Document 13445454
advertisement

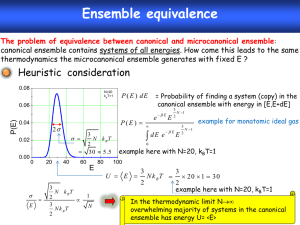
Canonical Ensemble
p(E)
p(E) ∝ e−E/kT
NOT!
E
p({p, q}) ∝ e−H({p,q})/kT
8.044 L13B1
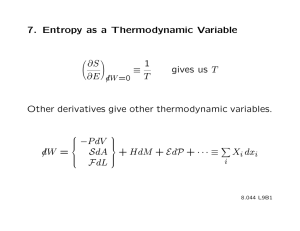
ADVANTAGES OF CANONICAL OVER MICROCANONICAL ENSEMBLE
1) ONE INTEGRATES OVER ALL PHASE SPACE
MICROCANONICAL
Ω
Φ
CANONICAL
Ζ
SURFACE OF
CONSTANT E
8.044 L13B2
2) SEPARATION
let H = Ha + Hb ,
then e−H/kT = e−Ha/kT e−Hb/kT
⇒ p({p, q}) = p({p, q}a) p({p, q}b) (a & b are SI) ⇒ Z = Za Zb ⇒ F = Fa + Fb ⇒ S = Sa + Sb etc.
8.044 L13B3
⇒ For N similar, non-interacting systems
Z = (Z1)N ,
F = N F1 ,
S = N S1
⇒ For N indistinguishable particles
(Z1)N
Z=
,
N!
correct Boltzmann counting
8.044 L13B4
Example Non-interacting classical monatomic gas
N
N
N
N
pi · p i
H =
=
Hi
i=1 2m
i=1
H1(p
p, p
r) =
(Z1)N
⇒ Z=
N!
px2 + py2 + pz2
2m
2
2
−(p2
x +py +pz )/2mkT
p1(p,
p p
r) = e
/(Z1h3)
2 + p2 >= 3mkT
Gaussian px ⇒< p · p >=< p2
+
p
x
y
z
< H1 >= 3/2 kT
8.044 L13B5
Z1 =
�
2
2
−(p2
x +py +pz )/2mkT
e
dpxdpy dpz dxdydz
h3
= (2πmkT )3/2LxLy Lz /h3 = V
2πmkT 3/2
V
=
2
h
λ(T )3
√
Where λ(T ) (or Λ(T ) ) ≡ h/ 2πmkT , the thermal
de Broglie wavelength.
⎛
⎞N
1 ⎝ V ⎠
Z(T, V, N ) =
N ! λ(T )3
8.044 L13B6
F = −kT ln Z
⎡
=
=
−kT
⎛
⎣−N
ln N + N + N
⎧
⎨
ln ⎝
⎞⎤
V ⎠⎦
λ(T )3
⎫
⎬
V
−kT N ln ⎩
−kT N
⎭
3
N λ(T )
'
'V
∝ T −3/2
"
8.044 L13B7
∂F
1 {}
N kT
P = −
= (−1)(−kT N )
=
∂V T,N
{} V
V
�
�
⎛
⎞
∂F
3 1 {} ⎠
⎝
S = −
= kN ln{} − kT N −
+ kN
∂T V,N
2 {} T
�
�
⎧
⎨
⎫
⎬
V
= kN ln ⎩
+ (5/2)N k
⎭
3
N λ(T )
E = F + T S = (3/2) N kT
8.044 L13B8
Find the adiabatic path, ΔS = 0.
⎧
⎨
⎫
⎬
V
V
is constant ⇒ 3/2 is constant
ΔS = 0 ⇒ ⎩
⎭
3
N λ(T )
T
⎛
⎞−3/2
V
T⎠
⎝
=
V0
T0
8.044 L13B9
Example Classical Harmonic Oscillator
1
p2
H1(p, x) =
+ Kx2
2m
2
1
p2
p(p, x) = √
exp[−
]
2mkT
2πmkT
x2
×
exp[−
]
2(kT /K)
2π(kT /K)
1
2π m
Z1 =
kT
h K
�
8.044 L13B10
Now assume there are N similar stationary oscilla­
tors so that we can extract thermodynamic infor­
mation.
Z = Z1N
2π m
F = −kT ln Z = −kT N ln
kT
h K
�
�
�
∂F
1 {}
S = −
= kN ln{} + kT N
∂T N
{} T
�
�
2π m
= kN ln
kT + N k
h K
�
�
�
This shows that an adiabatic path for a collection
of classical harmonic oscillators is one of constant
temperature.
8.044 L13B11
E = F + T S = N kT
This shows that the heat capacity is a constant
C = N k independent of temperature. This would
be true even if the oscillators had a variety of dif­
ferent frequencies.
8.044 L13B12
Canonical Ensemble
CLASSICAL
p({p, q}) =
Z=
e−H({p,q})/kT /Zhα
e−H/kT
{dp, dq}/hα
QUANTUM
p( state) = e−Estate/kT /Z
e−Estate/kT
Z=
states
where α depends on the dimensionality of the phase
space.
8.044 L13B13
EXAMPLE 2 LEVEL SYSTEM: STATES OF AN IMPURITY IN A SOLID
ε
g
E=0
1
E=
*
EXCITED
GROUND
STATE
g-FOLD
DEGENERACY
LOCATION
ENERGY LEVELS
INTERNAL
PHYSICAL DIFFERENCE
8.044 L13B1
STATES:
|0 >/ , \|1 >, · · · |g >
/
\
E=0
E=E
e−Estate/kT = 1 × e0 + g × e−E/kT = 1 + ge−E/kT
Z1 =
states
p(state) = e−Estate/kT /Z1
1
=
1 + ge
−E/kT
for |0 >
e−E/kT
=
1 + ge−E/kT
for |i > i = 1, · · · g
8.044 L13B15
g
1+ g
p(E= ε)
1
1+ g
p(state = i = 0)
ε/ k
T
8.044 L13B1
Heat Capacity of a Two Level System 1.60 1.40 g=1 1.20 g=2 g=3 C/Nk 1.00 g=4 g=5 0.80 0.60 0.40 0.20 0.00 0.0 0.3 0.5 0.8 1.0 1.3 1.5 1.8 2.0 2.3 2.5 2.8 kT/ε
8.044 L13B17
ε
Assume
• N impurities (N » 1)
ε0
• E = E0(V /V0)−γ
V0
Z = Z1N
V
F (T, V, N ) = −kT ln Z = −N kT ln Z1
∂F
S=−
=
∂T V
E )e−E/kT
g(
2
⎟
⎝
kT
⎠
N k ln Z1 + N kT
⎜
−E/kT
1 + ge
⎛
⎞
8.044 L13B18
−E/kT
E
e
S = N k ln(1 + ge−E/kT ) + gN k
kT 1 + ge−E/kT
g E e−E/kT
U = F + TS = N
= N E p(E = E)
−E/kT
1 + ge
∂F
∂F
∂E
P =−
=−
∂V T,N
∂E T � ∂V�� T�
−γE
V
= N kT
g
) e−E/kT
−( kT
1 + ge−E/kT
γE
γU
−
=
V
V
8.044 L13B19
ALTERNATIVE WAY OF FINDING U
Usually (but not always) U =< H > .
If so, U =
But Z = c
�
H({p, q}) p({p, q}) {dp, dq}
�
e−H({p,q})β {dp, dq}
β ≡ 1/kT
8.044 L13B20
⎛
⎝
⎞
∂Z ⎠
=c
∂β
N,V
⎛
−H({p, q})e−H({p,q})β {dp, dq}
1
⎝ ∂Z ⎠
−
=
Z
∂β N,V
⎛
e−H({p,q})β
⎞
H({p, q})
' '
e−H({p ,q })β
"
{dp', dq '}
p({p,q})
{dp, dq}
}
⎞
1
⎝ ∂Z ⎠
−
=U
Z
∂β N,V
8.044 L13B21
Example Monatomic Gas
1 N 2πmkT 3N/2
−3N/2
Z=
V
=
α
β
N!
h2
U =−
1
α β −3N/2
⎛
⎝−
⎞
3N 1 ⎠
3
−3N/2
αβ
= N kT
2 β
2
8.044 L13B22
Example 2 Level System
Z =
�
1 + ge−E β
�N
�
�−N
U = − 1 + ge−E β
�
�N −1 �
N 1 + ge−E β
−Ege−E β
�
gN E e−E/kT
=
1 + ge−E/kT
8.044 L13B23
MIT OpenCourseWare
http://ocw.mit.edu
8.044 Statistical Physics I
Spring 2013
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.