About this wheel
MEDICAL ELIGIBILITY CRITERIA WHEEL
FOR CONTRACEPTIVE USE
© World Health Organization 2009
All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World
Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791
4857; e-mail: bookorders@who.int). Requests for permission to reproduce or translate WHO publications –
whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above
address (fax: +41 22 791 4806; e-mail: permissions@who.int).
2008 UPDATE
The designations employed and the presentation of the material in this publication do not imply the
expression of any opinion whatsoever on the part of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers
or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full
agreement.
Acute
tme
ombi
nt)
ned
pills
and
co
mb
i
Pr o
ge
T-E
N
B
N THROM
P VEI
DEE
2
Impla
nts
Copp
er
I
J
1
35
ory
Hist
lants
Imp
UD
er I
p
p
1
ills
and
P ro ge s
com
t oge
bin
n -o
nl y
ed
DMPA
pi l
ls
&N
(
E
Ps
ISBN 978 92 4 154771 0
PA
DM
1
CICs)
Cs &
(CO
les
tab
ec
inj
)
Printed in
O
s (C
e
l
b
OPs )
cta
l ls (P
i
p
nje
i
d
n ly
ne
n -o
e
g
ET-EN
s to
&N
Co
N
ex
Un
9
15
0- 9
14 90-9
All reasonable precautions have been taken by the World Health Organization to verify the information
contained in this publication. However, the published material is being distributed without warranty of any
kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with
the reader. In no event shall the World Health Organization be liable for damages arising from its use.
N
SIO
EN
00
RT
>1
PE
60/
>1
HY
EXUALLY TRANSMITTED
HIV/
Y S
R
INFECTIONS
O
T
AID
MA
S
M
A
r
S
e
T
h
L
t
I
s
O
I
a
n
e
c
F
o
r
h
e
ased
IN EASE
Gonorr iaC
H
n
d
a
I
C
V
SM
I
r
i
d
infe
sk of
LV DIS
Chlamy
Past
OK
vaginitis
or AI ction
STI E
PE
D
ING
Age
S
L
nt
A
e
<
r
r
N NG
35
u
I
C
G I
1
Combin
Ag
VA EED ined
CICs)
e>
&
L
ed p
a
l
s
C
B
p
The mention of specific companies or of certain manufacturers’ products does not imply that they are
endorsed or recommended by the World Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by
initial capital letters.
UD
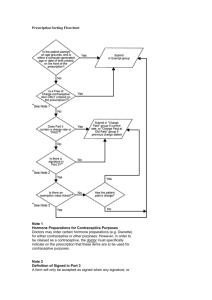
No restrictions for some conditions: There are many medical conditions
when ALL methods can be used (that is, all the methods are either a
category 1 or 2). These conditions are listed on the back of the wheel.
WHO
(NLM classification: WP 630)
PO
The wheel matches up the contraceptive methods, shown on the inner
disk, with specific medical conditions shown around the outer rim. The
numbers shown in the viewing slot tell you whether the woman who has
this known condition is able to start use of the contraceptive method:
1 = Yes: Use the method in any circumstance
2 = Yes: Generally use the method
3 = No: Use of the method is not usually recommended unless other
more appropriate methods are not available or acceptable
4 = No: Method NOT to be used
Categories 1 and 4 are clearly defined recommendations. For categories 2
or 3, greater clinical judgement will be needed and careful follow-up may
be required. If clinical judgement is limited, categories 1 and 2 both mean
the method can be used, and categories 3 and 4 both mean the method
should not be used.
ISBN 978 92 4 154771 0
CI
How to use this wheel
1.Contraception - methods. 2.Family planning services - methods. 3.Eligibility determination - standards.
4.Guidelines. I.World Health Organization. II.Title: Medical eligibility criteria wheel for contraceptive use.
CAN
The guidance in the wheel applies to initiation of contraceptive methods.
Recommendations for continuation of method use, when a woman
develops a medical condition while using the method, can be found in
the Medical Eligibility Criteria for Contraceptive Use guideline.
WHO medical eligibility criteria wheel for contraceptive use - 2008 update.
CER
Puerpera
S
l
and post
B
r
e
ast
can
abortion
Cer
(curr cer
ent)
c vic
(pre ance al
-tre
a r
C
The wheel includes recommendations on initiating use of six common
types of contraceptives:
1. Combined pills (low dose combined oral contraceptives, with < 35 μg
ethinylestradiol)
2. Combined injectable contraceptives (Cyclofem and Mesigyna)
3. Progestogen-only pills
4. Progestogen-only injectables, DMPA (a 3-monthly injectable) and
NET-EN (a 2-monthly injectable)
5. Progestogen-only implants (Norplant, Jadelle, and Implanon)
6. Copper-bearing IUD
WHO Library Cataloguing-in-Publication Data
SEPSIS
This wheel contains the medical eligibility criteria for starting use of
contraceptive methods. It is based on Medical Eligibility Criteria for
Contraceptive Use, 3rd edition (2004) and its 2008 Update, one of WHO’s
evidence-based guidelines. It tells family planning providers if a woman
presenting with a known medical or physical condition is able to use
various contraceptive methods safely and effectively.
Acknowledgements
The Medical Eligibility Criteria for Contraceptive Use and this version of the Medical Eligibility Criteria Wheel were
developed by the World Health Organization’s Department of Reproductive Health and Research. This wheel is
based on similar medical eligibility criteria wheels developed independently in Ghana and Jordan. We would like
to thank the responsible groups for their innovative work:
Ghana: The Department of Obstetrics and Gynaecology at the Korle Bu Teaching Hospital, part of the
University of Ghana Medical School; and the WHO country office in Ghana. Further technical or financial
support was provided by: the Ghana Health Service, UNFPA/Ghana, Gold Fields Ghana Ltd., JHPIEGO,
EngenderHealth, WHO/AFRO, USAID, and WHO/Eritrea.
Jordan: The Jordan Health Communication Partnership and the Near East Division, led by the late
Dr Alfred Yassa, in the Health Communication Partnership at the Johns Hopkins Bloomberg School of Public
Health/Center for Communication Programs, in collaboration with the Jordan Ministry of Health, and with
support from USAID.
We would like to thank the following individuals for their work on the original wheel: Dr Charles Fleischer-Djoleto,
WHO/Ghana; Dr Enyonam Kwawukume, Korle Bu Teaching Hospital; Mr Ward Rinehart of the INFO Project,
JHSPH/CCP; Dr Therese Lesikel, WHO/AFRO; Dr Kathryn Curtis, Centers for Disease Control and Prevention;
Ms Kathryn Church, Dr Catherine d’Arcangues, Dr Nuriye Ortayli, Ms Sarah Johnson, and Dr Paul Van Look,
Department of Reproductive Health and Research, WHO.
Dr Mario Festin, Dr Mary Lyn Gaffield, and Ms Sarah Johnson were responsible for this 2008 Update.
Layout and design: Ms Cath Hamill and Ms Janet Petitpierre.
Ordering copies and further information
Detailed information on the medical eligibility criteria, including guidance on other contraceptive methods,
appears in the Medical Eligibility Criteria for Contraceptive Use, 3rd edition, 2004 and the 2008 Update. This can be
accessed at http://www.who.int/reproductive-health or copies can be ordered from the address below.
Sample copies of the wheel can be ordered from:
Documentation Centre
Department of Reproductive Health and Research
World Health Organization
1211 Geneva 27
Switzerland
Fax: +41 22 791 4189. Phone: +41 22 791 4447.
Email: rhrpublications@who.int
Bulk orders to supply programmes can be ordered from:
WHO Press
World Health Organization
1211 Geneva 27
Switzerland
Fax: +41 22 791 48 57. Phone: +41 22 791 32 64.
Email: bookorders@who.int
Order online: http://www.who.int/bookorders
HE
AD
N
CI
2
2
2
4
1
1
O
4
TES
BE
DIA
r
Live ur
o
tum
4
3
2-3P
3
4
3
1
1
3
1
1
1
1
1
1-2
T
2
1
1
1
2G
s Rifampicin/ Cer
Hepatiti e rifabutin co tain antinvulsant Q
flar
s
acute /
EASES
R DIS
LIVE
1
1
1
1
2
2-3 P
4
3
2
3
2
nt
rre
Cu
1-2V 1-2 V
1
N
4
4
1 4 1
2 A
4
1
1
2
4
ine M
gra ra
Mi th au
wi
1
2
3
2
1
1
1
1
O
Cer
1
2
2
1
2
can vica
c
trea er (pr l
tme ent)
2
1
CAN
CER
S
2
3
1
1
2
2
1S
1
1 1 2R
1 1
1
2
mi Non
g
r
a
ino
us
S
HE
AC
1
2
2
1
Ut
fib erin
roi e
ds
2
2G
ARV
thera
py
DRUG INTERA
CTION
S
e
rch s
na 8 yr
e
1
M <
to
Mu
lti
ri ple
fac sk
tor L
s
3 /4
Ischa
em
hea ic
disea rt
se
4
2
1
M
I
S
CE
LL
A
N
EO
US
Obe
sity
rs
yea r
40 olde
and
1
2
1
CARD
I
O
V
A
SCU
ALR
D
ISE
AS
E
Stroke
3
3
1
us
iparo
Null
3
2
2
E
AG
4
Major surgery
4
2
2
with prolonged
immobilization
2
2
2
6 weeks to Puerper
a
6 months and pos l
Brea
tcanc st
abortion
(curr er
ent)
3
Acute
4
3U
3U
3U
1
Up to
6 weeks
2
2
2
1 1 1
1 1
1
BOSIS
N THROM
P VEI
DEE
ory
Hist
4
3
2
SEPSIS
4
2
K
1
1
ITY
PAR
J
K
3
K
1
2
K
4
2
1B 4 2 /3 2 H 1
1
D
4
1
POSTPARTUM
EDING
BREASTFE
9
15
0- 9
14 90-9
N
SIO
EN
00
RT
>1
PE
60/
>1
HY
EXUALLY TRANSMITTED
HIV/
Y S
R
INFECTIONS
O
T
AID
MA
S
M
A
r
S
e
T
h
L
t
I
s
O
I
a
n
e
c
F
o
r
h
eased
IN EASE
Gonorr iaC
n
d
a
C
SM
I
r
isk of
d
HIV
LV DIS
Chlamy
Past
OK
vaginitis
infec
STI E
PE
t
ING
Age
L
or AI ion
nt
A
e
<
r
D
r
N NG
S
3
u
I
5
C
1
G I
1
1
Ag
VA EED ined
1G
1
e>
L
a
B expl
35
2
n
1
1
U
1
1
1G
1
3I
2
1
1
1
1
1
1
1G
2
1
1
1
1
1 1 1G
3
1 1
1
1
1
3
D
F
Conditions that are
category 1 and 2 for all methods (method can be used)
Age 18–39
High risk for HIV
Schistosomiasis (bilharzia)
Anaemias, including sickle-cell
disease and thalassaemia
History of gestational diabetes
Surgery without prolonged
immobilization
History of high blood pressure
during pregnancy
Benign ovarian tumors,
including cysts
History of pelvic surgery,
including caeserean section
Breast disease: family history,
benign breast disease and
undiagnosed mass
Depression
Dysmenorrhoea
Tuberculosis (but if pelvic, cannot
use IUD)
Malaria
Uncomplicated valvular heart
disease
Past ectopic pregnancy
Epilepsy
Thyroid disorders
Irregular, heavy or prolonged
menstrual bleeding
Mild cirrhosis
Endometriosis
Taking antibiotics
(excluding rifampicin/rifabutin)
Post-abortion (no sepsis)
Varicose veins
Viral hepatitis (carrier or chronic)
Notes to the conditions
A Can insert copper IUD < 48 hrs after delivery or > 4 weeks.
M To check if migraine has aura, ask: “Do you see a bright spot in your
vision before bad headaches?”
B If she had no subsequent pregnancy, IUD = 2.
C Or other forms of purulent cervicitis.
D If she develops this condition while using the IUD, she can keep
using it during treatment.
E If at increased risk of STIs or HIV, advise condom use.
F If very high likelihood of exposure to gonorrhoea or chlamydia = 3.
G If on ARV Therapy = 2, except ritonavir-boosted ARVs = 3.
H AIDS, but not clinically well on ARV Therapy = 3 for insertion.
N Migraine without aura and < 35 years old, COCs and CICs = 2.
Migraine without aura and > 35 years old, COCs and CICs = 3.
O For complicated diabetes, or having diabetes for more than 20
years, COCs, CICs, DMPA and NET-EN = 3–4.
P COCs = 3; CICs = 2.
Q Phenytoin, carbamezepine, barbiturates, primidone, topiramate,
oxcarbazepine. For lamotrigine COCs/CICs = 3. Other methods = 1.
R If she is not clinically well, IUD = 3.
I COCs and heavy smoking = 4. CICs and light smoking = 2.
J If blood pressure cannot be measured, and she has no known
history of hypertension, all methods can be used.
K The same category applies to controlled hypertension.
L Risk factors include: older age, smoking, diabetes, hypertension.
S If the uterine cavity is distorted, cannot use IUD.
T > 45 yrs. = 2.
U If established on anticoagulant therapy = 2.
V DMPA = 1; NET-EN = 2.
Developed in collaboration with:
Communication Partnership
for Family Health
Johns Hopkins Center for
Communication Programs
© World Health Organization, 2009
University of Ghana
Medical School
Co
Comb
ined
pills
and
co
mb
i
Pro
ge
CICs)
Cs &
(CO
les
tab
ec
inj
)
Impla
nts
Copp
er
I
UD
lants
Imp
UD
er I
p
p
ETEN
Ps
A&
P
DM
Combin
ed p
ills
and
P r o g es
com
t og e
bin
n- o
nl y
ed
DMPA
pi l
ls
&N
(
PO
ICs)
s&C
C
O
s (C
ble
a
t
OP s )
c
ll s (P
i
p
nje
i
d
nly
ne
n -o
EN
e
g
NETsto
1 Use the method in any
circumstance
3
Use of the method not usually
recommended unless other, more
appropriate methods are not
available or acceptable
2 Generally use the method
4
Method NOT to be used
WHO Medical Eligibility Criteria Wheel
for contraceptive use (2008 update)
This wheel contains medical eligibility criteria for starting use of selected
contraceptive methods. It is based on WHO's guideline Medical Eligibility Criteria
for Contraceptive Use, 3rd edition, 2004 and its 2008 Update.