MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department 8.044 Statistical Physics I Spring Term 2013
advertisement
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
Physics Department
8.044 Statistical Physics I
Spring Term 2013
Solutions, Practice Exam #2
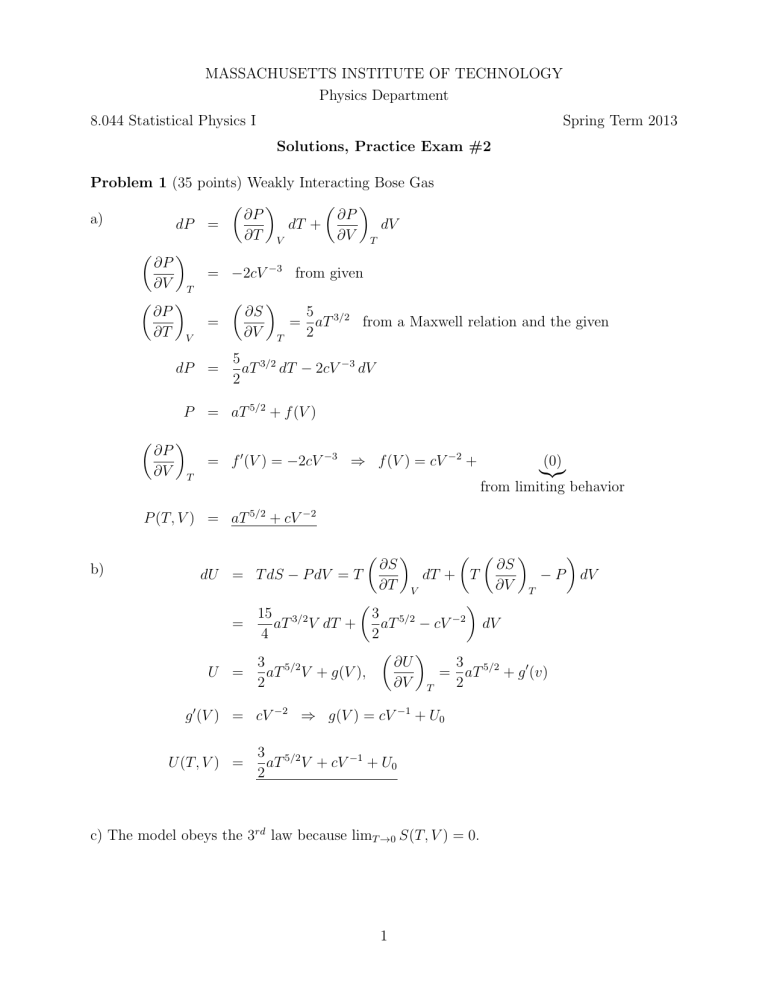
Problem 1 (35 points) Weakly Interacting Bose Gas
∂P
∂P
a)
dP =
dT +
dV
∂T V
∂V T
∂P
= −2cV −3 from given
∂V T
∂P
∂S
5
=
= aT 3/2 from a Maxwell relation and the given
∂T V
∂V T
2
dP =
5 3/2
aT dT − 2cV −3 dV
2
P = aT 5/2 + f (V )
∂P
∂V
= f 0 (V ) = −2cV −3 ⇒ f (V ) = cV −2 +
T
(0)
|{z}
from limiting behavior
P (T, V ) = aT 5/2 + cV −2
b)
∂S
dU = T dS − P dV = T
dT + T
− P dV
∂V T
V
15 3/2
3 5/2
−2
=
aT V dT +
aT − cV
dV
4
2
3 5/2
∂U
3
U =
aT V + g(V ),
= aT 5/2 + g 0 (v)
2
∂V T
2
∂S
∂T
g 0 (V ) = cV −2 ⇒ g(V ) = cV −1 + U0
U (T, V ) =
3 5/2
aT V + cV −1 + U0
2
c) The model obeys the 3rd law because limT →0 S(T, V ) = 0.
1
Problem 2 (30 points) Carnot heat engine
a) For a reversible process d/Q = T dS. Also, for a constant volume process, dQ = CV dT .
Thus
dQ
dS =
T
Z T1
Z T1
CV
dQ
dT
∆S =
=
T
T
T2
T2
= CV ln T1 /T2 = −CV ln T2 /T1
b) The efficiency of an engine cycle η is defined as (work out)/(heat extracted at the higher
temperature). Thus
dWout = η |dQ2 |
∆Wout
2→1
T1
=
1−
(−CV dT2 ) for a Carnot cycle
T2
Z T1 T1
= −
1−
(CV dT2 )
T2
T2
= −CV [(T1 − T2 ) − T1 ln(T1 /T2 )]
= CV [(T2 − T1 ) − T1 ln(T2 /T1 )]
c) Since the engine is run in cycles and entropy is a state function, the entropy change in
each cycle is zero, as is the the total entropy change in the process.
One can see this as well by applying conservation of energy.
Heat out at high T - Heat dumped at low T = Work out
∆Q1 = CV (T2 − T1 ) − ∆Wout
2→1
= T1 ln(T2 /T1 )
∆S1 = ∆Q1 /T1 = CV ln(T2 /T1 ) = −∆S2 found in a) above
2
Problem 3 (35 points) A Classical Ultra-relativistic Gas
a) For one atom
Z
exp[−/kB T ]dp3 dV /h3
Z1 =
V
= 3
h
= 4π
∞
Z
Z
2
exp[−cp/kT ]p dp
0
V
h3
|0
= 8πV
3 Z
kb T
c
kb T
hc
π
Z
sin θ dθ
{z
2π
dφ
}
0
4π
∞
exp[−y ]y 2 dy
}
{z
|0
2
3
For the whole gas
1 N
z
N! 1
b) p(p) ∝ exp[−cp/kB T ] p2 . The normalization integral was done in a).
Z=
p(p)
p(p) =
1
2
c
kb T
3
exp[−cp/kB T ] p2 for p ≥ 0
p
c) Note Z = Aβ −3N .
d)
1
U =−
Z
∂Z
∂β
V
1
Z
= (− )(−3N ) = 3N kB T
Z
β
F = −kB T ln Z
P = −
e)
∂F
∂V
= kB T
T
∂ ln Z
∂V
T
1
= kB T
Z
|
∂Z
∂V
{z
N/V
=
T
N kB T
V
}
F = U − T S → S = (U − F )/T = 3N kB + kB ln Z
"
3 #N
1
kB T
S(T, V, N ) = 3N kB + kB ln
8πV
N!
hc
3
MIT OpenCourseWare
http://ocw.mit.edu
8.044 Statistical Physics I
Spring 2013
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.