Information for Prospective Collaborators

Information for Prospective Collaborators
PRIMENT Clinical Trials Unit (CTU) is a partnership between the UCL Research Department of Primary Care & Population
Health, Mental Health Sciences Unit and the Department of Statistical Science. PRIMENT CTU collaborates with researchers to develop and evaluate interventions in primary care, psychiatric and community settings. The unit has a particular focus on mental health and behavioural change interventions.
PRIMENT's mission is to conduct high quality randomised trials and related studies in mental health and primary care.
PRIMENT is a UK Clinical Research Collaboration (UKCRC) registered clinical trials unit.
Collaboration with PRIMENT CTU
PRIMENT provides support to researchers conducting clinical trials and other well designed studies in primary care, psychiatric and community settings. We provide support from the early stages of protocol development through to study closure and dissemination of results.
The core support services provided by PRIMENT, which are normally provided for all studies are:
• Advice on study design and protocol development, regulatory issues, trial management, data management, safety and reporting
• Advice on development of study databases and randomisation services
• Statistical analysis
• Health economics evaluations as applied to trials
Additionally, subject to agreement of the PRIMENT Steering Group and availability of resources, PRIMENT will undertake operational responsibility for Trial Management and/or other aspects of the trial.
Please see the full list of the core support services below.
Involvement of PRIMENT in your study requires the inclusion of the following staff as co-applicants on the grant application:
• A PRIMENT trialist
• A PRIMENT statistician
• A PRIMENT health economist
The involvement of these staff for the duration of the trial and publication and dissemination of results ensures PRIMENT is able to work effectively with your team and satisfies the requirement of most major funding bodies that a registered CTU is closely involved in your trial.
PRIMENT members should be invited to attend Trial Management Group, Data Monitoring and Ethics Committee and Trial
Steering Committee meetings as considered appropriate.
PRIMENT members named on the grant application are expected to be authors and PRIMENT must be acknowledged on relevant publications.
FOR ALL CTIMP STUDIES (trials involving investigational medical products):
There will be additional costings for CTU support. For more information about this please discuss with the PRIMENT contact.
In addition the UCL Joint Research Office (JRO) should be contacted as soon as possible to discuss sponsorship and full trial costings. Please email the JRO on CTIMPS@ucl.ac.uk
.
Version 1 last updated 04/06/14
Page 1 of 3
By entering into collaboration with PRIMENT the CI agrees to undertake the following responsibilities:
• Provide copies of essential trial documentation to PRIMENT as and when requested
• Adhere to PRIMENT SOPs
• Inform PRIMENT of any deviations from, and substantial amendments to, the protocol
• Ensure PRIMENT has a copy of the latest version of the protocol at all times
• Inform PRIMENT of any correspondence with regulatory bodies or the funder
• Inform PRIMENT of any internal monitoring and audit activity
• Ensure PRIMENT collaborators are able to attend Trial Management Group (TMG) meetings as appropriate. If any collaborator is unable to attend, the terms of reference must be followed to ensure the meeting is quorate.
• Ensure one PRIMENT collaborator is able to attend Trial Steering Committee (TSC) and Data Monitoring Committee
•
(DMC) meetings as appropriate
Comply with ICMJE Uniform Requirements for Manuscripts Submitted to Biomedical Journals http://www.icmje.org/urm_main.html
Adhering to these requirements ensures PRIMENT can maintain oversight and ensure the quality of research being conducted.
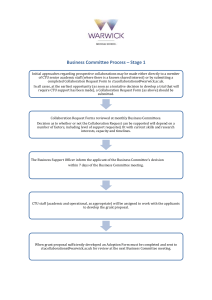
To request PRIMENT collaboration:
• Complete a copy of the collaboration request form
• Email the form and your draft proposal or grant application form to the CTU administrator priment@ucl.ac.uk
• The PRIMENT Steering Group will review the request and make a decision on whether to accept the request for collaboration
Please ensure you submit this form with a draft proposal or application form at least 12 weeks in advance of your submission deadline. We understand that occasionally external constraints may mean that 12 weeks is not feasible, and in these circumstances applications may be considered subject to resource constraints.
PRIMENT cannot confirm involvement in a trial nor provide cost estimates until the PRIMENT Steering Group has reviewed the proposal and agreed to adopt the study. The Steering Group meets every 2 weeks to review applications.
Version 1 last updated 04/06/14
Page 2 of 3
PRIMENT core support services:
1.
Protocol development
1.1.
Input into study/ trial design
1.2.
Input into statistical analysis plan
1.3.
Input into health economic analysis plan
1.4.
Costings for PRIMENT staff contribution
2.
Regulatory issues/approvals
2.1.
Advice on ethical approval
2.2.
Advice on NHS research governance procedures
2.3.
Advice on Competent Authority approval
2.4.
Advice on trial registration (EudraCT & ISRCTN)
2.5.
Advice on R&D approval
2.6.
Advice on honorary contracts/ research passports
2.7.
Advice on sponsorship arrangements
3.
Trial/study management
3.1.
Advice on funding administration
3.2.
Advice on clinical trial risk assessment
3.3.
Advice on monitoring arrangements/ plan
3.4.
Advice on setting up and maintaining Trial Master Files
3.5.
Advice on site initiation and set up of Investigator Site Files
3.6.
Advice on developing of study specific working practice documents and SOPs
3.7.
Advice on staff training
3.8.
Advice on Investigational Medicinal Product (IMP) processing arrangements
3.9.
Advice on protocol amendments
3.10.
Advice on the notification of regulatory authorities of study closure
3.11.
Advice on the notification of R&D departments of study closure
3.12.
Advice on the notification of Sponsor of study closure
3.13.
Advice on preparing final study report for the funding committee
3.14.
Advice on study close out at investigator sites
4.
Data management
4.1.
Advice on randomisation
4.2.
Carrying out online randomisation
4.3.
Advice on developing data collection instruments/ CRFs
4.4.
Advice on database development
4.5.
Creating and managing the trial database
4.6.
Advice on data entry
4.7.
Advice on data cleaning/ query generation
4.8.
Carrying out data cleaning/ query generation
4.9.
Carrying out statistical analysis
4.10.
Carrying out health economic analysis
4.11.
Advice on archiving
5.
Study/Trial safety and quality assurance
5.1.
Advice on Serious Adverse Events reporting
5.2.
Advice on implementing of urgent safety measures
5.3.
Advice on site monitoring/ source data verification
5.4.
Advice on conducting an audit/ inspection
6.
Study/Trial reporting
6.1.
Advice on periodic safety reports to MHRA & Ethics committee
6.2.
Advice on progress reports to ethics committee
6.3.
Advice on annual reports to funding committee
6.4.
Advice on Trial Steering Group reports
6.5.
Advice on Data Monitoring Committee reports
Version 1 last updated 04/06/14
Page 3 of 3