A., Kaariainen, L., Lvov, D.K., Porterfield, J.S., Russell, P.K. and Trent, D.W. (1985). Togaviridae. Intervirology, 24: 125-139. 300. Westenbrink, F., Middel, W.G.J., Straver, P.J. and de Leeuw, P.W. (1986). A blocking enzyme-linked immunosorbent assay (ELISA) for bovine virus diarrhoea virus serology. Journal o f Veterinary Medicine, B, 33: 354-361. 301. Westenbrink, F., Straver, P.J., Kimman, T.G. and de Leeuw, P.W. (1989). Development of a neutralisisng antibody response to an inoculated cytopathic strain of bovine virus diarrhoea virus. Veterinary Record, 125: 262-265. 136 302. Whitmore, H.L., Zemjanis, R. and Olson, J. (1981). Effect of bovine viral diarrhea virus on conception in cattle. Journal o f the American Veterinary Medical Association, 178: 1065-1067. 303. Wilks, C R ., Abraham, G. and Blackmore, D.K. (1989). Bovine pestivirus and human infection. Lancet, 8629: 107. 304. Wray, C. and Roeder, P.L. (1987). Effect of bovine virus diarrhoea-mucosal disease virus infection on salmonella infection in calves. Research in Veterinary Science, 42: 213-218. 305. Yolken, R., Leister, F., Almeido-Hill, J., Dubovi, E., Reid, R. and Santosham, M. (1989). Infantile gastroenteritis associated with excretion of ■ pestivirus antigens. Lancet, 8637: 517-520. 306. Zeegers, J.J.W. and Horzinek, M.C. (1977). Some properties of bovine viral diarrhoea virus and hog cholera virus and their RNA’s. In: Hog Cholera/Classical Swine Fever and African Swine Fever, Commission of the European Communities (EUR 5904 EN), Brussels, p. 52-57. 307. Zhou, Y., Moennig, V., Coulibaly, C.O.Z., Dahle, J. and Liess, B. (1989). Differentiation of hog cholera and bovine virus diarrhoea viruses in pigs using monoclonal antibodies. Journal o f Veterinary Medicine, B, 36: 76-80. 137 APPENDIX Abbreviations AMD actinomycin D Amp ampicillin ATCC American Type Culture Collection BDV Border disease virus BLF bovine lung fibroblast (cell line) BSA bovine serum albumin BT bovine turbinate (cell line) BVD bovine virus diarrhoea BVDV bovine virus diarrhoea virus cBVDV cytopathic BVDV cDNA complementary DNA cpe cytopathic effect CIP calf intestinal alkaline phosphatase CTP cytidine 5’-triphosphate dATP 2’-deoxyadenosine 5’-triphosphate dCTP 2’-deoxycytidine 5’-triphosphate DME Dulbecco’s modified Eagle’s medium DMSO dimethyl sulphoxide DNase deoxyribonuclease dNTP deoxynucleotide triphosphate dsRNA double stranded RNA 138 DTT dithiothreitol EDTA ethylenediaminetetraacetic acid ELISA enzyme linked immunosorbent assay EPDMA end point dilution microtitration assay ER endoplasmic reticulum gP glycoprotein HCV hog cholera/European swine fever virus HEPES N-2-hydroxyethylpiperazine-N’-2-ethanesulphonic acid IAA isopentanol ID50 50% infectious dose IFN interferon IU infectious unit (equivalent to PFU) kb kilobase kDa kilodalton LB Luria broth LSB low salt buffer MD mucosal disease MDBK Madin-Darby bovine kidney (cell line) moi multiplicity of infection MOPS 3-[N-morpholino]propanesulphomc acid Mr relative molecular weight mRNA messenger RNA NADC National Animal Disease Center ncBVDV noncytopathic BVDV NP-40 nonidet P-40 NTP nucleotide triphosphate 139 ORF open reading frame PBL peripheral blood leucocyte PBS Dulbecco’s phosphate buffered saline PFU plaque forming unit Pi postinfection PIPES piperazine-N,N’-bis[2-ethanesulphonic acid] PK proteinase K PNK polynucleotide kinase PVP polyvinylpyrrolidone RF replicative form RNA RI replicative intermediate RNA RNase ribonuclease rRNA ribosomal RNA S Svedberg unit (IO"13 sec) SDS sodium dodecyl sulphate SV40 simian virus 40 TCA trichloroacetic acid tcf tissue culture fluid TES N-tris[hydroxymethyl]methyl-2-aminoethanesulphonic acid Tris tris[hydroxymethyl]aminomethane tRNA transfer RNA VD virus diarrhoea VRC vanadyl ribonucleoside complexes vRNA viral genomic RNA VSV vesicular stomatitis virus MONTANA STATE UNIVERSITY LIBRARIES 762 10071955 6