Nuclear Calculations
advertisement

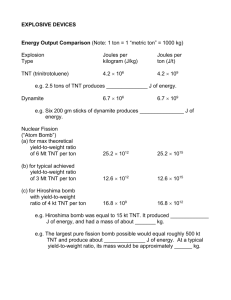
Nuclear Calculations Name:____________________ Date:___________ Directions: Use pencil only. Show all of your work. Round your answers down to two decimals. Use complete sentences on written answers. Write legibly and use structure in your work. Use standard SI units in your calculations. Rescaling a hydrogen atom. BB diameter = 4.5 mm BB radius=_________ Calculate the volume of the BB: VolumeBB=__________ Calculate the volume of a proton. ( radius = 8E-16 m) Volume p+ =_________ Calculate the density of a proton. (1.67E-27 kg) Density (ρ) =_________ If your BB had the same density as a proton, how much would it weigh? (in tons) 1 kg = 2.2 lb 2000lb = 1 ton WeightBB =________ If the proton in the nucleus of a H atom was the same radius as a BB, on average, how far away would the electron be? (in feet) (Hint: Use the proportionality equation from geometry.) Bohr radius = 5.29E-11 meters 1 meter = 3.28 feet Proton radius = 8E-16 meters. Distance = _________ Your BB is mostly Cu (ρ = 8960 kg/m3), find the mass of your BB: Mass = _________ Einstein’s famous equation ___________ says that mass is energy is mass is energy is….. If your BB were converted from mass to pure energy, what would the energy equivalence, in joules, of your BB be? c = 3E8 m/s Energy=__________ How many tanks of gasoline would that be equal to? 1 tank = 20 gallons 131E6 J/gallon Number of Tanks:_________ Nuclear weapon shots are measured in ktons of TNT. How many tons of TNT are contained in your BB if it was converted to pure energy? 1 ton of TNT = 4.18E9 Joules Tons of TNT = _________ If your BB were made of pure U-235 (ρ = 18,950 kg/m3), how many atoms would be in it? Number of atoms=_________ If each atom in your BB were to undergo a fission reaction, how many gallons of gas would it be equal to? How many tons of TNT? 1 fission = 3.2E-11 joules Gallons of gas = __________ Tons of TNT = _________