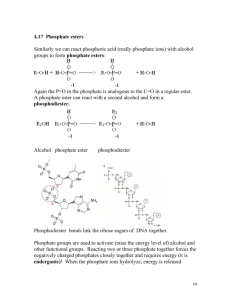
Estrogens and progesterone in the posterior vena cava and estrogens... by Pran Nath Varman
advertisement

Estrogens and progesterone in the posterior vena cava and estrogens in the urine of cattle by Pran Nath Varman A thesis submitted to the Graduate Faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Animal Science (Animal Physiology) Montana State University © Copyright by Pran Nath Varman (1964) Abstract: Cannulation of the posterior vena cava of four Jerseys and one Holstein (non-gravid) and one Jersey (gravid) was performed. Blood samples were collected every other day until the tubing plugged* Urine was also sampled at the same time* The samples of blood were analyzed for the estrogens and progesterone and of urine for the estrogens* Two peaks in the total blood estrogens occurred in all cows sampled* In the majority of the cows the first peak occurred during the period of days 6 to 8 and the second during the period of days 12 to 17 after heat* Similarly, the quantities of the excretion of estrogens in the urine reached two peaks* In most of the cows the first occurred during a period between days 3 and 8, whereas the second peak occurred between days 15 to 20, after heat* The levels of progesterone reached a peak level on day. 17 Of the estrous cycle* During thirty-four days postconception only one peak of the total estrogens in the blood and urine, corresponding to the first peak observed during estrous cycle, was found to occur. The possibility of the interplay of the gonadotropic hormones, progestins, estrogens and the estrogen-inactivating mechanism has been postulated. ) ESTROGENS AND PROGESTERONE IN THE POSTERIOR VENA CAVA AND ESTROGENS IN THE URINE OF CATTLE by PRAN NATH VARMAN A thesis submitted .to the Graduate Faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Animal Science (Animal Physiology) Approved; - ------H e a d e Major Department .— ^ ______ ________ Chairman, Examining Committee '.Dean9 Graduate Division MONTANA STATE COLLEGE ! Bozeman„ Montana December, 1964 -iiiACKNOWLEDGEMENTS The author expresses his sincere appreciation to all the members on the staff of the Animal and Range Sciences Department for their cooperation and encouragement throughout the period of his stay in Bozeman, Special reference must be made to acknowledge the generous help and valuable guidance given by D r 0 Ervin P 0 Smith during the entire course of this work and in preparation of the thesis. Thanks are also due to D r 0 J 0 C 0'Boyd for awarding the author a research assistanship in the form of a grant, which made it possible for him to come to the United States and pursue graduate work at Montana State College 0 Sincere appreciation is expressed to Professor F 0 S 0 Willson, D r 0 D 0 W 0 Blackmore, D r 0 El-Negoumy and D r 0 L 0 G 0 Young for helpful suggestions during the research program. The author is grateful to D r 0 Graeme Baker, who allowed the author to work in the Chemistry Laboratoryj to D r 0 M 0 W 0 Hull fgr performing the surgical operations and cannulation; to D r 0 R 0 R 0 Roehm and the Home Economics Department who made the D 0U 0 Spectrpphotometer available and from time to time provided the necessary guidancej and to Jack Taylor for taking the photographs 0 Sincere appreciation is extended to the Department of Veterinary Science and Animal Husbandry and "The Ministry of Agriculture", Punjab State, India, for the opportunity which they gave to the author to come to the United States for higher studies. ; t I,.....'.i =>iv~ L a s t , but not the least, the thanks are due to the parents and fnendj? an Ipdaa who made it possible for the author to pursue the post-graduate studies. Perhaps without their sacrifice, it would have /■ ■ not been possible to reach the United States of America, z TABLE OF CONTENTS r Page 11 VITA 0 0 0 0 0 6 0 0 0 0 6 0 0 0 0 0 0 0 0 0 0 6 6 0 0 0 ACKNrOWLEDdEMBNTS 0 0 0 0 INDEX TO TABLES 0 0 0 0 0 6 0 6 0 6 0 6 0 0 0 . 0 0 6 6 O 0 O 6 0 6 0 0 6 0 0 0 0 6 0 O 0 0 0 0 0 iii 0 0 0 0 yii INDEX TO FIGURES o o a o o o o o o o o o o o o o o o o o viii ABSTRACT o o a o o o o o o o o o o o o o o o o o o a o o ix INTRODUCTION 0 0 0 LITERATURE REVTEWo 0 0 0 0 0 0 0 0 6 0 0 0 0 0 0 0 0 0 0 0 0 0 0 I 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3 DEFINITIONS OF ESTROGENS AND PROGESTINS = , » , » 10 Ho Estrogens 0 0 0 Progestins , 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3 0 6 0 0 0 0 0 0 0 0 0 0 0 0 0 0 METABOLISM OF ESTROGENS AND PROGESTERONE> A0 0 '3 0 0 0 0 0 0 0 0 0,0 0 4 0 Biogensis> 6 0 0 0 6 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 10 Ho Estrogens 0 0 0 0 Progesterone = 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 6 0 0 0 0 0 0 0 6 0 0 Bo Interconversion of Estrogens Co Inactivation of Estrogens and Progesterone I, Estrogens> 0 0 0 0 0 0 0 0 0 0 0 0 0 6 6 0 0 0 Progesterone O o o o - o o o o e o o o o o o o Bound Progesterone » EXCRETION OF ESTROGENS0 v 0 0 0 0 0 0 0 6 0 0 0 0 0 0 0 0 6 0 0 0 0 0 0 0 7 8 9 Conjugated and Bound Estrogens O O O O 0 O Ho 4 4 o o o o o o o o o o o 0 4 9 10 12 13 14 -VX- Page z EXTRACTIONit ISOLATION AND MEASUREMENT OF ESTROGENS AND PROGESTERONE 0 0 0 0 0 0 » » « » » » 0 1 1 0 0 0 A. Extraction of Estrogens Bo Isolation of Estrogens» Co 6 6 0 6 6 0 6 6 6 6 6 6 0 19 6 0 6 6 19 6 By Paper Chromatography 2« Adsorption Column Chromatography 6 0 6 0 6 0 6 20 3« Thin Layer Chromatography » /« « » 20 6 6 6 6 6 Quantitative Measurement of Estrogens O 6 0 6 0 0 6 - 0 O O O 6 0 6 O 0 O E» Isolation Of Progesterone Fo Quantitative Measurement Of Progesterone 0 0 0 . 0 6 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 6 0 0 0 0 0 0 0 6 0 0 - 0 0 0 0 - 0 0 24 0 6 6 0 0 6 0 0 0 0 0 0 0 0 0 0 0 0 DISCUSSION AND C O N C L U S I O N .................... .. 0 0 0 6 0 0 0 0 6' 0 LITERATURE CITED o 0 0 0 0 0 0 0 0 0 0 6 0 0 0 0 0 0 43 0 « 0 0 55 59 . . . » 0 26 28 6 68 SUMMARY o o o o o o o o o o o o o o o o o o o o o o o o A P P E N D IX o 25 0 6 Levels, of Estrogens in Blood Plasma and Urine During the Estrous .Cycle.,. 0 » » » » 0 » » » » ». » .» Levels.of Estrogens.in Blood Plasma.and Urine During First 34 Days After Conception 0 0 6 0 0 21 21 O Extraction -Of Progesterone FyomrBlood And Other Tissues »' » » » . » » » » » » » » » RESULTS o o . ' O o !I. 6 Io EXPERIMENTAL PROCEDURE. I. 15 0 6 6 6 0 0 0 6 0 6 6 6 6 0 ■Kober Reaction.. « » » « -» » »' » D. 15 6 0 0 0 0 o o o o o e o o o o e o o e o o o o o o o o - o o 70 81 INDEX TO TABLES Showing the R values of progesterone and contaminating substances contained in the plasma e x t r a c t .on thin layer chromatography „ „ „ A measure of the quantities, of estrogen ..secretion .at different .periods .of the estrous..cycle* ., . .Cows ..no* I. to. 5 & i & 0 - 0 0 - 0 0 6 6 6 0 6 6 • 6 0 0 0 0 A measure of the quantities of estrogen, secretion at 'different .periods., of..the. estrous.. cycle during po&tconception,period*...Cow,.no* 6 0 0 j-Aa O 6 6 6 .0 6 A measure of the quantities of estrogen excreted at different.periods of the estrous cycle from the , uri n e .. Cows no* I to 5 0 0 . 0 0 0 0 O.e 0 0 6 0 0 0 A measure of the quantities of estrogen excreted in the-urine, at ..different, periods, of the estrous .cycle during postconception.period* .Cow.no* 6, '0 0 6 0 0 0 Plasma progesterone at various states,“of the estrous' cycle 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 6 0 -vili-INDEX TO FIGURES Figure Io Page The occuranee of a double peak in the levels of estrogens during menstrual cycle of a female ■ chimpanzee o o o o o o o o o o o o o o o o o o 15 2o Standard curves of pure estrogens for Kober1reaction . 4-3 3, The levels .of .estrogens, (micrograms, per, milliliter) . in-.bloo4 -plasma. during,..estrous... cycle, of ..cow., no 0.Io e„ o. o « 46 . 4o 5o The levels ,of .estrogens (micrograms per,.milliliter) • in'blood plasma...during..estrous .,cycle, of cow. no, 2 » » « o o The levels of estrogens (micrograms per milliliter) in blood plasma during., estrous .cycle., of : COW -no o -3 and .4 o . O O - a . O . O - O O . o O O - O O . o O - O . O O O 0 O O 47 48 6, The l'evels..of. estrogens, (micrograms,..per .milliliter), ............ in blood. p.lasma._during..estr6us1..cycle..of.cow.;nOo S o . o ^ . o , .49 7o The. levels ,.of ,estrogens ...(micrdgrains-. per .liter) excreted in urine during...estrous. cycle, of. cow ..no o. I .o,o .o ..o o,. o.o 52 The levels of .estrogens ..(micrograms, per. liter)'excreted . in- urine .during, estrous cycle of .cows .No.,.2 and 3. ... .. 53 The l!eyels,,of..estrogens,, (micrograms,..per.liter) excreted in urine during, estrous cycle, of ,,cows. no,. ..4. and.. 5.. ... .... . 54 8o 9. IOo The levels, of.estrogens .(micrograms per milliliter) in ■ - - blood plasma, of cow no. 6 during, posteonception. period.,. . . Ho 55 The. levels of estrogens (micrograms per .liter) excreted in the urine, of cow no. 6 during postconception period .= 56 12o Standard curve of progesterone.for.ultraviolet assay « .. 57 13. The levels of progesterone (microjgrams per milliliter) in blood plasma during estrous cycle . . . . . . . . . . . 58 -ix™ • ABSTRACT Cannulatipn of the posterior vena cava of four Jersqys and one Holstein (non-gravid) and one Jersey (gravid) was performed6 Blood samples were collected every other day until the tubing plugged. was also sampled at the same time. Urine The samples of blood were analyzed for the estrogens and progesterone and of urine for the estrogens. Two peaks in the total blood estrogens occurred in all cows sampled. In the majority of the cows the first peak occurred during the period of days 6 to 8 and the second during the period of days 12 to 17 after heat. Similarly, the quantities of the excretion of estrogens in the urine reached two pea k s , In most of the cows the first occurred during a period between days 3 qnd 8, whereas the second peak occurred between days 15 to 20, after heat. The levels of progesterone reached a peak level on day. 17 Of-the estrous cycle. During thirty-four days postconception only one peak of the total estrogens in the blood and uri n e , corresponding to the first peak observed during estrous cycle, was found to occur. The possibility of the interplay of the gonadotropic hormones, progestins, estrogens and the estrogen-inactivating mechanism has been postulated, ) INTRODUCTION It has been recognized for many years that one of the larger problems concerned in the physiology of reproduction of animals (especially the cattle) is low fertility rather than sterility. Many factors contribute to low fertility and thus cause great economic loss to the animal industry. Disorder of the endocrine glands is probably one of the most important factors, Another problem being faced by research workers interested i n ' the reproductive physiology is inconsistent results of synchronization of the estrous cycle of domestic animals. Similarly the physiologists engaged in the study of ova-transplantation in cattle have not as yet completely succeeded. It seems evident that there is a lack of knowledge concerning the physiological levels and the interactions of various hormones concerned with reproduction. This lack of knowledge may be responsible for the Veterinarians not being able to satisfactorily diagnose or treat some of the endocrine disorders of the animals. This may also very well be responsible for the unsatisfactory responses in synchronization and ova-transplantation, The levels of the estrogens circulating in the blood„ as well as being excreted in the urine of normal women during menstrual cycle, have already been established. Whereas there has been no report which has shown the day-to-day changes in the levels of these hormones during the estrual cycle of domestic animals. In contrast to relatively higher concentration of estrogens in a=2the urine 8 the quantities of these hormones in the blopd obtained from the jugular vein of the cattle are extremely Iow0 It is known that the posterior vena cava drains bipod from the hind portion of the body and also receives blood from the ovaries 0 uterus (also placenta in pregnant individuals) and the adrenals„ Collection of the blood from this vein before much dilution and inacti­ vation of the sex hormones has taken place can possibly be advantageous„ Considering the problems mentioned above9 the purpose of this study was s (1) To cannulate the posterior vena cava at a site close to all the points of secretion of the sex hormones„ (2) To determine the day-to-day changes in the levels of the endogenous estrogens and progesterone in the blopd and estrogens in the urine of cattle during the estrous cycle., (3) To determine the relationship of the secretion and urinary excretion of the estrogens <, REVIEW.O F .LITERATURE. Io DEFINITIONS OF ESTROGENS AND PROGESTINE A* Estrogens: Estrogens s, a Greek W o r d 9 is made up of two words 9 Oistros0 meaning "mad desire" and "Gen" referring to "to beget" (Stedman6s Medical Dictionary)„ It i s „ therefore6 evident that an estrpgenic compound refers to any substance which produces estrus whether derived from the ovary or not. In general9 estrogenic compounds may also produce growth of secondary sexual characteristics* changes in vaginal m u c o s a 9 and proliferation of the endometrium= A hormone is defined as a physiologic* organic substance liberated by living cells of a restricted area of the organism which diffuses or is transported to a site in the same organism where it brings about an adjustment that tends to integrate the component parts and actions of the organism (Zarrow9 1962)„ Estrogenic substances found in animals are referred to as natural estrogenic hormones because these compounds do fit the preceeding definition of hormones„ Chemically 0 estrogens are steroids and are characterized by an aromatic Ring=A and a hydroxyl group at carbon atom-3= Estrane is considered to be the parent compound of the estrogens„ The major ' estrogens of mammals are estrone* IVs^ and 17B=estradiol0 and estriol (Fieser and Fieser 1959)= The nomenclature of thef-two" isdmdrs of estradiol refers to the "cis" or "trans" position of the hydroxyl group-at carhop-17 position= “4“ The isomer with "cis"-hydroxyl group is designated by the prefix "B" while that with "trans" hydroxyl group as the VJ' (Turner, 1961)„ Progestlns s Another major group of hormones of reproduction is known as Progestins0 "Pro" (Greek) means "in favor of" and gestatio (Latin)s from gastatuss meaning "to bear" (Stedman’s Medical Dictionary). This generic term was suggested for substances regardless of chemical purity, which cause changes in the endometrium, adapting it for the ■■ ■■ reception of the fertilized ovum (ova in.multiparous animals). Progestins also, may partially, regulate the periodicity of the sexual cycle, stimulate the mammary glands, inhibit uterine musculature and relax the pelvic ligaments (Turner, 1961). Progesterone, also a steroid, is the most potent natural progestin. Theoretically, allopregnane and pregnane are the parent substances for the progestins. II. METABOLISM OF ESTROGENS AND PROGESTERONE A. BIOGENESIS Estrogens s The estrogens have been isolated and identified from various tissues and endocrine glands of the body. These hormones, in the female, are produced by the ovary, placenta and adrenal cortex and in the male by the testis and the adrenal cortex. Like other steroid hormones and unlike nitrogen containing hormones', -estrogens are not stored in the organs concerned with their b i o genesis■( O ’Donnell and -5Preedy„ 1961)„ It is generally thought that estradiol-17B is the principal estrogenic hormone produced by the ovary and is the mother substance of estrone and estriol (Boscott6 1 9 6 2 6 Harrow and Mazur9 1962)» To determine the sites of biogenesis of estrogens„ extraction and identi­ fication of these hormones from various tissues have been performed„ H o w e v e r 9 Nyman e t . a l 0 (1959) have pointed out that the presence of a steroid in an endocrine organ is no proof of its synthesis at that site* nor does the ability of the tissue to metabolize a steroid prove that the products ,are released into the blood stream in physiologically significant amounts* Human ovaries obtained from patients primed with FSH are able to convert acetate into estrone* estradiol* and cholesterol (Ryan and Smith* 1961a)* Similarly* Heard e t * a l * (1956)* and Werthessen e t * a l * •(1953) Jiave reported the conversion of acetate to the estrogens in pregnant mare and sow ovaries respectively* but Hollander and Hollander (1958) during in vitro studies with dog ovaries could not demonstrate the conversion of acetate-l-Cl4 to C11^estradiol-ITB and -instead some radioactive cholesterol was isolated* Conversion of acetate to choles­ terol in the rat has also been reported (Caspi e t * a l * 1962), Bloch (1945) pointed out that the animal organism is capable of synthesizing the steroids which it normally requires, It was* therefore* thought that the compounds possessing the cyclopentanophenanthrene structure arise either individually by synthesis or by degrade= —6— tion from a common precursor0 Cholesterol has been suggested to be the precursor of the steroid hormones (Bloch, 1945, Ryan and Smith, 1961c, and Werbin e t o a l 0 1957)» Huffman e t o a l o (1940) proposed that progesterone could function as an obligatory or main intermediate in normal estrogen formation» Ovaries from already primed humans are able to convert p r ogesterone-C^ into radioactive estrone and estradiol (Ryan and Smith, 1961b)6 Although estrogens have been isolated from the human placenta^ Ryan and Smith (1961b) were unable to detect any in vitro conversion of pro= gesterone to estrogens in human placental system. Very recently Aasted and Stakemann. (1963) have reported that the estrogens of human pregnancy are produced neither by the placenta nor by the fetal adrenal aloxie but by complex procedure involving both tissues. It has, therefore, been suggested that fetal adrenals produce a steroid precursor which is metabolized into estrogens by the placenta, Nathanson and Towne (1939) demonstrated that the use of testos­ terone propionate in human female castrates leads to vaginal cornification and increased urinary estrogen levels, decreased follicle stimulat­ ing hormone titres and increased urinary androgens„ The in vivo conver­ sion of testosterone propionate by. ovariectomized and andrenalectomized w o m e n , to estrone and estradiol-17B has been reported by West e t , a l , (1956), Estrone and estradiol are promptly synthesized when testis of the gonodotropin primed stallion are perfused with horse blood and sodium acetate (Nyman et; al, 1959), This may be due to the presence -7of a relatively high proportion of L e y d i g 's cells in stallion testis0 Similarly pregnant mares have also been found to be able to convert testosterone into the estrogens (Heard e t „ a l 0. 1956), and for the con­ version of testosterone to estrogens in human ovarian tissue (Bagget e t a a l a 1956, Wotiz e t a a l a 1956), in placental tissue (Ryan, 1959a) and in rat ovaries (Stitch e t « a l a 1962), Ryan (1959b) demonstrated that 16ot-hydroxyestrone (a metabolite product of androstenedione) is metabolized by the placental system to form estriola It was suggested that aromatization of an already 16- hydroxy lated androgen may also give rise to estriola It is known that 19-nortestosterone when administered to humans is converted to estrone and the magnitude of conversion is similar to the one observed on the administration of testosterone« These findings indicate that the absence of the angular methyl-group ( 0 1 9 ) does not significantly influence the extent of the conversion (Engel e t a a l a 1958)a Progesterone s Allen and Wintersteiner (1934) were the first to isolate pro­ gesterone from extracts of corpus Iuteum0 It is generally believed that progesterone is synthesized by all the steroid-producing glands via the acetate^cholesterolepregnenolone^progesterone pathway (Samuels 1955) a Although there is no direct evidence, a general agreement exists that the cells of the theca interna in the graafian follicle are ” 8” responsible for the estrogen production« The granulosa cells s" after they have hypertrophied to form the corpus Iuteum9 are responsible for the production of progesterone (Short 1961)0 Teledy eto a l » (1963) have reported that in dogs 9 during estrous cycle 8 no progesterone could be detected in ovarian venous blood when the corpus luteum was not presento Similarly, Huang and Pearlman (1962) have indicated that the r a t ’s corpus luteum in contrast to h u m a n ’s does not form estrogens and ■. ' instead is responsible for the formation of progesterone0 It is interesting to note that cow adrenals are capable of secreting as muph as Io5 mgo progesterone per hour (Balfour and Comline 1957)« ' Bo INTERCONVERSION OF ESTROGENS Following the isolation of estrone from the urine of pregnant women by Doisy e t o a l 0 (1929)9 this ketonic estrogen was considered to be the hormone of the ovarian follicle = In 1936 „however 9 MacCorquodale e t o alo were able to identify ITekestradiol as the principal estrogenic hormone of the liquor folliculi of the swine ovary 0 From time to time various workers have demonstrated the interconversion of estrogens» The conversion of eO-estradiol into estrone in man (Heard and Hoffman 1941) and into estrone and B-estradiol in guinea pigs and rabbits (Fish and Dorfman 1941) has been demonstrated* of estrone to estriol and estradiol-17^, Similarly the conversion in rats (Wotiz e t * a l * 1958b) and in humans (PearIman and Pincus 1943) has been demonstrated* It has been suggested that both estradiol-17bC and estriol may very well be the metabolites of estrone* Intestinal microorganisms have also been -9shown to be able.to reduce estrone to estradiol (Beck 1950)6 The conversion of estradiol=17B to other estrogens zIiag also been VS reported by many w o r k e r s 0 Engel et, al, (1959) demonstrated that the human fetal liver slices are capable of converting estradioL=17B into • estriolo The conversion in man of estradiol=17B into 16-Ketoestradiol- 17B (Levitz e t 0 a l e 1956) ? and 2«=methoxyestrone (Kfaychy and Gallagher 1957) have also been demonstrated0 Ce INACTIVATION OF EgTROGENS AND PROGESTERONE Ie'ESTROGENS The maintenance of circulating estrogens at a physiological level is dependant to a considerable extent on the inactivation qf the estrogens by metabolic conversions o * A highly effective mechanism for » « ■ the capture of the free estrogens is in existence in the body tissuesD It has been demonstrated in rats (Oakey e t 0 a l 0 1962) and in humans (Fishman et„ a l 0 1960) that within 30 minutes after the administration of e x o genous'estrogens9 abqyt 98 percent of the total free hormone cannot be recovered from the blood 0 If an ovary is transplanted into the mesentery„ or estrogen pellets are implanted intrasplenically„ the hormone would be inacti­ vated by its passage through the liver prior to its entrance into the systemic circulation0 This shows that the liver is a very important i' ■" organ for the inactivation of the estrogens (Coppedge e t i -a l 0 1950)„ Inactivation of estrogens may take the form of conjugation and elimination from the body 9■and metabolism to known and some unknown =10= compounds„ and/or oxidation (Eccles e t c a l p 1962)6 Very little is known about the nature of the inactivation products, of gstrogens but the evidence exists that estbbnq and estradiol are .attacked by an oxidative enzyme system (HellersI 19’ij-O)„ The liver is thought to form non-steroid estrogen metabolites as degradation products of estrone (Jellinck9 1959)0 Similarly* Werthessen etc a l 0 (1950r) have demonstrated that estrone on incubation with blood medium loses its IV=Ketosteroid group as well as its estro­ genic activityo The possibility of the presence in blood of an enzyme capable of inducing oxidation of estrohd tp a form whose nature is unknown has therefore been suggested. Conjugated and Bound Estrogens: The term conjugated means a probably chemical union such as sulfuric acid or glucuronic acid with a hydroxyl group on a steroid molecule to form an ester. Whereas binding means any association of a steroid with a protein (Antoniades e t . a l . 1957). In 1938* Schachter and Marrian reported the isolation of potassium salt of estrone sulfate from the urine of pregnant mares. Similarly the occurance of estrone-hydrogen sulfate in the urine of pregnant women (McKenna et. al. 1961) and estriol in women (Levitz et. a l . 1958) has also been established. Conjugation of diethylstilbestrol with sulfuric acid was suggested to be occurring in rat* cat nnd dog but not in rabbits (Starnes and Teague 1949)* whereas its conjugation with glucuronic acid in the rabbit had already been demonstrated by -11Mazur and Shorr (1942). Zimmerberg (1946)sindicated that the conjuga­ tion of diethylstilbestrol is perhaps the preferred method of inactiva­ tion. Oxidation of this synthetic estrogen occurs only when the liver cannot handle it by conjugation. Diczfalusy (1953) reported that a large proportion of estrogens in the pregnancy blood or plasma occurs as conjugated, presumably glucuronides and sulfates. Both conjugated and unconjugated radio­ activity in extracts of various protein fractions of plasma have been demonstrated by Antoniads et_. a l „ (1957), Similarly Purdy et, al. (1961) found that plasma obtained two and one-half hours after the administration of C1^ e s t r a d i o l to women* contained C14-estrpne sulfate as the principal radioactive metabolite. It wag, therefore* syggested that estrone sulfate may be ah important transport form of estrogens in human plasma. DeMeio et. al. (1958) have shown that Mg++ and adenosine triphosphate are essehtial for the biosynthesis of the sulfates of estrone and estradiol-17B. The presence of estrogenic lipoprotein complex in the circula­ tion of several species has been reported by many workers. Estrogen- serum protein binding in the presence of surviving rat liver tissue occurs selectively with albumin (Szego and Sidney* 1956) and this mechanism is correlated with the functional state of the liver (Szego9 1953),. It has also been reported that the enzymetically catalyzed association of estrone or its metabolites with albumin is inhibited or a b o lished'by increasing the concentrations of cortisol. This shows a “12— competitive phenomenon exhibited by the estrogens and certain cortico­ steroids in vivo (Szego and Sidney9 1956)» Reigel and Mueller (1954) have indicated that the Iiver8 to a much greater extent than other tissues (uterus 9 submaxillary glands and kidneys)8contains an enzyme system which catalyzes the formation of a protein-bound estrogen» This, system requires an electron-ddhating Substrate9 an electron-transporting coenzyme (TPN) and Oxyg e h 9 suggesting thereby an oxidative or perioxidative step in the binding process, Erlanger e t , a l , (1959) h a v e estimated that at legst twenty estrone residues are linked to each molecule of bovine serum albumin, B-glucuronidase 9 an enzyme which can hydrolyze estrogenglucuronides9 has been found to exist in the liver and spleen of calves (Bernfeld e t , a l , 1953)j spleen of calf (Bernfeld and Fishman 1953)j ox spleen (Mills 1948), The major portion of the glucuronidase activity of the bipod is concentrated in the leucocytes and platelets, B-glueuronidase activity has also been demonstrated in Saliva9 gastric ju i c e 9 spinal fl u i d 9 urine and tears (Fishman e t , a l , 1948), 2, PROGESTERONE S The inactivation of progesterone is brought about either by its ' conversion to other metabolites or the binding with serum proteins. Short (1961) has indicated that if an assumption is made that the progesterone molecule can undergo reduction at C ^ 8 Cj. and ^ theoreti­ cally, twenty-six possible progesterone metabolites can be formed. Eleven of these are already known to occur in human tissues qr urine. "13” The generally accepted scheme for representing reduction of progesterone involves arrangement of the metabolites in order of increasing state of reduction (Taylor 1955)» The scheme indipates that progesterone is reduced initially, at the double bond in Ring=A to yield 5ot and 5B"pregnene"3s20"dione which undergo reduction ft C™3 to the three pregnanolones9 Sp^hydroxy-5o<"pregnan”20"one 9 SB-hydroxy-So^ pregnan"20?phe and 3o(=hydr6xy"5B"pregnan"20-one0 The latter is then reduced at C^20 to give BB-pregnane-Sots 2OcXrdiol (pregnanedipl) „ Bound-Progesterone g There has been no report which has shown that progesterone exists in the body in a conjugated form. When incubated with whole blood very little progesterone enters the red-cells and instead its major portion remains confined to the plasma (Short9 1958a)„ • Westphal e t 0 a l 0 (1955) observed that when progesterone is added into human serum albumin or blood serum and the mixture is ^un on paper electrophoresis9 over 90 percent of the added progesterone migrates with the protein fraction0 . When it is added to rat serum albumin only 50 percent migrates with the protein fraction9 indicating prpnounced spedies difference0 It has also been indicated that not more than one albumin mtilecule associates with each molecule of progesterone (Short 1961)o' Eik-Nes e t o a l p (1954) have reported that the albumin fraction of plasma proteins is responsible for the solubility of the progesterone in the blqod-whereas globulins have no measurable effect of such kind. “14“ !Ho EXCRETION OF ESTROGENS Between 50-80 percent of the estrogens in the blood are present 5 as closely bound to plasma proteins, Presumably this prevents the bound hormone from being filtered out of the blood as it passes through the glomerulus of the kidney (Villee 1961)» Excretion of estrogens in various excretory products has.been studied by many workers» It has been observed that the major portion of estrogens excreted in the urine is in a conjugated form, As such the estrogens are firmly united to such hydrophilic groups as sulfuric or glucuronic acid so that their solubility in the urine is increased» The low binding capacity of these conjugates with serum albumin explains their rapid filtration through the glomeruli of the kidney into the urine (Eik-Nes e t » g.l» 1954)» In cattle the excretion of the estrogens in urine during gestation is lowest on the 50th day and an increase in the excretion at an accelerated rate occurs during the last part of gestation (Nelson and Smith 1963)o Similarly the urinary estrogenic content on the IOth day after parturition has been shown to be equivalent to 251 ug» of estrone per liter as compared to a value of 642»5 ug» found on the 275th day of pregnancy (Smith e t . a l » 1956)» Assay of urinary estrogens in primates has often shown double peaks (Everett 1961)„ The occurrence of a double peak in the levels of estrogen excretion in the urine by a female chimpanzee (Fish et» a l » 1941) is shown in figure no» 1» :- - - !- - - >- - - S- - - !- - - 6- - - 1- - J- - - 6- - - 1- - - »- - - ) - - S - - - 1_ _ _ I_ _ _ c_ _ _ 'I 5 10 ' r 15 fr « ■_ > » 20 » ) I ^ < 25 d - 30 Days Of Menstrual Cycle .Figure.-Is T.o.tal. ^ s t r o g e n .excretidn-by _a-female-chimpanzee. ... (from Fish et^> al» 1941) . . The occurrance of such two peaks in normal human female's has also been demonstrated (Markee and Berg 1944j Pedersen-Bjergeiard and Pedersen-Bjergaard 1948)0 The first peak has been observed to occur on day 12 and the second on day 21 of the menstrual cycle„ Nd sudh peaks have ever been shown to occur in the bovines=, I V a EXTRACTION* ISOLATION AND MEASUREMENT OF ESTROGENS AND PROGESTERONE A c -EXTRACTION OF ESTROGENS It is known that the urinary estrogens exist in a conjugated F o r m 6 To free these hormones some kind of hydrolysis is necessary=, Stimmel (1946) acidified urine samples (to Congo red) with hydrochloric acid and then extracted with Oi125 volume of butyl alcoholi After £he extractionj the butyl alcohol was evaporated and the residue was taken up in 100 m l o of -0„2 N NaOH solution which was further diluted to 400 ml. = 16™ volume with W a t e r 0 This mixture was then acidified with I O 0O m l 0 of concentrated hydrochloric acid and autoclaved at 15 pound steam pressure for 3 hours 0 The ffed estrogen Cphtent9 after hydrolysis9 was then extracted with Pther which was ultimately washed with 9 percent ' y' - NaHCOg splution and finally with water. ' The recoveries of estrogens added t'q ap essentially estrogen™free urine (from castrated female) extract for estrone and Ot-Pstfadiol9 and Pstriol were 80 to H O percent ' . and 57 to 94 percent respectively, F r i e d g o o d .et, a l , (1948) hydrolyzed the urine samples by using ■ 30 volume percent of 6N sulfuric acid (equivalent to 15 volume percent of concentrated hydrochloric acid)« The mixture was refluxed for -10 ■■■ minutes and extraction was then achieved with ether. The quantitative removal Qf estrogens from the ethereal extract was accomplished through a reduction in their solubility by the addition of carbon tetrachloride. The estrogens were then extracted with IN=KOH solution whereas the neutral steroids were found to remain in the organic phase (pthers carbon tetrachloride as Is 18), Potassium hydroxide phase was then acidified with 6N=sulfuric acid (to Congo red) and extracted with ether which was ultimately washed with sodium bicarbonate solution and water. The recoveries of added estrone 9 estradiol and estriol were found to be ‘ 91,5-99,9 percent, 91,6-100 percent and 91,0-98,0 percent respectively, Stimmel (1949) studied the effect of the addition of 4 mg, percent zinc along with 15 volume percent hydrochloric acid on the hydrolysis pf urinary conjugated.estrogens. It was o b s e r v e d t h a t under -17such treatment estrone was converted to estradiole whereas 15 volume percent of hydrochloric acid alone and refluxing for 10 minutes gave the maximum recovery of 90 percent. The next highest recovery was obtained with 5 volume percent of sulfuric acid and refluxing for 10 minutes. Similarly Engel e t . a l , (1950) reported that higher yields of estrogens are obtained after autoclaving human pregnancy urine with 15 volume percent of 12N sulfuric acid for 5-10 minutes than after refluxing for 10 minutes with the same concentration of the acid. In 1 9 5 4 9 Katzman e t , a l , found that pregnancy urine containing 15 volume percent concentrated hydrochloric acid when boiled for 30 minutes gives the highest recovery whereas Preedy and Aitken (1961) have reported that maximum recoveries are obtained by refluxing the urine samples with 15 volume percent concentrated HCl for 45 minutes, Anyway9On hydrolysis with such a concentration of acid for 30 minutesB the free estrogens are found to be quite stable (Katzman e t , a l , 1954), Even though acid hydrolysis for extraction of urinary estrogens is so widely used, the use of some enzyme preparations for this purpose is another alternative, Katzman e t , a l , (1954) have indicated that the presence of chromogenic material in urinary extracts when acid hydrolysis is used, can be a major obstacle to the use of the Kober method for the estimation of e s t r o g e n s T h e h a s , therefore„ been advocated. use of enzyme preparation Beer and Gallagher (1955a) have supported the report of Katzmah et, al, (1954) that glucuronides form the major fraction and-sulfate esters a very minor fraction of estrogen;= eoISe= esters in the urine» This is perhaps the most probable reason why only B-glucuronidase enzyme has been widely used (Kinsella et» a l 6 1956; Beer and Gallagher 1955a and 1955b)„ P-myI a s e 9 which contains phenolsulfatase-enzyme and "glusulase"* an enzyme preparation which contains both glucuronidase and Sulfatase9 have also been vised (Kinsella eta alo 1956; Givner et» alo 1960; Ladany and Finkelstein 1963; Veldhuis 1953), Mather (1942) studied the distribution of estrogens between immiscible solvents a The distributions between benzene of ether and 0a3-M sodium carbonate solution were recommended for separating estriol from other estrogens0 It has well been established that a large proportion of the estrogens circulating in the blood are in a conjugated form (Antoniads > !I e t a a l a!957; Purdy et» ala 1961)» It is also known that estrogens are transported in the blood in the form of an estrogenic-lipo-protein™ complex (Szego and Sidney 1956)» I t 9 therefore* seems evident: that i. h some kind of hydrolysis is essential to set the estrogens free a Preedy and Aitken (1961) have pointed out that there is no difference in the -. ■ ' procedure, for the urinary and plasma estrogen isolations except some minor differences in the handling", : Similarly the procedures for extraction of estrogens from ovarian follicles (MacCorquodale e t » a l » 1936)9 endometrium (Szego and Samuels 1943)* ovaries (Westerfeld et» a l » 1938) and fetal cotyledons ' (Veenhuizen eti a l l '‘I960)"resemble the procedure used for urine or ■ / “19blood estrogenso B 0 ISOLATION AND PURIFICATION OF ESTROGENS It is possible that the unwanted substances present in the biological extracts may interfere in the quantitative analysis* It i s 8 therefore9 advisable to isolate the concerned products in their pure forms o Several chromatographic procedures which can successfully be applied for this purpose are available* I* By Paper Chromatography: Paper chromatographyg which has been used for the separation of estrogens and other compounds9 is relatively simple* By this method homogenous material is concentrated in one position rather than distri­ buted over a series of fractions as in column partition chromatography* This technique has some disadvantages ass I) large quantities of the * material cannot be handled so that the plasma extracts containing much lipids cannot be chromatographed directly9 2) the resolution is poor ( O ’Donnell and Preedy 1961)* Many solvent systems with various solvent combinations have been reported (Oakey 1961; Axelrod 1953; Reineke 1956; Veenhuizen e t * a l * 1960; Burton et* al* 1951a)* Burton et* al* (1951b) observed that replacement of water by certain polar organic solvents overcomes the tailing and that the bulky urine extracts can be applied in a narrower •zone along the starting line when formamide rather than propylene glycol is used as the impregnating solvent* absolute methanol ( 1 1 1 by v o l u m e i s Formamide„ when added to considered to be a good stationary I ="2P=> phase (Burton e t a a l a 1951b; Axelrod 1953)« 2a ' Adsorption Column Chromatography; Adsorption column chromatography has been used very extensively for isolation and purification- of estrogens in the extracts of biological fluids and tissues. The method has the advantage that large amounts of the material can be processed. The disadvantages of this procedure are the difficulty in the standardization of the adsorbent, moisture content, tailing and relatively large volume of the eluate ( 0 ,Donnell and Preedy 1961), Adsorbents such as celite (Bauld 1955; Givner e t , al, 1960), alumina (Stimmel 1944; Umberger and Curtis 1949) and silica=gel (Beer and Gallagher 1955a arid 1955b; Leyitz d t , a l , 1956) have been used to ■-prepare the column fpr this technique, - •• 3, Thin Layer Chromatography; ^ Lisboa and Diezfalusy (1962) have very recently reported the ;■ use of thin layer chromatography for the separation and characterization of the estrogens, The said authors have reported .the Rp V a l ties for i':’ ' ' 24 phenolic estrogens in various solvent systems," This method has also been found to be very useful by Ladany and Finkelstein (1963), The method has a favorable resolution, reproducibility and high precision. Time given for running chromatograms is less than 2 hours. When applied ■ as a strip line, sufficiently large quantities of the extract,can be handled, ... ^ • • Similarly, countercurrent distribution ‘had also been uded for J -21= the isolation of the estrogens (Engel et_0 a l 6 1950), Co QUANTITATIVE MEASUREMENT OF ESTROGENS - Various methods for the detection of estrogens in paper chromato- • graphy and thin layer chromatography have been successfully used, Turnbull's blue method (estrogen spots when treated with solution con= taining equal volume of I percent aqueous ferric chloride and I percent aqueous potassium ferricyanide9 give blue coloration) which in fact is i a general method for the detection of phenols3 has proved to be of parti­ cular importance ( O ’Donnell and Preedy 1961), Axelrod (1954) has cautioned that even corticosteroids* androgens9 urinary pigments and « ".,V 1 impurities can give positive results with this .method0 The technique of dipping the chromatographic strip in 15 percent fuming sulfuric acid for one minute and then observing under an ultra-violet-lamp for fluorescence* has also been used (Axelrod 1953)« The fluorimetric (Goldzieher e t 0 alo 19j52 and 1 9 5 4 1 Veldjhuis 1 9 5 3 1 Slaunwhite e t c, a l « 1951% Nako and Aizawae Nocke 1961% Purdy -eto a l 0 1961)* enzymatic (Hurlock and Talalay 1957) and bioassay techniques (Allen and Doisy 1923% Emmens 1950* Freud 1939* Lauson e t o a l 0 1939e Evans and Varney 1941) have also been quite extensively used for the quantitative measurement of estrogens0 Kober color method which has been extensively used for the estimation' of estrogens is reviewed in the following Sectiqn0 'Kober R e a ctions In 1931* Kober discovered that when estrin is heated with con= ' -22centrated sulfuric acid and phenol it gives rise to a yellow color0 On addition of water and reheating the mixture„ the yellow color is con­ verted into a highly specific pink color (W x 9 522 mu). Anyway9 the pink color thus produced is not very stable and the impurities contained ' in the biological extracts containing estrin produce an intense brown color which can mask the color due to estrin. Venning et. al. (1935) ewe# ivmonis indicated that the light absorption of the test mixture should be measured not only at 522 mu but also at 420 m u e the latter being the wavelength of maximum absorption of brown color. By doing so the light absorption due to brown component at 522 mu could therefore be calculated from its absorption at 420 mu and thereby by subtraction, the absorption at 522 mu due to the pink component is obtained. It has also been st^dwn that there is no other substance* except estrogensg in the iirine. of humans which can produce a pink color with the Kober reagent (Venning e t . a l . 1935). The compositions of the reagent and its amount used* the preheating time to produce yellow color * amount of water added to the yellow mixture and the reheating time are the factors which can influence the test results. Bachman and Pettit (1941) found that the treatment of the final •" ■■ crude color product with hydrogen peroxide * as originally suggested by Kober (1938)* reduces but does not eliminate the absorption due to the impurities. The said authors also pointed out that the methpd for the correction of absorption *•due to the brown color * proposed by Venning et. al. (1935)* does not permit reliable determination of the estrogens -22abecause the spectra of the individual brown colors from different samples vary too much. In 1950, Allen suggested a correction formula which permits the analysis of mixed absorption curves provided the absorption curves of the contaminating substances closely approximate a straight Iine0 This has been found to be true in the case of urinary estrogenic extracts (Allen 1950), The correction equation is as follows: CDXx = -ODx - t■•,PPb , Where ODx = observed density ,at wavelength "x" as the absorption maximum OD q and OD^ ■= the observed densities at wavelengths "a" and "b", "a" and "b" must be equidistant from "x" CDXx = calculated density due to substance "x" having an adsorp= tion maximum at wavelength "x" The actual amount of "X" is then calculated from CDXxo Bauld (1954) observed that a ;depression of about 15-40 percent in the final color produced by Kober reaction can be caused dye to the presence of solvent residues. The three possible types of inhibitions which can be responsible for interference in the color development are a s 'follows: a) failtupe to form the initial yellow complex (inhibition type I) b) failure to convert the yellow complex fully into the pink (inhibition type II) c ) instability .of the pink complex (fading). “23= The type I inhibition in the case of estriol is due to diminished reducing power of the reagent. The second stage of the Kober reaction9 involving conversion of the yellow complex into the pink o n e ? involves oxidation (Nocke 1961) which if excessive causes fading. The Kober reagents used by Bauld (1954) for estriolg estrone 6 dnd estradiol con= twined percent* 66 percent and 60 percent V/v of sulfuric acid respectively. The modifications suggested by Bauld (1954) were incorporated into Brown's method for the urinary estrogens (Brown 1955). According to this method the concentration of sulfuric acid in the reagents is the same as used by Bauld (1954). added into each reagent. Quinol at the rate of 2 percent is It was suggested that addition of quinol to the estrogen fractions^before evaporating the solvent* can prevent any oxidation of the hormones. Chromogenic impurities from the crude urine extracts were separated by methylating the estrogens in alkaline solu= tion with dimethyl sulfate and then oxidizing the contaminants with hydrogen peroxide and extracting estriol methyl ether with benzene and the estrone= and estradiol=methyl ethers with light petroleum. This was based on the fact that methylation of the estrogens does not limit the estimation of the hormones by the Kober reaction (Marlow 1950). The appropriate quinol=sulfuric acid reagent (3 ml) for the particular estro= gen is added to the estrogen fraction. minutes, Preheating is done for 20 After cooling* I ml* 0.5 ml and 0.2 ml water is added to the tubes .containing estriol* estrone and estradiol respectively. The tubes -24ar.e then reheated for 10 minutes«, The readings for light absorption are taken at 480g 615e and 552 mu for estriol and estrone and 4809 518* and 556 mu for estradiol. In 1 9 5 6 8 Bauld modified the method used by Brown (1955), The composition of the reagents was kept the same whereas their amount added to the estrogen and quinol residue* the heating periods and the final concentration of the acid were modified. The appropriate reagents .?• as described by Brown (1955) (2,6 ml for estradiol=17B and estriol and 3,0 ml for estrone) were added. Preheating in a boiling water bath was followed by cooling in water bath at 15° C, At this stage 50+ 5 mg, of quinol was added and the dilutions were carried out as follows: ■ estradiol-17B with 0,7 ml of the reagent* estriol with 0,7 ml of water and estrone with 0,3 ml of water. Reheating time allowed was 15 minutes and after cooling the absorbancy was measured at 480* 512* and 544 mu for estrone and estriol and at 480* 515* and 545 mu for estradiol^lTB, The method was used later on'by Givner et, al, (1960) and was found to be quite satisfactory, A similar method was reported by Hunt (1957), The Kober reagents for this procedure contain sodium nitrate* quinone and hydroquine, No quinol is"added after the preheating interval'and the final concentra­ tion of sulfuric acid is 60 percent. This method has successfully been used by Nelsdn and Smith (1963), : - ■, ' ' i - D, EXTRACTION,OF PROGESTERONE FROM BLOOD AND-OTHER TISSUES As already stated* the progesterone in the blood exists as “25” bound to the plasma proteins 0 It I s g therefore 9 evident that to iso­ late the hormone its binding with the protein part should be broken« Zander and Simmer (1954) and Oertel etc al« (1959) have pointed put that the proteih-steroid binding is broken down even with the organic solventso It is also known that pretreatment of plasma with a solution of sodium hydroxide breaks the .progesterone-protein binding apd while extracting with ether prevents any emulsion formation (Short 1 9 5 8 a and 1958b j Sommerville and Deshpande 1 9 5 8 1 Sommerville et» a l 0 1963),„ Lombardo e t c a l e (1955) have reported the possibility of the application of dialysis for the extraction of progesterone from the b l o o d 6 E o 'ISOLATION OF PROGESTERONE The paper chromatography method for progesterone with petroleum ether and 80 percent methanol system as recommended b y Bush (1952) has been used quite extensively (Gomes e t 0 a l 0 1963| Raeside and Turner ' 1 9 5 6 1 Short 1958a and 1958b)» • :■ In 1961 Short reported that decalins m e t hanolsw a t e r s methy!cyclohexane 5 acetic-acid swater and methycyplohexane water §methanol systems can also prove to be quite satisfactory 0 Simi­ larly 6 methanolsbenzene system has been used (Oertel e t 0•a l 0 1959) with satisfactory resolution. The adsorption "column chromatography with silica^gel (Sommerville : etc alo 1 9 6 3 ) g aluminum-oxide (Noall et. a l 9 ,1953$ Loy eto alo 1957) and f thin layer chromatography (Lisboa 1963$ Futterweit e t o al. 1963) have also* successfully$ been applied. z =26= F o QUANTITATIVE MEASUREMENT OF PROGESTERONE Progesterone has been described as the least polor of all the naturally occurring steroid hormones and no other B-unsaturated oxo- steroid has as yet been identified in vivo with the same Rp value as •progesterone in paper chromatographic system. all steroids possessing the (Short 1961)o It is also known that ^ S - k e t o n e group absdfb ultraviolet light Progesterone being a member of this group of steroids and at the same time having a definite characteristic Rp-value can , easily be identified, I Progesterone can also be identified from its color reaction.with many reagents, Dinitrophenylhydrazine reacts with B-unsaturated ketone group of progesterone to give an orange-red color., whereas m-diriitrobenzene reacts with the 2 0 -keto carbopyl to give a brown purple color (Raeside and Turner 1956), Because of its unsaturated ketone grouping progesterone exhibits a characteristic absorption maximum in ethanol at 240 mu (Short 1961), This single characteristic for its quantitation has been used by many workers (Raeside and Turner 1 9 5 6 1 Gomes ,et, al- 1963J Loy C t v a l , 1957) even though Zaffaroni and Burton (1951) have pointed out that the other lipids contained in the extract also absorb in the ultra-violet spectrum. And when the hormone is separated from the contaminants with paper chromatography 9 and eluted, some background material from the chromato­ gram itself can enhance the absorbance. It has, therefores been emphasized by Lombardo (1955) that the readings-of the densities of I =27= the eluate of an unused area of the chromatogram must be taken into consideration 0 Samuels (1947) has indicated the solution of progeste­ rone in ethanol should possess a well defined peak at 240 mu and there should be very little or no absorption at 280 mu. To correct the effect of the interference due to contaminants in the final progesterone elu a t e „ the readings are generally taken at 230 m u 8 240 mu and 250 mu. The corrected absorbance due to progesterone is then calculated by applying the Allen's correction formula (Gqmes e t , al, 1963; Loy et, al, 1957), Oertel et, al, (1959) have advocated the use of sulfuric acid=ethanol reaction. absorption is obtained at 295 mu, By this method the maximum However 9 this method* due to its low sensitivity* has not proved to be very useful (Short 1961), A gas chromatographic technique for the identification and measurement of progesterone has been reported by Futterweit et, al, (1963,), EXPERIMENTAL PROCEDURE ...... aurmu^fiKm ; ■ Five Jerseys and one Holstein Iactatiiig cowj from the d#iry herd at Montana State College^ Bozeman 9 were used in this study* Laparotomy -I' - ■from the right si<Je of the animal was performed and the posterior vena cava was cannulated at a distance of about one inch backward from the level of the right kidney using Silastic Silicone rubber tubing* f* 1 The tubing was very kindly donated by Dow Corning Corporation 9 Midland 9 Michigan* The internal and external diameter of the tubing were 0*1325 cms* and 0*2625 cms* respectively* was about 72*5 cms* The total length of the tubing The length of the tubing inside the vein was approximately 37*5 cms* whereas about 30*0 cms* portion outside the vein was anchored to the abdominal'w a l l * The remaining 5*5 cms* was kept outside the body and a syringe adapter was fitted onto this end of the tubing* Except when the blood was actually drawn this end was always kept sealed with a metal plug* An iron ring with an internal and external diameter and thickness of 9 cms* g 7*5 cms* and 0*1 cms* respectively 9 and having a spongy pad fixed on the under surface was sutured to the skin* An aluminum lid of the same size as that of the ring was then bolted onto the ring* The lid could thus very easily be removed at the time of the sampling of the blood* About 250 ml* blood was collected with the help of a syringe every other day* Five ml* of sodium citrate solution in distilled water (0*5 g,*/ml») was used as an anticoagulant* After the collection of the sample* the cannula was flushed with about 5-10 ml* heparin solution (200 I 0 U* per ml* normal saline)* Heparin solution was -29injected dyery other day even though no samples were collected* To prevent the growth of fungus or other micro-organisms in the heparin solution Terratnycin■ was included in the stock solution (I m g 0 / m l * )„ Urine samples were also collected immediately after the blood was drawn * The samples were immediately brought to the laboratory and the blood was centrifuged at a rate of 3000 rpm, If the samples could not be analyzed immediately the plasma was stored in the freezing chamber of the refrigerator at a temperature of about -10 C?to -S 0 G e, The urine samples were stored at a temperature of about +5 C?* Sample collections were made ■during the e'strdus cycle of four Jerseys and o n e .Holstein cow and during the first 34 days post-conception period from one Jersey cow, c The urine samples were extracted using the method outlined by Nelson (1959) which is a modification of the methods of Friedgood (1948) and Stimrnel (1946)* no * The procedure followed is outlined on flow sheet io As recommended by Preedy and Aitken (1961) the extraction.pror cedure for estrogens from the plasma was the same as for the urinary estrogens except for the following changes ; ": t . . C: - 1) About 30 m l * plasma filtered through glasswool was diluted with distilled water to make 500 ml. 2) To avoid any bumping during hydrolysis with 15 volume percent of concentrated hydrochloric acid, the acidified diluted plasma was heated very gently to boiling till the bumping -30FLOW SHEET NO. I 250 ml. Urine Filtered through glasswool and hydrolyzed for 18 minutes with 15 volume percent concentrated H C l . After cooling extracted Pooled Ether Washed 4 x and then 2 Evaporated Extract 30 ml. 9 percent NaHCOg solution x 30 ml. of distilled water. ether to dryness under vacuum. Aqueous Phase (Discarded) v Residue Dissolved in 6 ml, ether and then added 108 ml, CCl4 . Extracted the phenolic phase with 3 x 50 ml, N KOH solution and I x 15 ml. distilled water. 't " -------------Pooled KOH and Water-Fractions Washed I x 20 ml. ether, acidified to pH 3,0 with 6 N HgSOy. Extracted 3 x 80 ml. ether, -------------- * — ------- — Ether Extract Washed 3 x 30 ml, 9 percent NaHCOg solution and then 2 x 30 ml. distilled water. Ether Extract Extracted 5 x 20 ml. 2,5 percent NagCOg solution and then 2 x 30 ml, distilled water. VEther Phase Evaporated ether to dryness under vacuum. Dissolved the residue in 10 ml. 95 percent methanol. Ether-Carbontetrachloride (Discarded) & Aqueous Phase (Discarded) Aqueous Phase (Discarded) * Aqueous Phase (Discarded) 'V Extract stored in the refrigerator ready for chromatography. ” 31“ stopped (about 1 0 minutes) and then the boiling was con­ tinued for 20 minuteso The volume of the hydrolyzed’plasma left after boiling was on an average, about 405 m l 0 3) Extraction from the hydrolysate was performed three times with 1 0 0 tively, ml,, 2 0 0 m l , , and 2 0 0 ml, ether each time respec­ The emulsion formed with ether and plasma was broken by centrifugation, 4) Ether extract was washed 3 x 50 ml, 9 percent NaHCO 3 solution and then 2 x 50 ml, distilled water, 5) Washing of the ether phase to remove estriol With 2,5 percent NagCO^ solution was limited to three times instead of 5 times as in the case of urinary extract. Almost all the yellow pigments contained in the ether extract were left behind in the ethers carbon-tetrachloride fraction. While extracting the phenolic phase (estrogens) with IN-KOH solution from ethers carbon tetrachloride, some emulsion formation was observed which broke itself on standing for a few minutes, ■ - •■ The method for the extraction of progesterone from plasma used was the modification of the methods of Short (1958) and Sommerville et, al# (1963), The procedure adapted by the author is outlined on the flpw sheet number II, All of the three., estrogenic compounds obtained from the urine jand blood were separated from!contaminating substances descending paper chromatography. The procedure used was the same as developed by -32FLOW SHEET N O 6 II Plasma Diluted with equal volume of distilled water; added KaOH solution (0,2 g, per m l , ) to make 1.5 g, NaOH per 100 ml, diluted plasma. Extracted 3 x 1/3 volume ether. Emulsion broken by centrifugation, I I Pooled Ether Extract Washed 2 x 1/10 volume distilled water. Aqueous Phase (Discarded) Ether Phase J Ether evaporated under vacuum. Aqueous Phase (Discarded) Residue Dissolved in 20 ml, petroleum ether (30° - 60° C), Rinsed the flask 5 x 20 ml, freshly prepared 70 percent methanol each time before transferring to separatory funnel for extraction of progesterone from petroleum ether, r Pooled 70 percent methanolic Phase ^Eva p o r a t e d under vacuum. Residue Dissolved in 5 ml, and then rinsed the flask 3 x 3 ml, 95 percent methanol. Nz Ready for chromatography, 4 Petroleum Ether (Discarded) *= 3 3 = Burton e t « a l 0 (1951) and later on modified by Axelrod (1953) and Gorski (1959)» This method had already been used by Nelson (1959) and is shown as followss 1) Chromatographic paper strips, about 56 cms„ l o n g 8 were cut from a roll of Whatman filter paper no, 1 » The.strips were boiled in absolute methanol for one-half hour, then washed once with redistilled methanol and air-dried 0 of a hard lead pencil two lines and 8 9 With the help each at a distance of 6 cms 0 ‘ Cjns0 from one end of the strip were marked = 2) Two m l 0 of the urine extract in 95 percent methanol and 5 m l 0 of the plasma extract in 95 percent methanol yere taken separately in conical centrifuge tubes (15 m l 0) and the solvent was evaporated in the presence of nitrogen g as 0 ' 3) The chromatographic paper strip, already boiled, rinsed and air-dried 9 was dipped in sufficient quantity of formamide s methanol (Isl) which acted as a stationary ph a s e 0 4) The strips were then laid down on a thick sheet of blotting paper= Within about one m i n u t e , the end of the strip with marked lines was picked up with the help of a clean pair of forceps and placed in the solvent trough assembly so that the 6 cmso mark would rest on the glass r o d 0 5) The residue was dissolved in three drops of ethyl-acetates methanol ( 5 si) solution and applied in the form of a narrow band along the starting line ( B c m s 0 mark). This was repeated = SUe3 twice with 2 drops of ethyl~acetateSmethanol 0 tions were made with the help of a lt) 0 The applica­ micropipette 0 Sufficient time was allowed between the two consecutive applications to prevent the spreading of the applied materials 0 6 ) To keep the amount of the stationary phase approximately constant.from strip to strip the time spent in applying the estrogenic material over the chromatographic paper? strips was kept constant (30 minutes at room temperature ) 0 The strips were then ready to be hung in the developing chamber. 7) The developing chamber made from a 30 x 30 x 60 c ms 0 glass tank'kept in a wooden box to give good insulation was used. The inside of the tank was lined with filter paper 9 the lower end of which was always kept dipping in about 3.5 cms. deep mixture of equal volumes of benzene and normal-hexane. Two filter paper strips were also hung separately near the side walls and their lower ends were kept dipping in formamide 9 contained in two small glass jars. The glass lid of the tank was made to remain sticking firmly by applying starph-glycerql paste. The developing chamber was p laceji in a cold r o o m 8 the temperature of which was being maintained et 8 1 0 Q-t Io C 0 ) The solvent trough assembly with the strips hanging down,, with their 6 cms. mark on the glass rod of the assemblys was placed on tjie supporting frame inside the developing tank. =SSea Benzene and n-hexane ( I s l ) which served as the mobile phase * was kept equilibrated with formamide in a brown bottle 0 The amount of the solvents put in the trough and the number of the strips hung from each';trough were kept constant* 9) One blank strip treated exactly in the same manner as the ones V- having the unknown estrogenic material and another having pure estrogens as a reference strip* was also included* 10) The strips were taken out of the tank after a 24 hour period and hung for drying* 11) A representative strip* 1,33 cms* wide* was removed from one side of the 4 cms, wide strip and then dipped in a freshly prepared solution of equal volumes of 2 percent (weight/volume) each of ferric chloride and potassium ferric cyanide* The strips were allowed to.remain in the developing reagent for about 30=45 seconds * them for about 2 These were then washed by immersing minutes successively in each of four beakers containing 5 percent HCl * 5 percent H C l * distilled water and distilled water respectively* ''A It was observed that washing ■ : lV' the developed strips in this manner removed most of the back= ground material and the blue spots * due to an interaction . b e t w e e n ■the estrogens and the developing reagent * could be more clearly visualized* . Under the conditions described for the paper chromatography the distances gravelled from the starting line by all the three estrogens =>36“ % were as follows Estradiol“17B = 5o7 + O 036 c ms 0 (7 observations) Estradiol”17oC = + 0=72 C m g 6 (7 observations) Estrone = 29o9 8 = 6 + 1°14 C ms 0 (7 observations) The reference strips having pure estrogens also helped in locating the spo t s o It was noticed, however 9 that the estrogens from the urinary extracts moved slightly faster than the pure estrogens on the reference stripso This may be due to the extract being overloaded with the contaminantso The corresponding areas from the undeveloped strips were eluted with absolute methanol„ The pieces of the chromatographic paper having individual estrogens were folded with the help of a pair of forceps to g i v e 'them the shape of a funnel 0 They were then inserted and kept near the mouth of the 15 ml, capacity conical centrifuge tubes 0 About 10-12 m l o methanol was allowed to falls, drop by drop, from a buret over t h e m 0 and its elution 9 While cutting the piece of the chromatographic paper great care was taken not to let any paper fringes fall into and remain in the eluate 0 The purification of the plasma extract for progesterone was done by using standard thin layer chromatographic technique 0 The procedure used for preparing the slurry, process of coating the plates, drying and prewashing them is outlined in the appendixo The procedure used for applying the unknown samples, developing the chromatogram and elution was as follows s I) Two starting points were marked, each at a distance of 1„5 ■=37” c m s 0 from the right and left edge and.about 1»5 cms, from the lower edge of the already prewashed and activated layer* These points were marked with the aid of a template and were ' used to apply the pure progesterones hereafter called the reference spots 0 The distance between these two points was marked off into four equal parts* To stop the solvent from overrunning the chromatogram* the layer was divided at a distance of 15 cms* by making a mark at right angle to the direction of development* 2) The methanol from the plasma extract was evaporated in the presence of nitrogen gas* 3) The residue was applied to the starting point as a narrow I. cm* long band in the center of one of the four areas ,already marked* This was accomplished by dissolving the residue in three drops of benzene and then rinsing the tube with two drops of benzene each time* A microsyringe* (10 ul*) was used for applying the samples* 4) The plates were placed in the tank already saturated with the solvent system which was a mixture of ml* ether and 2 1 2 0 ml* n=hqxanee 80 ml* glacial acetic acid (hereafter referred to as System " A " ) * 5) After developing* the reference spots were detected and marked *Microliter #701 Hamilton Co* *■Inc* * Whittier* California = 38" by noting the absorption of ultraviolet light by progesterone. The corresponding areas of the unknown samples were then scraped off into a buchner funnel fitted with a fritted disc of fine porosity, A filter-vac was used to place the funnel over a filtering flask. was used for the elution. About 10=12 ml, of absolute methanol Vacuum was then applied to suck the eluate down into a 15 ml, conical centrifuge tube, 6 ) The methanol from the eluate was evaporated in the presence of nitrogen gas and the residue was once again applied on to another Chromatogram 9 exactly in the same manner as previously. I described. The developing system used was a mixture of 160 ml, b e n zene$ 40 ml, absolute methanol and 2 ml, glacial acetic a c i d 9 hereafter referred to as System "B", 7) The elution of the corresponding areas was done with 10=12 ml, absolute methanol as it was explained previously, 8 ) Methanol was evaporated and the centrifuge tubes containing the residue were kept in the freezing chamber of the refri= gerator until they were read on the spectophotometer, 9) After scraping the corresponding areas to the reference points '■ as explained a b o v e 9 the rest of the layer was sprayed with 50 percent sulfuric acid and then heated in an oven at a temperature of about 150° C, for 15 to 30 minutes. Pure progesterone and the contaminants were located by visualizing the fluorescence give out by these compounds under the •=39™ ultraviolet light and the distance they had traveled was measured. The Rp values of pure progesterone and the contaminants contained in plasma extract separated by thin layer chromatography are shown in Table No, .1, . TABLE .NO, I Table Showing the Rp -Values of Progesterone and Contaminating Subst ances„ ..Contained...JLn„The ..PlasmeL Extract ,.Qn.. Thin Layer Chromatography ---------- s ^ E T m — Compounds Progesterone . Contaminant I, Contaminant II Contaminant III Contaminant IV " ■1 * System "A” **System "B”. 0,1063 0,1992 0,2612 0,4685 6,7958 s ’ ... System "B"-*.. •' + 0,0009 + 0,0003 + 0,0003. +0,0075 + 0 „ p 0 1 7 '.i' ■ 1 . . . : ..... , 0,61 + 0,0005 0,3867 ± 0,0029 0,8267 «»===360< = > ====== , -U^nr . . Hexane + Ether + glacial acetic acid (120:80:2) * Benzene +■• Absolute Methanol-.+ .glacial, acetic acid (160:40:2).... Estimation of estrogens was done by using the Kober Color i Reaction as modified by Hunt et, al, (1957) I m d is outlined below":..... - ' : ■ 1) The methanolic eluate of the individual component obtained ' . ;. "... v from the paper chromatogram was evaporated to about 2 ml, by . • - -s ’ ' 1 - e* . ^ ■ j [ / 'i1 heating in a hot water bath (not over 60° C) in the presence 1 ' ' ■ ..• .: I. of nitrogen gas. V' . . One tube containing an eluate of an equal piece of blank chromatographic paper was also included, 2) Two-tpnths ml, of hydroquinone solution (see appendix) was added to the eluate, The evaporation was started again until -40the crystalline residue was Idft behind 0 3) Kober reagent for estradiol (gee appendix) was added into the tubes containing the estradiol samples„ Similarly 3 ml, of the reagent for estrone were added to the tubes containing the unknown samples of estrone. One tube containing reagent blank was also included, 4) The t u b e s 9 capped with corks having aluminum foil wrapped around them were placed in a boiling water bath and allowed to remain there for 20 minutes (preheating period), Thq tubes were shaken twice within the first five minutes without taking them out of the water bath 8 tp ensure that the crystalline residues had gone into solution, 5) After 20 minutes 9 the tubes were transferred to a cold water bath (15° to 18° C) and kept there for 7 minutes,i 6 ) Seven-tenths milliliter of the Kojaer reagent for estradiol ■ was added to each of the preheated tubes containing the unknown estradiol samples and the reagent for estradiol. Similarly 0,3 ml, of distilled water was added to each of the preheated tubes containing unknown samples of estrone and the estrone reagentg so that the final concentration of the sulfuric acid was 60 percent, 7) The tubes were capped once again and shaken very gently to iTiix the-contents thoroughly 0 Care was taken not to" let the contents come into contact with the aluminum foil or the cork « 4 1 « Itself 0 8 ) The tubes were then placed in the boiling water bath and shaken once during th^ reheating period of 15 minutes (Reheating ) 0 9) After completion of the reheating t i m e 8 the tubes were transferred back to the cold water bath and allowed to remain there for 7 = 10 minutes= 10) The tubes were then left at room temperature for about 10 minutes= 11) Estrone samples were read at 5 44 9 512, and 480 mu and 17e>^> and 17B=estradiol at 5 48s 515, and 482 mu= Sulfuric acid of 60 percent concentration was used as the blank= 12) A l l e n ’s correction equation was used to obtain the corrected absorbancy= Any absorbance due to the filter paper blanks was also taken into account before the quantities against the corrected absorbance were read from the standard curve= To estimate the quantities of progesterone the re s i d u e 'obtained after the evaporation of the eluate was dissolved in I.ml= of 95 percent ethanol= The tubes were shaken on an electric shaker to get a homogenous solution and then the absorbance read on a spectrophotometer* at 2 3 0 8 240 a n d -250 mu= blank= Ninety=fivg.-percent ethanol was used as a -The corrected absorbance at 240 mu was-obtained by applying the A l l e n ’s correction, equation= .. •*Beckmari Model "DU" Spectrophotometer =42= A standard curve for pure progesterone with the quantities ranging from 0 to 10 micrograms per milliliter of 95 percent ethanol was preparedo The quantities in the unknown samples were obtained from the standard curve„ Three samples out of the ones W h i c h 9 by ultraviolet assay 9 were found to be positive for the presence of progesterone were checked by gas chromatography&c The gas chromatograph was set up as follows: A four foot pyrex colum with outer=diameter of 6 mm„ was packed with 3o8 percent SE=30 on siliconized 60/80 mesh Diatoport=S 0 The column temperature at 245°. C 9 flash heatdr at 310° C 0 and detector at 330° C 0 flame ionization with helium flow of 60 m l 0 per minute were maintained. * *F S M Model 400 Biornedical0 RESULTS Standard curves with pure estrone, ITKr and 17B-estradiol for the Kober reaction, prepared in the beginning of the experiment and then checked after about one year, are shown in Figure N o 0 2 0 The absor­ bancy obtained for the range of the estrogens used to establish the standard curves obeys the Beer's Law, ITB-Estradiol Estrone Micrograms Figure No, 2 Standard Curves of Pure Estrogens For Kober Reaction -44= I= The levels of the estrogens in the blood plasma and urine during the estrous cycle § It was noted that each of the five cows exhibited a higher level of the estrogens at one time before and one time after thp midcycle than any of the other periods sampled 0 There was a considerable variation among the cows as to the time when these high levels were reachedo The periods of the cycle when individual cows showed the higher levels are as follows § A0 Estrogens in the blood plasmas The fluctuations in the levels of the estrogens in the blood ■ ; plasma during the estrous cycle of five cows are shown in figure n o 0 3 to 6 o It is evident that three out of five cows (cows numbered 2„ 3 £ 5) had the first high level of 17e4estradiol during the period between days 6 £ S 9 whereas cows numbered I and 4 exhibited their first high levels during the periods between days 3 £ 5 and 9 £ I l 9 respec­ tively o The second high level of 17©6?estradiol occurred during a period between days 15 £ 17 in cows numbered 2 and 5 and during a period between days 18 £ 20 in cows numbered 3 and 4 0 had the second high level of IToGestradiol between days Cow numbered I 1 2 £ 14, which was earlier than the other four cows„ Similarly the first, high level of 17B-estradiol of three out of C- ' - ■ five cows (cows numbered I 9 2 £ 5) was noted during a period between days 6 £ S 9 whereas for cow numbered 3 this high level was noted between -45days 3 6 5 = Three out of the five cows (cows numbered I 9 3 I 6 5) showed the occurrence of the second high-level during a period between days 1 2 6 1 4 9 whereas cow numbered days 15 6 17„ showed the second high level between 2 Cow numbered 4 exhibited only one high level of 17B- estradiol which occurred between days 9 6 11« The levels of estrone in four out of five cows (cows numbered 2 9 / ! 39 4 5) reached the first high level between days 6 6 6 , whereas those 8 of fhe cow numbered one reached the first high level between days 3 6 5 = Similarly sthree out of five cows (cows numbered 2 9 3 second high level of estrone between days 15 two cows (cows numbered 1 6 4 ) level between days 18 6 2 0 6 5) had the 1 7 s whereas the other 6 had a gradual increase reaching the high = Total estrogens of three out of five cows (cows numbered 2 9 3 ; -■ ■ - 6 / 5) were observed to reach the first high level during a period between days 6 6 8 = Cow numbered one had the first high level between days 3 5 and cow numbered 4 had this high level between days 9 6 11= 6 Similarly the second high level of the total estrogens of two cows (cows numbered 2 6 5) was noted between days 15 6 17$ whereas this high level of cows numbered 3 and 4 was observed between days 18 6 20» The fifth cow (cow numbered I) had the second high level of the total estrogens between days 12 6 14= The data obtained regarding the levels of the estrogens in blood plasma obtained from the posterior vena cava and in the urine during estrous cycle; during the first 34 days after conception; and the levels -46of progesterone in blood plasma, are shown in Figures No. 3 to 13. * -------- -X It^-Estradiol -© 17B-Estradiol Estrone Total Estrogens O T cO 0-2 3-5 6 - 8 9-11 12-14 15-17 18-20 Days After Heat Cow No, I Figure No, 3: The levels of estrogens (micrograms per milliliter) in blood plasma during estrous cycle. 0 =35 -47■X IV0^-Estradiol ■€>IVB-Estradiol ♦ Estrone - Total Estrogens 12-14 9-11 Days After Heat Figure No, 4: 15-17 18-20 The levels of estrogens (piicrograms per milliliter) in blood plasma during estrous cycle. & 17o£Estradiol Q- "S ITB-Estradiol #- -# Estrone W 5 -- Total Estrogens ------- 0-2 3-5 •----------- ------6 - 8 9-11 12-14 ______ . - 15-17 18-20 Days After Heat Cow No. 3 Days After Heat Cow No. 4 Figure No. 5: The levels of estrogens (micrograms per milliliter) in blood plasma during estrous cycle. -49Xr ^ O- -G ITB-Estradiol 0 -# Estrone - I-Zr^-Estradiol o.go Total Estrogens \ \ / / / LO 1 \ \ / 2 \ 3 4 5 6 Days After Heat Cow No. 5 Figure No. 6: The levels of estrogens (micrograms per milliliter) in blood plasma during estrous cycle. 7 -50B. Estrogens in the urines Figures no, 7 to 9 show the variation in the levels of the estrogens excreted in the urine during the estrous cycle of five cows. It was noted that the first high level of IV^restradiol of two put of five cows (cows numbered 3 6 4 ) days 9 11, 6 occurred during a period between The other three cows (cows numbered I s 2 first high level during the periods between days 0 6 6 8 , respectively. 5) showed the 6 2, 3 6 6 5, and Four out of five cows (cows numbered I 9 2, 4 had the second high level of this hormone occurring between days 18 20, whereas cow numbered 3 had it between days 15 6 5) 6 6 17, The urinary excretion of 17B-estradiol by two out of five cows (cows numbered 1 6 3 ) reached the first high level between days 3 6 5 , whereas that of cows numbered 2 and 5 reached the high level between days 0 6 2 and numbered I, 2 days 18 6 15 6 17, 15 6 17, 2 0 6 6 6 8 respectively. Three out of the five cows (cows 5.) had the second high level during the period between , whereas cow numbered 3 had this high level between days Cow numbered 4 had only one high level occurring between days Simil-Sbly two out of five cows (cows numbered 2 6 3) had the first high level of estrone occurring between days 3 6 5 , whereas cows numbered I and 5 had this high level between days 6 respectively. 6 8 and 9 6 11, The second high level of estrone for cows numbered I and 5 occurred between days 18 6 20, whereas cows numbered 2 and 3 had this high level between days 12 6 14, Cow numbered 4 had only one high -51level of estrone occurring during a period between days 15 Three out of the five cows (cows numbered 2, 3 6 6 17« 5) had the first high level of total estrogens occurring between days 3 6 5 , whereas cows numbered I and 4 had the first peak of total estrogens between days 6 6 8 and 9 6 11, respectively. (cows numbered I, 2, 4 6 Similarly, four out of five cows 5) had the second high level of total estrogens during the period between days 18 6 2 0 , whereas the second peak occurred in cow numbered 3 between %days 15 6 17, -52X:--------- KITo^-Estradiol / I ©----------© 17B-Estradiol / •----------• Estrone / ----------- Total Estrogens Z / / / / / / / / / / / o O- CO 200 / C C• 0-2 3-5 6-8 9-11 12-14 15-17 18-20 Days After Heat Cow No. I Figure No. 7s The levels of estrogens (micrograms per liter) excreted in urine during estrous cycle. -53"* IT0^-Estradiol SiITB-Estradiol ■0 Estrone -- Total Estrogens 9-11 12-14 15-17 18-20 12-14 15-17 18-20 Days After Heat 9-11 Days After Heat Cow No. 3 Figure No. 8; The levels of estrogens (micrograms per liter) in urine during estrous cycle. -54X 17o(-Estradiol S 17B-Estradiol Estrone Total Estrogens v 9-11 12-14 15-17 18-20 Days After Heat Cow No, 4 9-11 12-14 15-17 18-20 Days After Heat Cow No o 5 Figure No, 9; The levels of estrogens (micrograms per liter) excreted in urine during estrous cycle. — 55— II „ Estrogens in Blood Plasma and Urine During First 34 Days After Conception: Figure No. 10 6 11 show the levels of plasma estrogens and the estrogens excreted in the urine during the first thirty-four days of pregnancy by cow no. 6 . The levels of 17B-estradiol in the blood plasma were higher on day 1 1 post-conception than on any other day. The levels of the total estrogens in the plasma show a small peak on day 7 and then a higher peak on day 11 after conception. The levels began to increase on day 19, reaching another peak on day 26 and then declined. The levels on day 34 were equal to the levels on day 19. Similarly the levels of urinary estrogenic excretion reached a peak on day 9 after conception. The levels started to increase on day 16, reaching a peak on day 31 and then declined once again. On day 34 after conception the levels are equal to the ones on day 15. o -X 1 % 6 -Estradiol 4) 17B-Estradiol -# Estrone Total Estrogens Days Post-conception Figure No. 10: The levels of estrogens (micrograms per milliliter) in blood plasma of cow no, 6 . -56— X ---a— X ITe^rEstradiol ---- ITB-Estradiol O ---- -----# Estrone Total Estrogens Micrograms __________ Days Post-conception Figure N o 0Il; The levels of estrogens (micrograms per liter) excreted in the urine of cow no„ 6 during post-conception period. -57Figure no. 12 shows a standard curve of pure progesterone prepared for ultraviolet assay. Quantity of Progesterone — Figure No, 12; micrograms per ml, 95 percent ethanol Standard curve of progesterone for ultraviolet assay. “58— The pooled data for progesterone are shown in Figure no, 13, It was observed that the level of progesterone in the plasma of the posterior vena cava blood was low during the first 9 days and then began to rise until it reached the maximum on day 17 of the cycle, The level of progesterone on day 19 had decreased and was equal to the level on day 13, Days After Heat Figure No, 13: The levels of progesterone (micrograms per milliliter) in blood plasma during estrous cycle. 0059” DISCUSSION AND CONCLUSIONS The levels of estrogens in the blood and urine of the higher primates during a menstrual cycle have been established (Everett, 1961)„ But, to the best of the author's knowledge, there has been no report 1 which has shown the day-to-day changes in the levels of these estrogenic hormones during an estrous cycle= Most workers have tri^d to study the fluctuation of estrogens in the urine on the day of estrus and during gestation. Various attempts to determine the estrogens in the jugular venous blood of animals during the estrous cycle have not I succeeded (O'Donnell and Pre e d y , 1961), One report describing the fluctuations in the levels of progestins during the estrous cycle of ■» bovines has been published (Gomes et, al, 1963), It has been indicated that the adrenals and the uterus (placenta in the case of pregnancy), in addition to the ovaries, are capable of synthesizing and perhaps secreting estrogens and progestins, To . understand the fluctuation in the secretion of these hormones in vivo., ■ at least two alternatives as mentioned below are possible: I, To separately, collect- and. analyze the blood coming from all the possible sources of their syntheses and secretion,. Then by simple addition the information regarding the total of these hormones entering the circulation % n the body could be obtained, 2, To collect the blood samples' from a location near to the .site of secretion of these hormones where the blood being *-60“ drained away from all these sources has already combined. The second alternative seemed more advantageous» The posterior vena cava drains blood from the hind portion of the body and also receives blood coming from the ovaries, uterus (placenta during preg­ nancy ) and adrenals„ It was for this reason that the posterior vena ■ cava was selected as a point of sampling of the bl o o d , In as much as it is known that considerable variation occurs in the levels of estrogens and progesterone among individual animals a it is thought to be advantageous to obtain as many samples as ppssible from each animal during any experimental period. To collect as many blood samples as possible from each animal during an estrous cycle, ' cannulation is perhaps the only alternative. In the beginning of this' study, a polyethylene tubing was used to cannulate the posterior vena cava of a cow. The cannula with such a material could not be kept open for more than 11 days,. It was found that a cannula made from the Silastic Silicone rubber tubing was definitely better than the one made from polyethylene tubing, As there was no precedent of such a cannulation experiment,'the author had to face many problems to keep the cannula open for as long a- period as possible. The longest dura­ tion for which the cannula could be kept open was 43 days. It was observed that the formation qf a blood clot inside the tubing was responsible for its being plugged up. During the present study it was found that the estrogens in the blood, as well.as in the urine of 1cattle^ during an estrous cycle show “■61“ ■great fluctuations« It was noted that in most of the cows these hor= mones reach high levels once before and once after the midcycle» Three out of the five cows had the first high level of the total estrogens in the blood plasma occurring between days 6 6 8 0 Similarly9 three out of the same five cows showed the occurrence of the first high level of the total urinary excretion of the estrogens during a period between days 3 S 5 0 The second high level of the total estrogens in the blood plasma occurred between days 12 & I H 9 15 6 17 9 and 18 & 20 for. O n e 9 two and two cows respectively» Similarly four out of the five cows had the second high level of tot^l urinary excretion of the estrogens between days 18 S 2 0 o It is evident that in the majority of the cows the first high levels of Yhbrand ITB-estradiol and estrone in the- blood plasma occurred between days 6 £ S 0 . Similarly in the majority of the cows the second high levels of the same hormones in the blood plasma were observed to occur between days 12 £ 17« The quantities of these estrogens excreted in urine by the majority of the cows reached the first high level during a period between days 3 £ 8 and the second high level during a period between days 15 £ 20„ It is known that a considerable portion of the estrogens is excreted in the urine0 It was 9 therefore s thought advisable to collect the urine samples at the same time= The urine-collecting-bag9 as has already been used by many workers ($mith e t „ a l o 1956| Nelson and Smith 1963) 9 was avoided to prevent the cannula from being .disturbed,,. . \ “62= The method of extraction and estimation of the estrogens in the urine was almost the same as had already been used and found satisfactory by Nelson and Smith (1963)„ As recommended by Preedy and Aitken (1961) the method used for the extraction of estrogens from the blood plasma was the same as for urine except some minor changes„ Although it is the first time that the occurrence of a double ■ I peak in the levels of estrogens during the estrous cycle of cattle is being reported it is not surprising because the occurrence of such two peaks in the levels of estrogens in the primates has already been established (Fish e t 0 a l o 1 9 4 1 | Markee and Berg 1948; Everett 1961).. It is also interesting to note that the individual animals showed considerable variation as to the time of the peaks during the cycle. It has been reported that 17B=estradiol is the most potent estrogen whereas Iy0^ e s tradiol is the least (Boscott 1962). It has been indicated that estrone $ Iy0^ e s t r a d i o l 8 Ie=Ketoestradiol=IyBsi 2=methoxyestrone and some other estrogenics as well as non=estrogenic8 compounds may very well be the degradation products of 17B=estradiol (Beck 1950; Engel e t . a l 0 1959; Heard and Huffman 1941; Pearlman and Pincus 1943; Wotiz e t . a l . 1958; Levitz e t . a l . 1956; Kraychy snd Gallagher 1957). Purdy et. a l . (1961) have suggested that estrone may perhaps be the major estrogenic hormone present in the systemic blood. Whereas l%^=estradiol and estrone represent the major portion of the estrogens excreted in the urine (Nelson 1959), - A mechanism 63- ofinactivation is known to exist in the body of an individual which helps to maintain the circulating estrogens at a physiological level (Oakey et, al. 1 9 6 2 j Fishman et„ al« i 9 6 0 eto alo 1950 Ecfcles e t 0 a l 0 1962), iCoppedge It seems evident that such a mechanism may be playing an important role to prevent the animal from coming in heat during the period when the first high levels of estro­ gens in the blood plasma were observed0 The occurrence of the first high levels of estrogens in the plasma was in general not followed by the first level of the urinary excretion of the estrogens ( 1 % & and 17B-estradiol and estrone together)0 It might be possible that during this period the estrogens were either metabolized to some weak estro' V• ' Ii genic or eyen rton-estrogenic compounds or the major portion of the estrogens was excreted in the feces0 On the other h a n d 8 the second i' high level of estrogens in the blood plasma was 9 in'general9 followed by a high level, of the excretion of the estrogens in the ur i n e „ The gradual increase in the levels of the excretion of the estrogens in the urine 8observed after the second peak of the estrogens in the blood- • had already occurred e might indicate that the magnitude of the inacti­ vation mechanism at this stage also rises gradually 0 The inactivation mechanism might g therefore„ reach a peak during the same period when the second peak of estrogens excreted in the urine was observed. According to the modern concept (Deane 1952) it is believed that a feedback mechanism is responsible for controlling the production and/ or release of-the"hormones concerned with estrus. It is generally “64thought that the level of estrogens during postestrous and diestrous periods is very I o w 9 whereas the level of progestins during the diestrous period is very high. During the last part of phe diestrous the level of progestins falls down. This fall* along with the low level of estrogens9 lowers the threshold for the release of the ; - follicle-stimulating-hormone which in turn stimulates the development of the vesicle follicles. Growth of the follicles is accompanied by • an increase in the levels of estrogens. An increase in the levels of estrogens at this stage when the level of progestins is very low is believed to bring the animal in heat. The present St u d y 9 in contrast to the concept that during an - ' ■. estrous cycle of cattle the levels of estrogens rise only once 9 has indicated that the total estrogens in the b l o o d 9 as well as excreted in the u r i n e 8 show two pe a k s . Although no report has shown that the levels of the secretion of gonadotropic hormones from the hypothysis of cattle show a double p e a k 8 the occurrence of such two peaks in the swine has already been reported (Robinson and Nalbandov 1951). two peaks were observed on days 8 £ 18 of the estrous cycle. These No such information is available to show the fluctuation in the levels of gonadotropic hormones in the cattle. It has recently been reported'by Gomes et. al. (1963) that the levels of progestins in the ovarian venous blood of cattle during an estrous cycle start increasing on day 6 and reach a peak level on day "15 after heat. ■ The results from the present study„ although from a =65” small number of animals 9 have indicated that the levels of progesterone in the blood from the posterior vena cava reach a peak on day 17 of the estrous cycle« An hypothesis as to how the first peak of the total estrogens in the blood and the urine occurs and why the animal during such a period does not come in heat is presented as follows 5 If the secretion of the gonadotropic hormones reaches two peaks in cattle, as have been shown in swine (Robinson and Nalbandpv 1951) and if the first peak occurs during the postestrous period, growth of the vesicle follicles during this period is possible= The development of the follicles at this stage would be accompanied by the synthesis , '? :■ and release of estrogens= If the levels of progestins start on day 6 of the estrous cycle (G'om'dsH'et,= al.= 1963), then the ■. increasing further growth-of the follicles decelerated and stopped and then they become atretic= At this stage the estrogen inactivation mechanism might be .very active so that the higher levels of the estrogens might immediately be disposed of and thus the animal be prevented from coming ip heat= This may possibly be due to the rise in the levels of progestins and/or due to some other unknown factors= The levels of the estrogens in the blood therefore fall down and are then maintained= This inhibitory mechanism due to the presence of very high levels of progestins and some other unknown factory continue up until the later part p.f the diestrous period= If the animal is not pregnant, the threshold due to prolactin (from the pituitary)-and some unknown factors which are . "6 Bae responsible for maintaining the high Jevels of progestins (Turner 1961)* is lowered, •This initiates the lowering of the levels of the progestins during the later part of the diestrous period. Low levels of progestins '' '' '• and/or the occurrence of the second peak of the gonadotropic hormqnes at this stage* initiate the growth of the follicle vesicles and hence the second peak of the estrogens in the blood occurs. During this period the mechanism of inactivation of the estrogens might not be very active so that the estrogens could get a chance to bring about the necessary changes and thus prepare the animal for coming in heat. Later on this mechanism becomes very active which might be responsible for the low levels of estrogens during the estrous and the postestrous period. The occurrence of a peak of the estrogens during the postconception period similar to the first peak of the estrogens during an estrous cycle was observed in cow no, 6, indicates that if the animal "conceives Data obtained from this cow . the threshold due to which the blood estrogens are maintained at low levels is not lowered and hence the estrogens do not show the second peak which should have otherwise occurred. The absence of the second peak of estrogens may possibly be used as a diagnostic measure for pregnancy test. The low levels of estrogens during the first 34 days after conception are consistent with the report of Nelson and Smith (1963), In conclusion* it can be said that the evidence presented indicates that the quantities of-the estrogens in the blood* as well as in the urine* of cattle during an estrous cycle reach a double peak. In most of the cows the first peak in the blood occurred between days 6 6 8 , -67whereas in the urine it occurred during a period between dayp 3 £ S 0 The second peak of the estrogens in the blood occurred on iihys 12''to 17 6 ,whereas the second peak in the utfihe. occurred on days 15 to 2 0 0 The levels of progesterone reached a peak on day 17 of the estrous cycle« It is also evident that the levels of the estrogens in the blood* as well as in the urine* were not high during a period of 34 days postconception* It seems possible that the absence of the second peak of estro­ gens may be used as an indication that th,e conception has occurred* SUMMARY Five Jerseys and one Holstein lactating cows were used for this Study0 Cannulation of the posterior vena C a v a s, about one inch backward from the level of the right R i d n ey8 by using Silastic Silicone rubber tubing was performed0 B l o o d 8 as well as urine samples8 were collected every other day during the estrous cycle of four Jerseys and one Holstein,, Samples were also collected during a period of 34 days postconpeption from one Jersey* The blood plasma samples were analyzed for the estro­ gens and progesteroneo measuredo Excretion of the estrogens in urine was also The quantitative measurement of the estrogens was done by the Kober reaction assay whereas progesterone estimation was done by an ultraviolet as s a y 0 The results have indicated that the measurement of estrogens and progesterone in the blood from the posterior vena cava of cattle during an estrous cycle is possible* The quantities of the total estrogens in the b l o o d 8 as well as urine of cattle during an estrous cycle 8 reached two peaks 0 In the majority of the cows the first high level in the blood occurred between days 6 6 8 whereas the first peak in the urine occurred during a period between days 3 6 S 8 after heat* The second peak of the blood estro­ gens occurred between days 12 6 17 whereas the second peak of the urinary estrogens occurred during a period between days 18 6 2 0 8 after the heat* ■ The levels of progesterone in the blood of the cows were found to reach a peak on day 17 after heat. Corresponding to the two peaks of the total estrogens found to “69“ occur in the blood and urine during an estrous Cycle9 only one peak was ' observed to occur during a period of 34 days postconception <, Low levels of estrogens in the b l o o d s as well as in the urine of cattle during the early days of the postconception peri o d 9 are consistent with the reports by the previous wo r k e r s „ APPENDIX ” 71 " The purification of the plasma extract for progesterone was done 1 ' by using standard thin layer chromatographic technique. .. ' '• ■ The.procedure ' used is outline below s 1) Three 20 x 20 cms, glass plates of equal thickness were placed on the aligning tray. Right and left hand ends of the row of plates were completed with two 5 x 20 cms, end=plates0 2) The spreader (Stahl type) was adjusted to give I mm, thick layers and then placed on the right hand e^B-plate, 3) Silica-Gel-G (Brinkman Instrument Inc,* batch no, 7731/T6722B grain size maximum 5-20 u) and distilled water* 50 g, and 80 ml, respectively8 were mixed in a mortar. Grinding was carried out very gently and without taking the pestle out of the slurry. This prevented the formation of any air bubbles. Time spent in preparing the emulsion was limited to one minute, 4) The suspension was immediately transferred to the spreader, V- " , When the slurry started to come out* the process of coating was begun. The spreader was then drawn very smoothly across the plates towards the left end-plate. The spreader* the end- plates and the mortar were immediately washed and dried, 5) The coated plates were left in the laboratory for air-drying and "setting" for 30 minutes and then were stacked in the drying rack. Activation of the Silica-Gel-G layers was carried out by heating the plates and keeping them in a vertical position (in the rack) in an oven at 102° - IlO0C, for 30 w72™ minutes» 6) The adsorbent sticking to the edges and about 2 Inm0 of each edge of the layer was wiped off with the thiiriib .and finger. 7) The prewashing mixture9 consisting of Chloroform (3,65 ml.) ; .■ ■ :■ .. . - and iridthanol (35 m l . ) was poured in the tank and mixed well by getitle shaking. 8) Six 5.3 x 20.0 cms. Beckman paper wicks were placed in a verti­ cal position with their lower ends dipping in the prewashing solvent system to saturate the tank. 9) When the solvent mixture had reached the top end of the wicks 9 two plates for prewashing were lowered down and placed in vertical position lying against the opposite walls of the tank. 10) After developingg the plates were air-dried and heated once again in the oven at 102° to 110° C. for 30 minutes. A 2.5 cms, wide strip of Silica-Gel-G was removed from the end of the plate towards which the solvent was traveling. The drying rack with the activated plates was stored in an improvised desiccator9 having calcium ,chloride as the.desiccant. -73 TABLE NO, II A Measure Of The Quantities Of Estrogen Secretion At Different Periods Of The Estrous Cycle (Micrograms per ml. plasma) Period I 2 I O - 2 3 - 5 6 - 8 9 - 11 12 - 14 15 - 17 18 - 20 2 2 5 8 11 14 17 20 3 4 5 6 7 O 3 6 9 12 15 18 I 2 3 4 5 6 7 O 3 6 9 12 15 18 2 5 8 11 14 17 20 4 I 2 O 3 6 9 12 15 18 2 5 8 11 14 17 20 5 3 I 2 3 4 5 6 7 I 2 3 3 4 5 6 7 O 3 6 9 12 15 18 Cow No. 2 5 8 11 14 17 20 4 5 6 7 a* Days After Heat No Sample - - IVjt Estradiol a* 0.052 0.006 b** 0.064 0.049 0.038 17BEstradiol a* Estrone a* 0.016 0.018 0,019 0.098 0.090 0.060 0.028 0.014 0,031 0.073 0.075 0.075 0.093 0.098 0.118 0.062 0.017 0.035 0.024 0.085 0.032 0.123 0.115 0,016 0.043 0.023 0.019 0.020 0.012 0.020 0.039 0.015 0.034 0.019 0.022 0.024 0.015 0.024 0.087 0.053 0.015 0.016 0.019 a* 0.016 0.021 a* 0.021 0.023 0,062 a* 0.032 0.024 a* 0.009 0.017 0.016 a* 0,023 0.032 a* 0.019 0.033 0.013 0.016 0.025 0.025 a* 0.031 0.040 0.021 0.045 0.024 0.016 0.068 0.129 0.093 0.034 0.086 0.078 0.087 0.018 0.106 0.081 0.014 0.023 0.010 0.014 0.001 0.012 0.005 0.015 0.019 0.037 a* b** Undetectable a* Total a* 0.096 0.038 0.050 0.235 0.214 0.173 0.265 0.148 0.357 0.258 0.047 0,101 0.057 0.059 0.042 0.048 0.040 0.078 0.101 0.124 a* 0.045 0.056 0.097 a* 0.071 0.077 a* 0,118 0.202 0.127 0.095 0.135 0.119 74 TABLE NO. Ill A Measure Of The Quantities Of Estrogen Secretion During Postconception Period (Micrograms per milliliter plasma) Cow No. 6 Days PostConceptions I 5 7 9 11 13 15 19 21 26 31 34 1706Estradiol 17BEstradiol Estrone Total 0.006 0.002 0,021 0.013 0.021 0.003 0.014 0.007 0.005 0.008 0.005 0.002 0.032 0.012 0.027 0.020 0.033 0.015 0.006 0.011 0.014 0.008 0.008 0.006 0.034 0.012 0,016 0.027 0,028 0.005 0.013 0.001 0.005 0.023 0,011 0.007 0,072 0.027 0.064 0,060 0,082 0.023 0.033 0,019 0.024 0,039 0.024 0.015 75TABLE NO. IV A Measure Of The Quantities Of Estrogens Excreted In The Urine At Different Periods Of The Estrous Cycle (Micrograms per liter of urine) Periods a* Days After Heat Cow No. 17c6 Estradiol 17BEstradiol Estrone Total I 2 3 4 5 6 7 O 3 6 9 12 15 18 2 5 8 11 14 17 20 I - a* 31.87 49.12 10.50 24.38 93,75 249.38 a* 46.87 17.25 50.61 73,50 43,50 184.88 a* 50,62 119.25 69,00 61.50 111.00 206,62 a* 129.37 185,62 130.12 159.38 248,25 640,88 I 2 3 4 5 6 7 O 3 6 9 12 15 18 - 2 - 5 8 - 11 - 14 17 - 20 2 40.02 25.80 18,00 21.30 22.42 33.75 36,12 20.18 18.00 15.82 12.75 6.45 12.75 23,55 19,56 63.90 51.90 0.90 27,90 30,00 24,90 79.76 107,70 85.72 34.95 56.77 76.50 84.57 I 2 3 4 5 6 7 O 3 6 9 12 15 18 - 2 5 8 11 14 17 20 3 18,00 31.05 39.00 53.10 7.95 22,05 7,95 1.50 6.60 9.60 8.25 11.92 8.25 11.40 21,90 15,00 12.54 19.50 15.15 13.80 30.90 59.55 54,00 75.24 35.70 49.12 30,00 I 2 3 4 5 6 7 O 3 6 9 12 15 18 - 2 5 8 11 14 17 20 4 a* 4.50 a* 64,05 a* 14.10 56.10 a* 6.00 a* 12,90 a* 15.30 14,40 a* 4,20 a* 9.30 a* 12,90 10,50 a* 14.70 a* 86,25 a* 42.30 81.00 I 2 3 4 5 6 7 O 3 6 9 12 15 18 - 2 5 8 11 14 17 20 5 a* 13,80 10.20 13.65 22.50 21,00 29.40 a* 10.50 13.95 9,60 27.75 24,68 32.25 a* 20,40 18,00 25.10 12.30 16,43 32.25 a* 44.70 42.15 48.45 62.55 62,11 93.55 No Sample b** b** Undectable -76TABLE NO. V A Measure Of The Quantities Of Estrogens Excreted In The Urine During Post-Conception Period (Micfograms per liter of urine) Cow No. 6 Days PostConception 5 7 9 11 13 15 19 21 26 31 34 17=1Estradiol 17BEstradiol Estrone 24.00 48.00 43.50 24.60 6.60 b** 12.00 2.40 33.60 5.10 b** b** 8.25 6.00 9,60 5.10 2,40 19,50 16.50 49,80 24.60 11.25 5.40 5.40 8.10 4,20 13.20 6.00 b** 7.80 5.25 17.10 0.30 Total 55.50 66.90 126,90 54.30 17.85 5.40 13.65 21.90 19.05 35.40 8,70 b** Undectable TABLE NO. VI Plasma Progesterone At Various States Of The Estrous Cycle (Micrograms per milliliter plasma) Days After Heat I 9 11 13 17 19 Cows I I I 2 I I Cow No, 2 62 2 2 6 78 2 2 Progesterone Content 0.004 0.001 0.014 0.031 0,037 0,031 - 77- PREPARATION OF .CHEMICALS AND REAGENTS ie Benzene8 n-Hexane8 Absolute Methyl alcohol and chloroform were redistilled before use, iio Ether anhydrous washed once with equal volume of one percent ' : (Weight/Volume) ferrous sulfate solution in distilled water and then once with equal volume of distilled wat e r 8 and redistilled. Stored in brown bottles in the refrigerator, iii, K o b e r 0S reagent for estrones Added 660 ml, concentrated sulfuric acid to 422 ml, distilled water. Added 10 mg, of sodium nitrate 8 dissolved* and then 20 mg, p-guinone added and dissolved by gentle warming. Then 20 g, of hydroquinone was added, and dissolved by warming and shaking. Reagent was thqn filtered through glasswool and stored in brown bottle, iv, K o b e r 0S reagent for estradiol isomerss Prepared in the same way as for estrone except that 600 ml, concentrated sulfuric acid was added to 480 ml, distilled water, v. Purified e t h anols Refluxed 750 ml, absolute ethanol with 37,5 g, 1 •• each of zinc dust and sodium hydroxide pellets for 12 hours continuously and distilled. Stored in brown bottle in the refrigerator, vi, Hydroquinone solution inpurified ethanols Dissolved 0,2 gm, . hydroquinone crystals in 10 ml, purified ethanol. freshly prepared. Always used -78- Appendix Figure I. The equipment used for cannulation; I 0 Silastic tubing Ho Metal plug kept In end of tubing IlIo Metal cover to protect end of tubing IV„ Ring sutured to skin to fasten metal cover V„ Two way valve used in blood sampling V l o Syringe used to apply negative pressure. -79- Appendix Figure 3, Drawing blood from cannula, - 80- Appendix Figure 4, Thin layer chromatography for four unknown sample extracts in solvent system "A", (The areas corresponding to the reference spot for pure progesterone have been removed for elution and separation in solvent system "B",) Appendix Figure 5. Thin layer chromatography for five unknown sample extracts in solvent system "B", (These unknown sample extracts are eluates from solvent system "A" as shown in appendix figure 4«) LITERATURE CITED A a s t e d 9 V 0 F 0 and G 0 Stakemann0 19630 The site of production of oestro­ genic hormones..in..human .pregnancyo. ..Ho.. Experimental..investigation . on the .role..o f .t h e .foetal adrenal*. Acta. Endocr0 43.sl840 Al l e n 9 E 0 and E 0 A 0 Doisy0 19230 J 0 A m 0 M e d 0 Assoc0 81§8190 (Cited by: E m m e n s 9 C 0 .W0 .1950o.. .Estrogens0. Chapter .XVIs391-4170 Emmens9 C 0 W 0 (edit)0._ Hormone..As s a y 0 . Academic Press 9..New..York). Al l e n 9 W 0 .M0 1 9 5 0 0 ,A simple method for analyzing complicated absorption Curves9 of. use in. the .colorimetric determination of urinary steroids. J 0 Clin0 Endocr0- IOaTO0 Allen8 W-0 M 0, and O 0 ,Wintersteiner-*. 19340... Crystalline. Progestins0 Science 8 0 a.!90o Antoniades9 H 0 N 09.R 0 B 0 ..Pennell9 .W0..R0 Slaunwhite9 J r 0 and A 0 A 0 Sandberg0 1 9 5 7 0 Binding and distribution of intravenously injected ci4=.steroids. and .their .metabolites' in human :plasma and'its fractions 0■ J 0 B i o L 0 Chem0 .229 sl071o Axelrod9 L 0 ,R0. 1 9 5 3 0 .. The .quantitative ,separatipn.,of,estrogens by paper . partition chromatography*,,.,J0 .Biol0.Chem0.. 201.a59o..... Axelrod9 L 0 R 0 1 9 5 4 0 The chromatographic fractionation and identifica­ tion- of, compounds ..related, to the estrogens* .. Rec0 ..Prog0 in .Hormone .. . Res 0 9 l69-0 . Ba c hman9 -C0- and D 0 S 0 Pet t i t 9 .1941, Photometric determination of estrogens, .,III0 A procedure .for. the- .estimation,, of .the,,estrogens, of pregnancy.urine*. J 0 .Biol0 Chem0-.138:689,. . . . . Baggett9 B-O9 L 0 .L0 Engel and S 0 K 0 Dorfman, 1956, The conversion of Testosterone??3= c 14 to.Cl4-Estradiol=17B by human.ovarian,tissues, J 0 B i o l 0 C h e m 0 221:931, Balfour9 W 0 E 0..and R 0 S 0 Comline0 . 1957, Secretion.of.progesterone by the adrenal.gland,.': Nature 180:1480»........... Ba u l d 9 W 0 ..S0 - 1954, Some errors in the colorimetric estimation of Oestriol9 oestrone and oestradiol. by the, Kober reaction0, Biochem0 J 0 56:42-6,...: Bau l d 9 W 0 .S0... 19550 .Separation, of. oestrogens..in. urinary. extracts...by partition . c h r o m a t o g r a p h y , .Biochem, J 0 59:294, Bauld9 Wo- S 0 1956, A method for the determination of Oestriol9 oestrone 9 and oestradiol in human urinary partition chromatography and color!- -82metric, estimation, .Biochem, J , .635488,. B e c k 9 A, Bo 1950,.. Studies .on .the. excretion .of .oeslrogens.. by,, pregnant e w e s , :Aust, „J , ..A g r , .Res, -ls322,-.. B e e r 8 C, T, and T„ F, Gallagher, 1955a, Excretion of estrogen metabo­ lites by-humans, .I o. The fate of..small doses.of estrone .-and estradiol17B, J., Biol, .Chem, .2141335, B e e r 9 C, T, and T, F, Gallagher9 1955b, Excretion of estrogen metabo­ lites by. humans, . .II, . .The fate.of.large doses of ..estradiol-17B after intramuscular .and..oral.administration.J, Biol, .Chem0 214s351, B e m f e l d 9 ’)?, .and-.W, .H, Fishman., 1953,, B-glucuronidase, I,. :Purification of -calf- spleen .E-glucuronidase, J, Biol, Chem-, 232:757, Bernfeld9 P , 9 J, S,. Nisselbaum and W, M, Fishman, 1953, B-glucuroni­ dase,- II,.. Purification of..calf, liver B-r-glucuronidase,, .J, Biol, Chem, 2.02-s .763,. . . . . . . Bloch „ K, 1945,.. The biological, conversion, of, cholesteroL-to.. pregnanediol, - J,..„Biolo..Chem, :157 5661,-, • Bosc o t t 9 -RVrJ,. .1962,... Chemistry, of. oestrogens in the. ovary, by Zucherman9 S, -S, -.(.edit). Academic. Press.New York, Bradbury9 iJ,- T, ^ .1961,.. Direct action o f .estrogen., on .the;.ovary ...of the •immature rat,....E n d o c r , .68.5.115., .L..... B r o w n 9 J, 'B, .1955, A .chemical..method..for., the ..determination ...of Oestriol9 oestrone and..oestradiol. in..human...urine, ...Biochem,. J , ,60.5185, Bur t o n 9 R, B , 9 A, Zaffaroni and E, H, Keutmann, 1951a, Paper chromato­ graphy of. steroids,... II, Corticosteroids, and..related compounds, J , Biol, Chem, 188:763, .... Burton9 R, ;B,' >Aa,^Zaffarorii,.ahd.;:E., Hi. Keutmann, 1951b, Corticosteroids in urine-of.n6fmai persons.determined.by paper.chromatography, J, Biol, Chem,.. 193.s769, . . B u s h 9 I, E, 1952, Methods of paper chromatography of steroids applied to the study of steroids in mammalian blood..and„.tissues,.„....Biochem, J, 50s370,. Caspi9 E, R , „ I, Dorfman9 B, T, K h a n 9 .G, Eosenfeld and W, Schmid, 1962, Origin of the carbon atoms of steroid hormones biosynthesized in vitro in the bovine adrenal from acetat e - l - C ^ , J, Biol, Chem, 237:2085, =■83— Coppedge8 R 0 L 0 8 A 0 Segaloff and H. Po Sarett0 19500 Diphosphopyridine nucleotide.in the inactivation o f .©^estradiol.by-rat Iiver0 J 0 B i o l 0 Chem0 .182jl810..................... Deane „ H 0 W 0 1952 0- Histochemical.. observations .on ^the .ovary and oviduct of the'.albino, rat Juringvthe -estrous: cycle 0.- A m 0.J 0 Anat0 91s3630DeMeio8 R 0 H 08 C 0 Lewyeka8 M 0 Wizerkaniuk and O 0 Salciunas0 19580 Biological..synthesis, of sulphuric acid esters .of steroid hormones or their metabolites^..Biochem0 J 0 68:1. . ........ Diczfalusy9 E 0' 1953„ . Acta.Endocr0 Suppl0 12:85. [Cited by O'Donnell, V 0 J 0 .and J 0 R 0 K 0 Preedy0 1961. The estrogens in hormones in blood (1st edit) by G r a y 9 C 0 H 0. and. A 0- L 0 Bacharach (edit). Academic • Press , .New .York]..... '-.................. Diczfalusy, E 0 and A 0 M 0 Magnusson0 1958. Tissue concentrations of oestrone, oestradiol-17B and oestriol in the human fetus. Acta Endo c r 0. 28:169.. Do i s y 8 E 0 'A08 C 0 D. Veler. and. S 0. A 0..Thayer0 1929.. Folliculin from urine of pregnant women. .Am. J0 .Physiol-.. 90:329. Eccl e s 8 S 0 S 0, R 0 E 0 Oaken and S 0 R 0 Stitch. 1962. The effect of whole body x-'irradiat ion .-.on.'the:.metabolism .of. Oestradiol=IV-B-. by rat kidney • and small ,intestinevin. vitro... .Biochem0 .J0 85 :38p0 .. Ei k -Nes, K 0 , J 0 A 0 Schellman8 R 0 Lumry and L 0 T 0 Samuels. 19540 The binding o f .steroids, to. protein... I 0 ..Solubility determinations. J 0 B i o l 0 C h e m . 206:411... . . E m m e n s 8 C.' W 0 1950. Estrogens.in.hormone assay by Emmens8 C 0-W0 (edit) Academic Press,..New York. ■ Engel,'L° L 0, W 0 R 0 Slaunwhite, J r 0, P 0 Carter and I 0 T 0 Nathanson0 1950. The separation .of ,natural .estrogens.by counter current distribution. J 0 B i o l 0 C h e m 0.. 185 :255. Engel, L 0 L 0., J 0 Alexander and .M0..Wheeler0.. .1958. Urinary metabolites of administered 19-nortestoesterone. .J 0 Biol0 Chem0 231:159. Engel, L 0 L 0, B 0 Baggett and M 0 Halla. 1959. Formation of C ^ labelled oestriol from 16-Cl^-oestradiol-17B by human.liver, s l i c e s " Biochem0 Biophys. A c t a 0 31:435. Erlanger8 B 0 F 0, F 0 Borek8 S 0 M 0 Beiser and S 0 Lieberman0 1959. Steroid protein conjugates. II0 Preparation and characterization of conju­ gates of bovine serum albumin with progesterone, deoxycortico-sterone ■' -84and estroneo J 6 B i o l 6 C h e m 6 234;1090, E v a n s 9 J 6 S 6-and R 6 F 6 Varney,. 1941«. Mouse uterine method.for estrogen assay, ...J ,..Biol6..Chem6 -.140 ipxxxviii',■ Everett6 J 6 W 6 1961, Mammalian reproductive cycle in sex and internal secretion.by .Young9- W 6 C-, (edit), The Williams, and Wilkens Company9 Baltimore, Fieser9 L 6 and M 6- Fieser6 1959 ,. .The Steroids6 Reinhold9 New York6 F i s h 9 W 6 R 6 and R 6 I, Dorfman6 1941, Metabolism of the steroid hormones, I 6 The conversion .of.. -estradiol to estrone .by .the-guinea., pig, . J 6 B i o l 6 C h e m 6 .140 :83, -F i s h 9 W 6 R 69 W 6 C 6 Young and R 6 I, Dorfman6 1941, Excretion of estro­ genic and.androgenic substances.by male and female chimpanzees-with known nating^behavior records., .Endocr,..28 :585, Fishman9 J V 9 .H6 .L6 Bradlow .and: Gallagher, I960,.. Oxidative metabolism of estradiol,. J 6 B i o l 6 Chem6 235:3104,■ Fishman9 W 6 H 6 B 6 Spinger and R 6 Brunetti, 1948, Application of an • improved glucuronidase assay method, to.the.study of. the. human blood B-glucuronidase.,..-.J 6. Biol6 Chem6 173:449, Friedgood9 H 6 B 6 9 J 6 B 6 Garst and A, J 6 Haagen-Smit6 1948, A new method for the separation of androgens and estrogens and for the partition of estriol from the estrone-estradiol fraction with- special reference to the identification,.and.-quantitative,, microdeterminatiqn. of estrogens by ultraviolet.absorption .spectrophotometry, .J6 B i o l V C h e m 6 174:523, Fr e u d 9 J 6 1939, Acta Brevia N e ar l a n d .Physiol, Pharmacol Microbiol, 9:11 [Cited by. Emm e n s 9. C 6 W 6 9 .1950, Estrogens in ..hormone., as say by E m m e n s 9, C 6.- W,-. (edit). Academic Press. New .York,.] . Futterweit9 W 6 9 N 6 L 6 McNiven and R 6 I 6 Forfman = 1963, Gas chromato­ graphic identification of progesterone 'in human pregnancy plasma, Biochemica et Biophysica Acta 71:474, Giv n e r 9 M 6 L 6 9 W 6 S 6 Bauld .and .K6 .Vagi, I960, . A chemical method for the quantitative determination of 2-methosyoestrone9 Oestrone9 Ring-D?KetolicToestrogens9 oestradiol-17B„ 16-epioestriol.and oestriol in human urine, Biochem,.J6 .77:406, Goldzieher9 J 6 W 69 J 6 M 6 Bodenchuk and P 6 Nolan, 1952, reactions of steroids, J 6 Biol, Chem6 199:621, The fluorescence -85Goldzieher8 J 0 W ,8 .J » .M0 B o d e n c h uk.and .P. .Nolan,. 1954, reactions of.steroids,. Anal, C h e m , .26:853, Fluorescence Go m e s 8 W, R , 8 V, L, Estergreen8 J r , 8 0, L, Frost and R, E, E r b , 1963, Progestin levels .in., jugular, and .ovarian...venous.blood 8_corpora. Iutea and ovaries of .the .non^pregnant. bovine, J ,. Dairy ..Sci. 46:553, Gors k i 8 J , 1959, [Cited by Nelson8 D. W „ 8 1959, Urinary excretion of Estrogenic substances .by .the b o v i n e .during- estrus and at different stages of gestation], Har r o w 8 B, and,A, Mazur, . .1962, Textbook of Biochemistry (8th ed , ) ■Wo B, Saunders. Company8 Philadelphia,........... H e a r d 8 R, 1D , .H „. and M, M, .Hoffman,...1941, Steroids :: IV of injected .c&estradiol, J, Biol,.Chem,.141:329, The fate in man H e a r d 8 R 0 D, H , 8 P, H, Jellink and V, J , O 9Donnell, 1955, Biogenesis of the estrogens,. .The conversion of testesterone-4-C^ to estrone in the pregnant mare, .Endo c r , .57:200, H e a r d 8 R, D, H , 8 E, G, B l i g h 8 M, C, Cann8 P, H, Jellinck9 V, J, O 1Donnell9 B, G, Rao and J, L, Webb, 1956, . Biogenesis of the sterols and steroid h o r mones,; R e c , Prog,- Hormone Res, 12:45, He l l e r 8 C,.G, 1940, Metabolism, of the, estrogens:.. The effect of liver and uterus upon, estrone9 estradiol and estriol, .Endocr, 26:619, H o l l a n d e r 8 N, and V, P, Hollander, 1958-, The effect of folliclestimulating hormone on the .biosynthesis .in vitro, of estradiol-17B from acetate-1-Cl^ and testosterone-4-Cl^T J , Biol, Chem, 233:1097, H u a n g 9 W, Y, 9 and W, H, Pearlman, 1962, The corpus luteum and steroid hormone formation:.. I, Studies on. luteinized.rat ovarian.tissue in vitro, J, Biol, Chem, 237:1060, . .. Huffman8 M, N , 9 S, A, Thayer and. E, A, Dqisy, 1940,..T h e .isolation of ^rdihydrotheelin from.human placenta, .J,..Biol, .Chem, .133:567, H u n t 8 M, L„ 1957, Analysis of extracts of bovine urine by the Kober color reaction (Cited by Nelson9 D 0 -W0 9 1959, M,S, Thesis9 Montana State College), H u r lock9 B, and.P, Tala l a y , 1957, Principles, of the.enzymatic measure­ ment of steroids, J , Biol, .Chem, 227:37, Jellinck9 P, H, 1959, Biochem, J, 71:665, The metabolism of [16-C1 4 ] oestrone in vitro, -86Katzman8 P 0 A d 8 R 0 F 0 Straw, H 0 J d ,Buehler..and E 0 A 0 Doisy0 19540 Hydrolysis of .conjugated.estrogens0. R e c 0- Prog0.Hormone..Res„ 9;450 Kinsella8 R 0 A 08 J r 0 8 F 0 E 0 Francis9 S 0 A 0 Trayer and E 0 A 0 Doisy0 19560 Enteric excretion .of metabolites, of .steroid hormones.in the human, J 0 Biol». Chem0.219:265, ■ K o b e r 8 S 0 1931, . Biochem0 L 0 239 :209, [Cited by Cohen9 S 0 L 0 9 and G 0 F 0 Marrian, 1934, The application of the Kober test to the quantitative estimation .of. oestrone and..oestradiol.in, human, pregnancy urine, .Biochem0 .J0 .28:1603],. K o b e r 8 S 0 . 1938, ..The. colorimetric .estimation, of the estrogenic hormones I I 0 Estrone.,., Biochem0.J, 3 2 : 3 5 7 , ...... s Kray c h y , Sf0 ..and. T 0 F, Gallagher,... 1957,. .2-methosyestrone „.a ,new ...metabolite.of:,estradiol?17B:.in; man,.. .-.J0 .iBiol.,. Chem0 229 :519, Lad a n y 9 S,' and.. M 0 ..Finkelstein,; .1963, Isolation .of .estrone,, estradiol17B and.,.estriol. from female human, urine.,... Steroids. 2,:297-, ' Lauson9 H 0 D 0 9 C, G, Hel l e r 9 J 0 B, Golden and .E0 L 0 Severinghaus0 1939 , The immature .rat uterus.i n .,t h e.assay of estrogenic ..substances and a comparison.of.estradiol, estrone...and estriol, Endocr0' 24:35, L e v i n 8 L 0 \ 1 9 4 5 0. The. fecal .excretion, of. estrogens, by, pregnant cows, ■ J 0 Biol, Chem0 157:407VLev i t z 9 M 0 J 0 9 R 0 Spitzer and G 0 H 0 Twombly0 1956, The conversion of estradiol-17B-16-Cl^ to radioactive 16-Detoestradiol-17B in man, J, B i o l 0 Chem0 222:981, ■ Levitz9 M 09 J 0 R 0 Spitzer and G 0 H 0 Twombly0 1958, Interconversion of 16-oxygenated.,.estrogens.:..I , The synthesis, of estriol^ie-C-^^ and its metabolism .in. man, ...J, B i o l0 Chem,. .231:787, . Lisboa9 B, P 0 1963, Separation and characterization of 3-ketone steroids of.the pregnahe^series by means of thin, l a y e r .chromatography0 Acta Endocr,. 43:47, Lisboa9 B 0 P 0 and E 0 Diqzfalusy, 1962, Separation and characteriza­ tion of steroid oestrogens by.means of thin-layer chromatography, A c t a 0 End o c r 0 .40:60, Lombardo„ M 0 E , 9 P 0 H 0 M a n n 9 T 0 A 0 Siscell and P 0 B, Hudson, 1955, V method for the extraction of steroids from blood, J 0 Biol0 Chem0 212:345. - 87- L o y 8 R 0 G o 8 W 0 H 0 McShan and L 0 E 0 Casida0 1957, . A rapid method for purification and qualitative ,estimation of progesterone, from luteal tissue, '-.J0 B i o l 0 .Chem0 229 ;583, MacCorquodale, D 0 W 0 9 S 0 A 0 Thayer and E 0 A 0 Doisy0 1936, The isola­ tion of the .principal estrogenic substances rof- liquor: f Olliculi0 J 0 B i o l 0...Chem0 .115 s435 0 ■ Mat h e r 8 Alien, . 1942, . Distribution .of .estrogens .between immiscible solvents., -..J0 .Biol0 .Chem0-144 s617. Mar k e e 8 J 0' E 0 .and.B 0 .Berg0 1944, Cyclic.fluctuations.in.blood estro­ gens as -.a possible.- cause, of menstruation.,.. Standard M e d 0 Bull, 2s55, Marlow8 H W 0, ..1950,...Groups.involved, i n .the. Zimmerman -and.. Kober , reactions,..J 0 Biol, Chem0-183:167, ..... Ma z u r 8 A 0 ’’and; E 0 .Shorr, 1942« _ The .isolation of. stilbestrol, monoglycuronide from.the urine pf rabbits,. J 0.B i o l 0 Chem0. 144:283, McKenna 8 J 08 E,.. Menini. and. J 0.K 0,Norymberski0 1961, in human pregnancy urine, - Biochem0 -J0 .79 slip,.. Oestrin sulphate Mills, G 0 T 0 ,.1948, The ..B-glucuronidase activity ..of ox spleen and the • assay of -B-glucuronidase .preparations,. .Biochem0- J « .43:125, N a k p 9 T 0 and Y, Aizawa0 1956, On microgluorometric separatory determination.of estrogens in the ..blood and.urine, EnJocr0 Japan 3«92o ., .. .Nathanson, I, T 0 and L 0 E 0 Towne0 1939, The urinary excretion of estrogens,- androgens and. ESH following the administration of testos- teron'e to -human, female castrates, Endocr0 25:754, Nelson, D 0. W 0 1959, Urinary excretion of estrogenic substances by the bovine during estrus and.at different, stages- of, gestation, M 0S 0 - Thesis Montana. State College, . N e l s o n 8 D 0 W 0 and E 0 P 0 Smith,. 1963, -Urinary excretion of estrogenic compounds during estrus. and. gestation, by the,bovine as determined by three methods, J 0 Dairy Sci0 X L V I ;.135, N o a l l , M 0 W 0, H 0 A 0 Salhanick8 G 0 M 0 Neher and M 0 X, Zarrow0 1953, Method.for the. isolation of progesterone from human placenta, J, B i o l 0 C h e m 0 201:321, N o c k e 0 W 0 19610 A study of the colorimetric estimation of oestradiol™ I V B 8 .OestradiolelVeC8 Oestrone8.oestriol,and. 16-ep-ioestriol by the Kober -reaction„,..'Biochem,, ■J«-. V8s5930 N y m a n 9 M 0 A 0 9 J 0 Geiger..and .J,0 W,0. Goldzieher0.. 19590 Biosynthesis of estrogen, by- the perfused; stallion testis,. . J 0 Biol0 Chem0 234:16» O a k e y 9 R 0 E 0 1961« The .separation of steroid oestrogens.by partition chromatography0 Biochem0..J0.-81:13p0 O a k e y 9 A 0 W 0 9 S 0 S 0 E c c l e s 9 and S 0 R 0 Stitch0 . 19620 „ Rapid, metabolism • of C-^ labelled oestrogen in the. r a t 0. .Biochem0.J 0'.82:2V p 0 O e r t e l 9 G 0'.W 0 9 .S0 .P0 .Weiss, and .K0 .B0 Eik=Nes0,.. 1959 0 . Determination of progesterone..in., human .'blood .plasma 0 ,J0 .Clin0 -Endocr0..Metab0 19:213« O eDonnell9. V 0 J 0 and J 0 R 0 K 0 Preedy0 1961« The estrogens in hormones in blood (1st edit).by...Gray 9 C 0 H 0 and. A 0 .L0 Bacharach..(edit) 0 Academic Press •New.. Y o r k 0 ■ 1 Pearlman9 W 0 H 0 and G 0 .Pineus0 1943« m a n 0 J 0. B i o l 0 .Chem0 14V:3V90 . ' . ■ The..metabolism of,estrone in ■ ’ ■Pedersen=Bjergaard9.G 0 and K 0 Pedersen-Bjergaard0 19480 Sex hormone analysis III0 Estrogenic and gonadotropic substances in the urine from women with normal menstrual cycles, and normal..pregnancies0 Acta0 Endocr0 1:263«.' P r e e d y 9 J 0 R 0 K 0 and E 0 H 0 Aitken0 1961« The determination of estrone 9 estradiol-lVB and estriol in urine and plasma with column partition chromatography*. J 0, B i o l 0 Chem0 .236:1300« P u r d y 9 R 0 - H 0 9 L 0 L 0 Engel and J 0, L 0 Oncley0 1961«. The .characterization of estrone sulfate from human plasma, J 0 .Biol0 .Chem0 236:1043« Raeside9 J 0 I 0 .and.C 0 W 0 Turner, 1956, Chemical,estimation of proges­ terone in the blood of cattle, sheep and goats, J 0 Dairy S ci0 38:1334« Reineke9 L 0.M 0 .1956« Paper chromatography in. steroid,.determination. Anal, Chem0 28:1853, Rieg e l 9 1« L 0 and G 0 C, Mueller, 1954, Formation of a protein-bound metabolite of e s t r a d i o l - i e - C ^ by rat liver homogenates, J 0 Biol0 C h e m 0 210:249, Robinson9 ;G0 E 0 9 J r 0 and A 0 V 0 Nalbandov0 1951« Changes in the hormone content os swine pituitaries during the estrual cycle, J 0 Animal S c i 0 10:469«' “ 89= R y a n 9 Ko Jo 1959a-o Biological .aromatization. of ..'steroids,= . Jo .Biol, Chem0 2 3.4 s26 8 o. : . • ■ ■ ■ R y a n 9 K 0 J 0 1959bo . Metabolism o f .C-lB-oxygenated. steroids by human placentae .The.formation,of.estribl*. ..J= Biol= Chem0 234;200.6» R y a n 9 K 0 Jo and O 0 W 0 Smith0 196I a 0 Biogenesis of estrogens by the human o v a r y : I 0 Conversion of acetate-l-C^^ to estrone and estradiol, J 0 Biol,. C h e m 0 236?7050 R y a n 9 K 0 J 0 and O 0 W 0 Smith0 1961b0 Biogenesis of estrogens by the human ovary? I I 0 'Conversion, of-progesterone=4=C^^- to estrone and estradiol,: J 0 B i o l 0 Chem0 236?710, ■ R y a n 9 K 0 J 0 and 0, W 0 Smith, 1961c, Biogenesis of estrogens by the human ovary? III0 .Conversion,, of .cholesterol?-4-?C^ to. estrone, ■ J 0 B i o l 0--Chem0 .236 ?2204O' • Samuels 9 L 0 T 0 1947, Metabolism of steroids by tissues? I 0 Determina­ tion of testosterone .and related steroids .in..tissue.extracts, J0 ■ B i o l 0 C h e m 0.. 168 ?.4710 ■ . Samuels9 L 0 T 0 1955, Progress in the chemistry of fats, and other lipids, 3 s396 [Cited by S h o r t 9 R 0 V 0 1961, Progesterone in hormones in blood by G f a y 9 C 0 .H0 and A 0 ,L 6 Bacharaeh. (edit).Academic Press • New Yo r k ] ......... Schachter9 B 0 and G 0 F 0 Marrian0 1938, . The isolation of estrone sulfate from the urine of pregnant mares, J 0 Biol, Chem01' 1 2 6 ?663, Sh o r t 9 R 0 V 0 1 9 5 8 a , . Progesterone in .blood?.I, The chemical.determina­ tion of,progesterone .in periphral .blood,.. ..J0 .Endocr0 16?415, S h o r t 9 R 0 V 0 1958b, Progesterone...in. b l o o d ?. .I l ,. Progesterone in the "peripheral, blood, of pregnant cows, , J 0 Endocr, 1 6 ?426, Short 9 R 0.V 0 19610.. .Progesterone 'in hormones, in blood, b y .G r a y 9 C 0 H 0 and A 0 L 0 Bacharach0 Academic Press..New York, Slaunwhite9 W 0 R 0,9 J r 0 9 L 0 L 9 E n g e l 9 P 0 C 0 ,Olmsted and P 0 Carter, 1951, The fluorescence, and extinction and partition.coefficients.of estro­ gens, ■ J 0 B i o l 0 Chem0 191?627, Smi t h 9 E 0 P 0 9 W 0 M 0 Dickson and R 0 E, E r b 0 1956, Fluorimetric estima­ tion of estrogens in bovine urine„ J 0 Dairy S ci0 39?162, - 90 “ Solomone S og W 0 Van De and S 0 Lieberman6 19560 The in vitro synthesis of 17 "hydroxy^progesterone-andr ^ a n d r o s t e n e - S i,. 1-7-d-ione.from pro--' '"gesf erone" by"bovine" ovarian'-f issues'o-""J 6'-Am^ 'Chem6 -Soc6--VS :5453 6 Sommerville8 I 6 F 6 and G 6 N 6 Deshpande6 1958, The quantitative deter- mi-nation of progesterone .a-nd-.pregnanediol. in. human, plasma,, ..J 6. Clin6 Endo c r 6:-.Me-ta,-. 18j-122-3».-i.-.-. ., Sommerville9 I 6 F 69 M 6 T 6 Pickett9 W 6 P 6 Collins and D 6 C 6 Deyer6 1963, ’ A- modified ,method-.-for- the.quantitative ^determination.,of progesterone •in human, p l a s m a , Acta6,.Endocr, -4S slOl6 -- : Starnes 9 W 0 R 0 and R 6 S 6 Teague, 1949, Metabolism of synthetic estrogen's. Urinary sulfur partitioning after-estrogen.-administration, J , B i o l 6 Chem,.179:43, Stedman,s ’Medical...Dictlonary, 1 9 6 1 , ■ The... Williams.: and, Wilkens ,...Company - Baltimore,. . . . . Stimmel8 B 6 F 6 1944, A quantitative study of the adsorption of estrone9 estriolg. and o^estradiol .on..a-chromatographic..column,. .J.., Biol, Chem6 153 S327 » ...... Stimmel, B'., ..F6 .1946, . The fractionation, and...photometric, estimation of - the estrogens .,in. human.- pregnancy, urine., .,J6.Biol,. Chem6 162 :99, Stimmel8 B 6 F 6^,_ 1949, .. The. effect..of.zinc:=hydrochloric..acid hydrolysis on estrogen, in- human, urine, ....J 6.Biol, Chem6 178:217, Stitch9.§l:,’..R, 9 .R 0--E6.Oakey ..and...S6...S0.Eccles6.,. .1962.,.. Biosynthesis of. oestrogen -by. rati-ovaries,,; Biochem6 J 6 .82:48p, Sz e g o 9.C 6 -M6 .1953,.. The influence., of...the- liver ..upon- .estrogen-protein binding.in. vitro, End o c r 6-.52:669,., S z e g o 9 C 6 M 6 and L 6 T 6 Samuels, 1943, Colorimetric -determination of the estrogens: A method for-the determination of ,,total...estrone and estradiol from tissue sources, .-,J6-.Biol6 ..Chem, .151:587, 1943, Sz e g o 9 C 6 M, and R 6 Sidney, 1956, .Hepatic intervention in the binding of estrogens to rat serum albumin in vitro, J 6 Biol6 .Chem6 221:619, Tayl o r 9 W 6 1955, The metabolism of progesterone by animal tissues in v i t r o : II, Factors influencing the metabolism of progesterone by rabbit, liver and the investigation of the products of metabolism, Biochem, J 6 60:380, -91- Teledy9 G o 9 Eo Endroczi and Ko Lissak0 1 9 6 3 0 Ovarian progesterone secretion during the oestrous cyc l e , pregnancy and lactation in dogSo A c t a 0 -Endocro 44s461« Turner9 C 0 D 0 1961« . General.Endocrinology (3rd edit). Wo B 0 Saunders ■ Company, Philadelphia0 ■ Umberger9 E 0 J 0 and J 0 M 0 Curtis0 1 9 4 9 0 The p-phenylazobenzoyl esters of the natural..estrogens.and ..their .separation by chromatographic . adsorption*. J 0 B i o l 0 ,Chem0 178:265* Veenhuizen9 E 0 L 0 9 R 0 E 0 Erb and J 0 Gorski0 19600 Quantitative deter­ mination of .free .estrone j- estradiol-17B.and estradiol-17o& in bovine fetal cotyledons.o-...,.J-o .Dairy S c i 0 43:27* . Veldhuis9 A 0 H 0 9 .19530 ..A chemical, method .for .the determination of • estrogens in ..plasma*. J 0. B i o l 0 Chem0 .202:107, Venning9 E 0 H 0 9 D 0 A, E v e l y n 9 E 0 A 0 Harkness and J, S 0 L 0 Browne* 1935* The determination of estrin in urine, with the photoelectric colori­ meter* J 0.B i o l 0 _Chem0 .120:225*. . Vil l e e 9 C 0 A 0 1961* S o m e .problems of the metabolism and mechanism of action of..steroid..sex .hormones, in. sex.and: internation ..secretion by Y o u n g 9.W 0 Co (edit-.)-*. Williams and Wilkins Company 9 Baltimore, W e r b i n 9 H 0 .J0..Pl o t z 9 ..-G0.V 0.LeRoy and. E 0 ,M0 .Davis* 1957, Cholesterol A precursor ...of..estrone-^in. vivo* ..J 0 .Am0 Chem0..S oc0 79 :1012, Werthesseri9-N0.T 0 9 C 0.F , .Baker.and N 0.S0 Field,. 1950, . Conversion, of. estrone ,on incubation .in .blood*. ..J0-Biol0 .Chem* .184:145* Werthesseh9, No T 09.E 0 Schwenk .and C 0 .Baker* .1953* .Biosynthesis of •estrone,..and .Brestradiol in., the perfused.ovary * Science. 117:380 0 W e s t 9 C 0 D 0 9 B 0 L 0 Damast9 S 0 D 0 Sarrow and O 0 H 0 Pearson* 1956* Conversion of testosterone to .estrogens in castrated adrenal ectomized human females* . J 0 .Biol0 C h e m 0 .218:409* W e s t p h a l 9 U 0 „ H 0 E 0 Firschein and E 0 M 0 Pearce* 1955* Binding of hydroc6fticosterone-4-Cl1+ and progesterone-4-cl1* to.serum.albumin demonstrated by paper electrophoresis* Science.121:601* Westerfeld9 W 0 W 0 9 S 0 A 0 T h a y e r 9 D, W 0 MacCorquodale and E 0 A 0 Doisy0 1938* The ketonic estrogen of sow ovaries, J 0 Biol0 Chem0 126:181* -92W o t i z 9 H 0 H o 8 Jo Wo D a v i s „ Ho M. Lemon and M« G u t o 1956« Studies on steroid m e t a b o l i s m .V, .The conversion, of, testosterone^U-G^. to estro■ gens by :human. ovarian^-tissueso-..' .Jo 'Biol, Chem-, -222-.:48?»- • W o t i z 9 Ho H «9 Co Botticelli9 F, L, Hi s a w 9 J r 0 and I, Ringler, 1958a„ Identification..of.. estradiols-17B-. from.-, dogfish., ova. (Squalus„ Suckley). Jo Biolo_ CheiTio .:231s589-o . W o t i z 9 Ho. H o 9 Bo So Ziskind and I, Ringler, 1958b« Studies in steroid metabolism, VIs The fate of estrone-lGr-C^ in .rat blood, J , Biol, C h e m o 231:593, Z a n d e r 9 J , and H, Simmer, 1954, Klin. W. Schr, 32:529. [Cited by S h o r t 9 R,. Vo 1961,' . Progesterone in .hormones, in blood, by Gray., C. H, and A, L, Bacharach9 Academic. Press New York], Zaffaroni9 A 6 and.R 6 B , Burton, 1951, Identification of corticosteroids of beef adrenal extract by paper . c h r o m a t o g r a p h y -J:, Biol, ■Chem, 193:749,.. ' Z a r r o w 9 M. X, 1962, The hormones of reproduction in.reproduction; in farm animals.by H a f e z 9 E, S, E, (edit). Lea and Febiger9 Phildelphia, Zimmerberg9 H, 1946, The inactivation of diethylstilbestrol in v i t r o 0 Jo Biol, Chem, 166:97, / 7 MONTANA STATE UNIVERSITY LIBRARIES 762 1002 !825 3 N378 V43 cop.2 Varman, P. N. Estrogens and progesterone in the posterior vena cava ... N A M E A N D AOOfir irvvJhA- — tiL.Xr