Document 13490350
advertisement
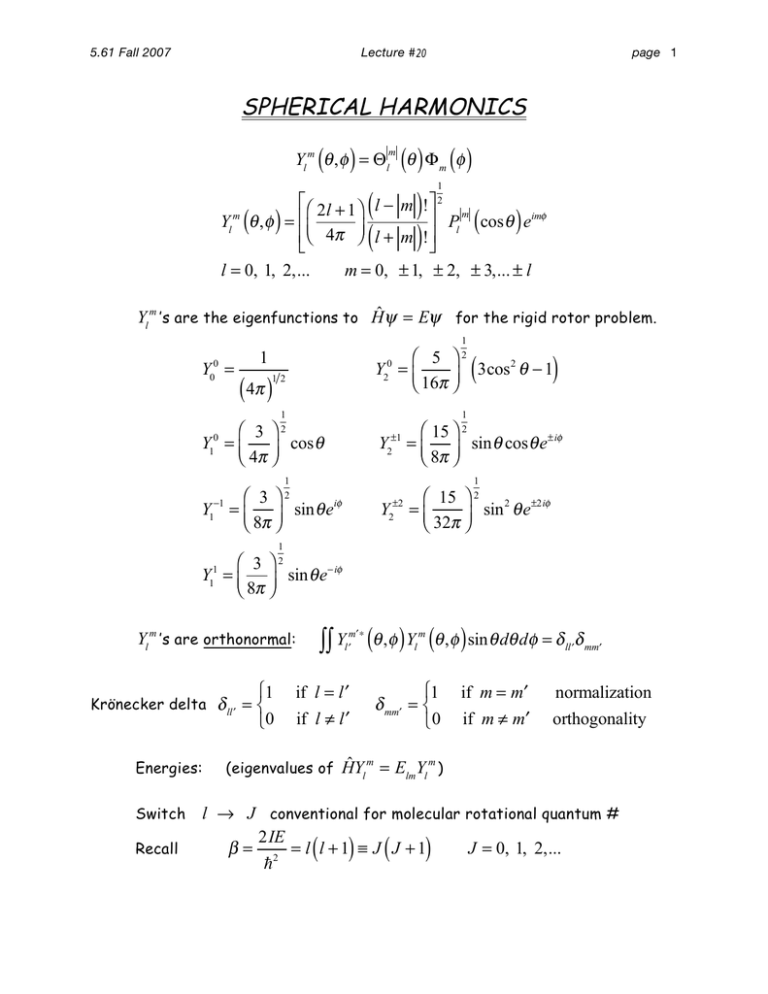
5.61 Fall 2007 Lecture # 20 page 1 SPHERICAL HARMONICS ( ) m () () Yl m θ , φ = Θ l θ Φ m φ ( ( 1 2 ) ) ⎡ ⎛ 2 l + 1⎞ l − m ! ⎤ ⎥ Pl m cos θ eimφ Yl θ , φ = ⎢⎜ ⎟ ⎢⎝ 4π ⎠ l + m ! ⎥ ⎣ ⎦ l = 0, 1, 2,... m = 0, ± 1, ± 2, ± 3,... ± l m ( ) ( ) Yl m ’s are the eigenfunctions to Ĥψ = Eψ for the rigid rotor problem. Y00 = ⎛ 5 ⎞ Y20 = ⎜ ⎝ 16π ⎟⎠ 1 ( 4π ) 12 1 1 2 (3cos θ − 1) 2 1 ⎛ 3 ⎞2 Y10 = ⎜ ⎟ cos θ ⎝ 4π ⎠ Y2±1 1 2 ⎛ 15 ⎞ 2 = ⎜ ⎟ sin θ cos θ e± iφ ⎝ 8π ⎠ 1 2 ⎛ 3⎞ Y1−1 = ⎜ ⎟ sin θ eiφ ⎝ 8π ⎠ ⎛ 15 ⎞ Y2±2 = ⎜ sin 2 θ e±2iφ ⎟ ⎝ 32π ⎠ 1 2 ⎛ 3⎞ Y11 = ⎜ ⎟ sin θ e− iφ ⎝ 8π ⎠ ∫∫ Y (θ ,φ ) Y (θ ,φ ) sin θ dθ dφ = δ Yl m ’s are orthonormal: ⎧1 if l = l′ ⎩0 if l ≠ l′ Krönecker delta δ ll′ = ⎨ Energies: Switch Recall m′∗ l′ m l ⎧1 δ mm′ = ⎨ ⎩0 ll′ δ mm′ if m = m′ normalization if m ≠ m′ orthogonality ˆ m = E Y m) (eigenvalues of HY l lm l l → J conventional for molecular rotational quantum # 2IE β = 2 = l l +1 ≡ J J +1 J = 0, 1, 2,... ! ( ) ( ) 5.61 Fall 2007 page 2 Lecture # 20 ∴ E !2 EJ = J J +1 2I ( ) J=3 6!2 E3 = I Y30 , Y3±1 , Y3±2 , Y3±3 7x degenerate J=2 3!2 E2 = I Y20 , Y2±1 , Y2±2 5x degenerate ( ( !2 E1 = I E0 = 0 J = 1 J=0 ( Y10 , Y10 2x degenerate ( Y00 nondegenerate ( ) ) ) ) g J = 2J + 1 Degeneracy of each state from m = 0, ± 1, ± 2,..., ± J Spacing between states ↑ as J ↑ !2 !2 ⎡ J +1 J + 2 − J J +1 ⎤ = E J +1 − E J = ⎦ I J +1 2I ⎣ ( )( ) ( ) ( ) Transitions between rotational states can be observed through spectroscopy, i.e. through absorption or emission of a photon δ+ hν δ+ Absorption δ- EJ δδ+ or EJ+1 δ+ Emission δ- EJ δ- EJ-1 hν ) Lecture # 20 5.61 Fall 2007 page 3 Molecules need a permanent dipole for rotational transitions. Oscillating electric field grabs charges and torques the molecule. Strength of transition 2 dμ dx I JJ (ξ ⋅ μ) J electric field of light J dx dipole moment of rotor Leads to selection rule for rotational transitions: J = ±1 Recall angular momentum is quantized (in units of ). Photon carries one quantum of angular momentum. J = ±1 Conservation of angular momentum Angular momentum of molecule changes by 1 quantum upon absorption or emission of a photon. 2 E photon = h J J +1 photon J J +1 = Erot = E J +1 ( J + 1) I EJ = photon J J +1 h = 4 Define B and B J J +1 h 8 2 rotational constant (Hz) I h rotational constant (cm-1) 2 8 cI ( ) (Hz) = 2B J + 1 J J +1 ( ) (cm -1 ) = 2B J + 1 2 I ( J + 1) 5.61 Fall 2007 page 4 Lecture # 20 E J=3 E3 = 12Bhc ΔE2→3 = 6Bhc J=2 E2 = 6Bhc ΔE1→2 = 4Bhc J=1 E1 = 2Bhc ΔE0→1 = 2Bhc This gives rise to a rigid rotor absorption spectrum with 00E= evenly spaced lines. J=0 2B νν 01 0→1 → νν 121→2 → νν 23 2→3 → νν 34 3→4 → νν νν 45 4→5 → 56 5→6 → ν 2B (Hz) or 2B (cm -1 ) Spacing between transitions is ( ) ( ) ν J +1→ J +2 − ν J → J +1 = 2B ⎣⎡ J + 1 + 1⎤⎦ − 2B J + 1 = 2B Use this to get microscopic structure of diatomic molecules directly from the absorption spectrum! Get B directly from the separation between lines in the spectrum. Use its value to determine the bond length r0! 5.61 Fall 2007 2B = h 4π 2 cI 1 ∴ page 5 Lecture # 20 I = µ r02 µ= m1 m2 m1 + m2 1 ⎡ h ⎤ 2 ⎡ h ⎤ 2 -1 r0 = ⎢ 2 (B in cm ) or r = ⎥ ⎢ 2 ⎥ (B in Hz) 0 8 π cB µ 8 π B µ ⎣ ⎦ ⎣ ⎦




