Endophyte‐assisted Phytoremediation of Trinitrotoluene (TNT)
advertisement

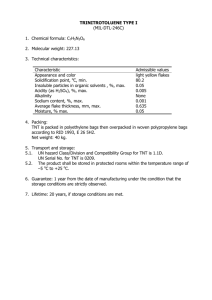
Endophyte‐assisted Phytoremediation of Trinitrotoluene (TNT) Camille Swezy Chris Sahari Advisors: Dr. Zareen Khan and Dr. Sharon Doty School of Environmental and Forest Sciences, University of Washington, June 2014 Methods: Fig 1. Structural formula of trinitrotoluene Objective: We tested to see if this endophytic strain degrades TNT in culture, and if it can assist in removal and degradation of TNT from soil by fescue grass. Phytoremediation and Endophytes Phytoremediation is the application of green plants to clean up and stabilize damaged soils and environments by removing pollutants and increasing overall ecosystem quality by their presence. Microbes beneficial to Photo: Zareen Khan plant growth and survival (referred Fig 2. An example of an endophyte colonizing plant tissue through fluorescent microscopy to as “endophytes”) can aid in this process. The interaction between endophytes and their host plant is Why Tall Fescue Grass? mutual and highly beneficial; these Tall fescue is suitable for military training ranges as they can regrow microbes can fix nitrogen, degrade pollutants, and allow for increased from rhizomes after explosive tolerance by the plant to stressful testing. Additionally tall fescue is conditions. known for its hardiness and fast germination rate. Background: PTA1 ‐Endophyte isolated from Populus tremula x alba 717‐1b4 culture ‐Found to grow in high TNT ‐Pseudomonas strain Diagram credit: Muhammad Nazmin Bin Yaapar, for Biology‐online.org Fig 3. A diagram illustrating the phytoextraction method of phytoremediation. Ideally, the grass will translocate the TNT molecules from the soil into its roots and tillers. Growth of PTA1 with/without presence of TNT 0.7 Part 1: Growing PTA1 in Presence of TNT The endophytic strains were grown in a rich media for 24 hours (see Fig. 5) before inoculating into 125 mL flasks with minimal media at a starting optical density of 0.2 at 600 nm. Flasks were spiked with TNT at a concentration of 5 mg/L (this was done by dissolving TNT powder in acetone, then adding the required amount of solution into each flask and waiting until the acetone evaporated out).The cultures were placed in an orbit shaker, and optical density measurements were taken daily for Fig 4. PTA1 growth on three to four days to measure growth, with the first reading rich media after 2 days 3‐4 hours after time zero. 1 mL samples were taken at each in 30°C incubator time point to analyze at a later time. Part 2: Reduction in Phytotoxicity of TNT to Grasses by PTA1 Soil was spiked at a concentration of 100 mg TNT per 1 kg soil (TNT powder was dissolved in 100 mL of acetone, then Fig 5. PTA1 inoculated Poured over soil and mixed evenly 2‐3 times per day until all acetone evaporated out). For each pot, 0.25 g of seeds in rich media culture, grown for 1 day in orbit were placed in conical tubes, containing 50 mL of MS shaker salts/vitamins broth inoculated with PTA1 culture at OD600 of 0.1. Tubes were put in a shaker for four hours, then the solution was poured over the soil. All groups were kept under equal conditions: under the same growth lamp, in the same fume hood, and all received watering when necessary with consistent levels of soil saturation. Growth was monitored periodically. Proper protection was used, including a lab coat and protective gloves and eye goggles. Optical density @ 600 nm Project Overview: The explosive compound trinitrotoluene (TNT) is still used by the military and is a significant environmental pollutant that is toxic to all organisms and possibly carcinogenic. To increase health of contaminated areas, the TNT must be extracted from the soil in a safe and sustainable method. There is a high need for remediation of TNT from military training ranges in order to prevent the compound spreading into neighboring communities, and research on phytoremediation can assist in cleanups of contaminated soil around the world. Previous research in Doty Lab has shown an endophyte, PTA1, isolated from popular, grew in presence of TNT and protected a grass variety against the phytotoxicity of TNT. The inoculated grasses had more biomass, more chlorophyll than the control. 0.6 PTA1 with TNT #1 0.5 PTA1 with TNT #2 0.4 PTA1 no TNT #1 PTA1 no TNT #2 0.3 0.2 0.1 0 Time 0 Time 1: +4.5 hours Time 2: +20 hours Time 3: +29.5 hours Time 4: +47 hours Fig 8: PTA1 grows in the presence of TNT Part 2: Reduction in Phytotoxicity of TNT to Grasses by PTA1 Seven weeks after sowing and inoculating the seeds, there did not appear to be any major differences in growth or responses of grasses to soil conditions among the different treatment groups. In order to determine the necessary TNT concentration in the soil to produce responses in growth, we watered the grasses with TNT dissolved in dimethylformamide, a solvent less toxic to forms of life. We found that after adding 6 mL of the TNT solution, the tips began to brown and tillers frayed. In uncontaminated soil, the inoculated grass grew taller and thicker than the uninoculated, but there was not much difference in response between the inoculated/uninoculated in TNT soil (see Fig 8+9). Further research needs to be done to test if PTA1 actually colonizes tall fescue grass, as well as whether the soil concentration of TNT decreased over time (due to uptake). Therefore this project is ongoing and not yet complete! Tall fescue in soil at concentration of 100mg Tall fescue in uncontaminated soil TNT/1 kg soil Treatment groups: 1. Control (no TNT, no inoculum) 2. Uninoculated (with TNT) 3. Inoculated (without TNT) 4. Inoculated (with TNT) 5. No grass, inoculated soil (with TNT) Fig 6. The experiment setup; groups in triplicates Results: Part 1: Growing PTA1 in Presence of TNT It took almost four trials to get clear data as it took us awhile to get accustomed to maintaining sterile conditions. We often found our flasks would become contaminated from other bacteria present in the lab. Eventually we obtained clear results, as graphed in Fig 7. We found that PTA1 still grew when TNT was present in solution, but after 2 days growth began to decline compared to the no‐TNT control. In order to determine if PTA1 consumed TNT as a source, and thus degrading it, samples taken from time points will need to be run in high‐performance liquid chromatography (HPLC). Therefore, our research is incomplete Fig 7. PTA1 culture with TNT present, at this time. 2 days after time 0. Fig 9. Inoculated (R) showing stronger growth than uninoculated (L) Fig 10. Inoculated (R) shows little difference in response to TNT than uninoculated (L) Discussion: Due to lack of time, both of our experiments are still on‐going and not complete at this time. We still need to: 1. Further analyze our samples in the HPLC to test for degradation of TNT in culture. 2. Compare the final soil concentration of TNT to the initial soil concentration to test that the fescue was actually extracting TNT molecules from the soil. 3. Perform fluorescent microscopy on the grass tissue to verify that PTA1 successfully colonizes tall fescue grass. Our biggest takeaway from this project is that we have realized that this area of research is long term and extensive; it can take time until clear data is properly obtained. Acknowledgements: Thank you to Zareen Khan and Sharon Doty for their guidance and mentorship, and to Emilie Viglino, Ivy Salim, Robert Tournay, Lisa Hannon, Shyam Kandel, Amy Baum, Neil Fleck, Andrew Sher, and Sherry Zheng for their constant assistance and words of encouragement.