Journal of Colloid and Interface Science 445 (2015) 252–261
Contents lists available at ScienceDirect
Journal of Colloid and Interface Science
www.elsevier.com/locate/jcis
Use of drinking water treatment solids for arsenate removal from
desalination concentrate
Xuesong Xu a, Lu Lin a, Charalambos Papelis a, Maung Myint a, Tzahi Y. Cath b, Pei Xu a,⇑
a
b
Department of Civil Engineering, New Mexico State University, NM 88003, United States
Department of Civil and Environmental Engineering, Colorado School of Mines, CO 80401, United States
g r a p h i c a l a b s t r a c t
Arsenate removal
100%
80%
60%
Screen
40%
Effluent
20%
Chemisorption
Arsenate
0%
0
200
400
600
800
Iron
hydroxide
Drinking water
treatment solids
Gravels
Screen
Arsenate
NOM
Peristaltic pump
Influent: RO concentrate
a r t i c l e
i n f o
Article history:
Received 10 October 2014
Accepted 27 December 2014
Available online 9 January 2015
Keywords:
Arsenate removal
Arsenic
Sorption
Drinking water treatment solids
Desalination concentrate
a b s t r a c t
Desalination of impaired water can be hindered by the limited options for concentrate disposal. Selective
removal of specific contaminants using inexpensive adsorbents is an attractive option to address the
challenges of concentrate management. In this study, two types of ferric-based drinking water treatment
solids (DWTS) were examined for arsenate removal from reverse osmosis concentrate during continuousflow once-through column experiments. Arsenate sorption was investigated under different operating
conditions including pH, arsenate concentration, hydraulic retention time, loading rate, temperature,
and moisture content of the DWTS. Arsenate removal by the DWTS was affected primarily by surface
complexation, electrostatic interactions, and arsenate speciation. Results indicated that arsenate sorption
was highly dependent on initial pH and initial arsenate concentration. Acidic conditions enhanced arsenate sorption as a result of weaker electrostatic repulsion between predominantly monovalent H2AsO
4
and negatively charged particles in the DWTS. High initial arsenate concentration increased the driving
force for arsenate sorption to the DWTS surface. Tests revealed that the potential risks associated with
the use of DWTS include the leaching of organic contaminants and ammonia, which can be alleviated
by using wet DWTS or discarding the initially treated effluent that contains high organic concentration.
Ó 2015 Elsevier Inc. All rights reserved.
1. Introduction
1.1. Reverse osmosis (RO) concentrate treatment
Desalination of seawater, brackish water, and reclaimed water
has been a viable solution to providing alternative water supplies.
Reverse osmosis (RO), nanofiltration, electrodialysis, and thermal
⇑ Corresponding author at: Department of Civil Engineering, New Mexico State
University, 3035 S Espina Street, NM 88003, United States.
E-mail address: pxu@nmsu.edu (P. Xu).
http://dx.doi.org/10.1016/j.jcis.2014.12.090
0021-9797/Ó 2015 Elsevier Inc. All rights reserved.
distillation are well-established desalination technologies, producing fresh water for industrial, domestic, and agricultural uses [1–4].
However, with increasing water recovery (ratio of product to feed
water) during desalination, the concentrations of dissolved constituents in the concentrate stream increase. Consequently, proper
disposal of brines, and particularly those containing elevated concentrations of toxic contaminants, including heavy metals, is one of
the primary impediments for implementation of desalination
technologies [5].
Ocean discharge is widely used by desalination facilities in
coastal areas, but concentrate disposal remains a major challenge
253
X. Xu et al. / Journal of Colloid and Interface Science 445 (2015) 252–261
for inland plants where disposal options are limited by the quantity
and quality of concentrate, regulations, and geographical and geological constrains. A recent review of technologies for concentrate
treatment was published, focusing on concentrate volume minimization and beneficial uses [6]. However, most of these technologies
are often costly and energy intensive. Selective removal of specific
contaminants (e.g., arsenic and heavy metals) using inexpensive
materials could be an alternative to meeting disposal requirements
or water quality criteria for beneficial use applications [5].
1.2. Arsenic removal technologies
If present in water and consumed in large quantities, arsenic, a
metalloid abundant in nature in the form of organic and inorganic
compounds, can cause a variety of pathological conditions, including cutaneous and visceral malignancies [7]. The acute minimal
lethal dose of arsenic in adults is 70–200 mg per day, or 1 mg per
kg weight per day [8]. The United States Environmental Protection
Agency (USEPA) has set an enforceable regulatory limit for arsenic
in drinking water at 0.01 mg L1 (10 ppb).
A variety of physicochemical techniques are being utilized for
arsenic removal, including coagulation [9], ion exchange [10], RO
[11], liquid–liquid extraction [12], and sorption [13]. Specifically,
sorption using ferric-based sorbents is an effective treatment technology because of the high sorption capacity of iron for arsenic and
simple operation. Table 1 summarizes the arsenic sorption capacity
of different sorbents. Studies have shown that these materials have
a strong affinity for arsenic under natural pH conditions compared
to commonly used activated alumina. For sorption processes, alternative sorbents that meet the requirement of cost-effectiveness
and eco-friendly disposal are needed. A low-cost and potentially
effective substitute for arsenic sorbents could be the solid residuals
from coagulation/flocculation water treatment processes, which
often utilize ferric-based coagulants [14].
1.3. Drinking water treatment solids (DWTS)
Drinking water treatment solids (DWTS) are the residuals
produced during water treatment using iron or aluminum salts
as primary coagulants. Laboratory studies have demonstrated
that DWTS have strong affinity to sorb contaminants such as
phosphorus [15,16], hydrogen sulfide [17], metals [18,19], fluoride [20], and arsenic [5,21]. Laboratory batch equilibration studies have shown that both ferric-based and aluminum-based
DWTS have a high affinity for arsenite [As(III)] and arsenate
[As(V)] species [22]. Gibbons and Gagnon examined the sorption
of arsenic from groundwater in batch and column experiments
using the solids from different water treatment plants [23].
The results showed that ferric and lime solids were effective sorbents for arsenic removal [23]. The arsenic removal mechanism
by DWTS involves mainly inner-sphere complexation with ferric
hydroxides [24], which is affected by the ferric mass in the
DWTS [25,26].
The factors influencing arsenic sorption include pH, loading
rate, initial arsenic concentration, competing ligands or complexing metals, temperature, and specific physicochemical properties
of the adsorbing solids (e.g., specific surface area, total carbon content, porosity) [22,27,28]. X-ray absorption spectroscopy demonstrated that strong, inner-sphere complexes are formed between
sorbed arsenic and the Al/Fe-hydroxide components of the solids
[29,30]. Arsenic leaching tests indicated that 50–60% of the total
arsenic in DWTS was bound strongly on amorphous iron hydroxides [31]. Maintaining non-alkaline and high redox potential conditions is critical for minimal arsenic mobilization in DWTS [31].
Thermodynamic studies indicate that arsenic sorption increases
with increasing water temperature [32].
Although previous studies have demonstrated the feasibility of
arsenic removal using DWTS (Table 1), there is lack of knowledge
on the removal efficiency of arsenic from saline water, such as
desalination concentrate, in which competition with co-existing
ions and high ionic strength may affect the selective sorption of
arsenic. Therefore, this study focused on investigating sorption of
arsenate from RO concentrate using different types of DWTS during continuous-flow operation. The impacts of operating conditions on arsenate removal were investigated by performing
experiments at different pH, initial arsenate concentration in RO
concentrate, hydraulic retention time, temperature, and loading
rate using different types of DWTS.
Table 1
Summary of results from arsenic sorption studies using various sorbents under different testing conditions.
Adsorbent
Char carbon
Activated carbon
Iron oxide coated sand
Ferric-based water
treatment residual
Lime-based water
treatment residual
Activated aluminum
MnO2
Hydrous ferric oxide
(HFO)
Zirconium-loaded
activated carbon
(Zr-AC)
Granular ferric
hydroxide (GFH)
Activated alumina
grains
FeCl3 treated tea
fungal biomass
Type of water
Synthetic 0.1 M
NaCl solution
Synthetic 0.1 M
NaCl solution
Drinking water
Ground water
Ground water
pH
As concentration
1
7.6
8.0–8.2
157-737 lg L for
193-992 lg L1 for
157-737 lg L1 for
193-992 lg L1 for
100 lg L1
38.8-47.2 lg L1
8.0–8.2
38.8-47.2 lg L1
2–3
6.4–7.5
Temp.
(°C)
As(V);
As(III)
As(V);
As(III)
1
Operating condition and models for
calculating adsorption capacity
Adsorption
capacity (mg/g
solids)
As(III)
As(V)
Refs.
25
Batch
89.0
34.46
[33]
25
Batch
29.9
30.48
[33]
22 ± 2
22
Batch & Langmuir
Batch & Langmuir
–
0.043
2.23
[34]
[35]
22
Batch & Langmuir
–
0.16
[35]
0.172
7.0
[36]
[37]
[38]
0.041
Drinking water
Drinking water
Drinking water
7.6
7.9
9.0
1 mg L
<1 mg L1
0-60 mg L1
25
25
22
Batch & Langmuir
Column & Langmuir
Batch
0.180
–
28.0
Drinking water
8–9
5-100 mg L1
25
Column
–
2.8
[39]
Drinking water
8–9
5-100 mg L1
25
Column
–
2.3
[39]
25
Batch & Langmuir
3.48
15.9
[40]
30
Batch & Freundlich
5.4
10.26
[41]
Drinking water
Ground water
5.2 for As(V);
7.0 for As(III)
7.20
1
2.85-11.5 mg L for As(V);
0.79-4.90 mg L1 for As(III)
0.9 mg L1 for As(V);
1.3 mg L1 for As(III)
–
254
X. Xu et al. / Journal of Colloid and Interface Science 445 (2015) 252–261
2. Materials and Methods
2.1. RO concentrate and water quality analysis
RO concentrate was collected from the Kay Bailey Hutchison
Desalination Plant in El Paso, Texas, the largest inland desalination
plant in North America that treats brackish groundwater. The total
dissolved solids (TDS) concentration of the RO concentrate was
10 ± 2.3 g L1. The major ions in the RO concentrate included
sodium (2660 ± 368 mg L1), calcium (673 ± 113 mg L1), chloride
(4993 ± 656 mg L1), and sulfate (1272 ± 226 mg L1), while minor
ions
included
potassium
(69 ± 5.4 mg L1),
magnesium
1
(168 ± 22 mg L ), manganese (332 ± 8.6 lg L1), and arsenic
(63 ± 11 lg L1). The brackish groundwater is chlorinated before
RO desalination for biofouling control; therefore, the arsenic in
the RO concentrate is oxidized to arsenate. The pH of the concentrate was 7.8 ± 0.4, the alkalinity 388 ± 6 mg L1 as CaCO3, and
the dissolved organic carbon (DOC) concentration in the RO concentrate was 4.1 ± 2.6 mg L1.
Throughout the study, all RO concentrate and treated samples
were diluted to levels suitable for analysis with the analytical
instruments. Sample collection and handling followed the guidelines in Section 1060 of Standard Methods [42]. Water samples
were filtered through 0.45 lm cellulose acetate filters (Toyo Roshi
Kaisha, Ltd., Japan) when applicable.
Common ions, including sodium, calcium, potassium, magnesium, chloride, phosphate, and sulfate were measured using ion
chromatography (ICS-2100, Dionex, Sunnyvale, CA, USA).
Concentrations of aluminum, arsenic, boron, chromium, copper,
iron, manganese, lead, and selenium were quantified using inductively coupled plasma mass spectrometry (ICP-MS PerkinElmer,
Elan DRC-e, Waltham, MA, USA). Alkalinity was measured using a
digital titrator (Hach, Colorado, USA) and titrated with 1.6 N sulfuric acid standard solutions to pH endpoint of 4.6. The pH was measured using a portable pH meter (Oakton 300 Series, Eutech
Instruments, Singapore), and electrical conductivity was measured
using a benchtop conductivity meter (Model 431-61, Cole-Parmer,
Vernon Hills, IL). DOC was analyzed using a TOC Analyzer (Shimadzu TOC-L, Kyoto, Japan), and TDS concentration was quantified by
evaporation at 180 °C after filtering the RO concentrate samples
through 0.45 lm filters.
2.2. DWTS and characterization
Two types of dewatered DWTS were studied to compare their
arsenate sorption capacities. Surface water DWTS (referred to as
SDWTS) was collected from a conventional surface water treatment plant in Colorado, USA, which uses coagulation, flocculation,
sedimentation, and green sand filtration to treat water diverted
from a creek to produce potable water. Ferric sulfate (20–
50 mg L1) is used as a coagulant to remove turbidity and natural
organic matter (NOM) from the river water. The SDWTS was collected from the dewatering filter press of the treatment plant.
Groundwater DWTS (referred to as GDWTS) was collected from a
groundwater treatment plant in Texas, USA, which removes NOM
and naturally occurring arsenic (concentrations in the range of
11–16 lg L1) with chlorination, coagulation using ferric chloride
(9–15 mg L1), followed by sand filtration. The filter backwash
GDWTS was collected from sand drying beds in the treatment
plant.
To compare the impact of moisture content (i.e., hydration of iron
oxide) on sorption capacity, both wet SDWTS (dewatered SDWTS)
and dry SDWTS (dried at 105 °C for 48 h) were investigated. The
water content of the DWTS was measured by standard thermal
evaporation method in an oven (OF-01E, Jeio Tech, Korea) at
105 °C for 24 h. The organic content was measured using ignition
test by heating the DWTS at 550 °C for 8 h in a muffle furnace (Furnace Vulcan 3–550, Dentsply International Inc., PA, USA). The elemental composition of the DWTS was analyzed using the acid
digestion method, namely mixing of 0.2 g DWTS with 5 mL 67–
70% nitric acid concentrate (Fisher Scientific, Canada), 10 mL deionized water, and 2 mL 12.1 N hydrochloric acid (Fisher Scientific,
Canada), followed by digestion in a microwave oven (Multiwave
3000, Anton Paar, Austria) [43]. The filtered digested samples were
then analyzed using ICP-MS. X-ray diffraction (XRD, MiniFlex II, Rigaku, Japan) was used for mineralogical analysis of the DWTS.
To quantify the contribution of alkalinity from the DWTS to the
water, the alkalinity of the suspension equilibrated with the DWTS
was determined by titration. Deionized water or a 10 g L1 NaCl
solution was used to simulate the leaching of alkalinity from the
DWTS to fresh water and the RO concentrate, respectively. Two
grams of the DWTS were mixed with 20 mL of deionized water
or 10 g L1 NaCl solution and stirred for 24 h before measuring
alkalinity.
The pH at the point of zero charge (pHPZC) was measured to
investigate the impact of DWTS charge on arsenate sorption. The
salt titration (ST) method was modified in the study based on the
methods developed by Sakurai et al. [44] and Jain et al. [45]. Two
grams of the DWTS were mixed with 20 mL of 0.02 M or 0.2 M NaCl
solution. Then 1 mL of 0.3 N HCl solution was added to each DWTS
suspension. The DWTS aqueous suspensions were stirred for 1 h
each day and equilibrated for 4 days. The equilibrium pH values
of the DWTS suspensions were measured every 24 h (pH1 for
0.02 M NaCl solution, pH2 for 0.2 M NaCl solution). The value of
DpH = pH2 pH1 was calculated, and the graph of DpH versus
pH1 was plotted to determine pHPZC at which DpH = 0. All the
DWTS samples were characterized using triplicate measurements.
2.3. Continuous-flow once-through column adsorption tests and
calculations
Continuous-flow sorption and leaching experiments were conducted in column reactors (5 cm in diameter and 30 cm long),
which were packed with 200 g wet DWTS or 42 g dry DWTS (with
the same amount of dry solids mass). Washed gravel was packed at
the bottom of the columns for water to percolate through (approximately 3 cm thickness). Wet DWTS were packed directly in the
columns. Dry solids were crushed and sieved to particle sizes
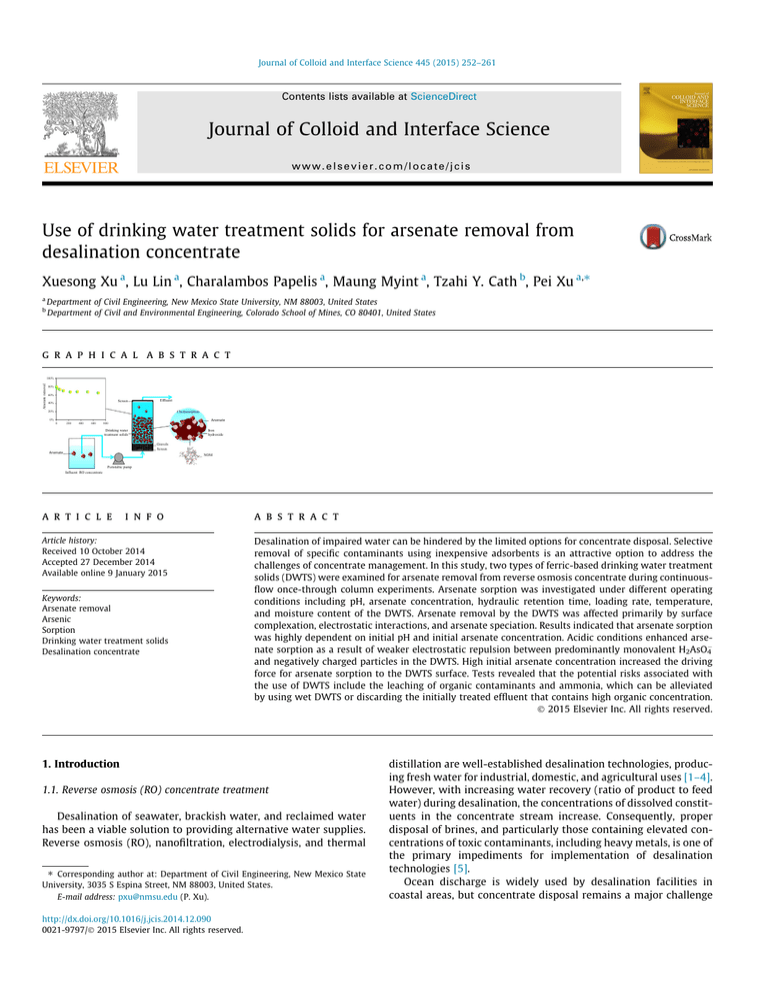
between 0.8 and 2.0 mm before packing. The schematic of the testing system is shown in Fig. 1.
Leaching tests were conducted to determine the leachability of
constituents from the SDWTS to deionized water. Dissolved metal
and metalloid ions (aluminum, arsenic, calcium, iron, magnesium,
Screen
Effluent
DWTS
Gravel
Screen
Peristaltic pump
Influent: RO concentrate
Fig. 1. Schematic of the continuous-flow column testing system.
255
X. Xu et al. / Journal of Colloid and Interface Science 445 (2015) 252–261
manganese, potassium, selenium, and sodium), organics (DOC),
inorganic ions (ammonium, bromide, fluoride, chloride, nitrate,
nitrite, phosphate, and sulfate), electrical conductivity, and pH
were measured at 0, 0.5, 2, 4, 8, 12, 18, and 24 h, during the continuous-flow leaching test.
Sorption experiments were conducted by pumping RO concentrate from a 20 L tank to the bottom of the column by a peristaltic
pump with Masterflex L/S Variable-Speed Drive (Cole-Parmer, Illinois, USA) at flow rates of 11–33 mL min1. The effects of operating
conditions on sorption processes, including influent pH, moisture
content, different DWTS, columns-in-series, and temperature, were
investigated. Effluent samples were collected at 0, 0.5, 2, 4, 8, 12,
18, and 24 h, and once a day for the following 15 days, aiming at
monitoring long-term removal efficiency. It should be noted that
the sorption experimental results throughout the study were
highly reproducible with percent removal standard deviation in
the range of 1–3% for duplicate samples.
The number of bed volumes (dimensionless), mass loading rate
(MLR, L kg1 h1), hydraulic retention time (HRT, min), arsenate
percent removal (%), and uptake rate (lg g1 h1) were calculated
as follows:
Bed volumes ¼ Volume of water processed=solids volume
Mass loading rate; MLR ¼ Water flow rate=mass of dry solids
Hydraulic retention time; HRT ¼ Solids volume=water flow rate
Percent removal ¼ ½ðFeed concentration
effluent concentrationÞ=feed concentration
100
Arsenate uptake rate ¼ ðFeed concentration
effluent concentrationÞ
Water flow rate =mass of dry solids
3. Results and discussion
3.1. Characteristics of the DWTS
The water and organic contents of the wet SDWTS were 78.9%
and 4.64%, respectively, and the organic content was 22% for the
dry SDWTS. The water and organic contents of the GDWTS were
4% and 14%, respectively. The major metals in the solids included
manganese, aluminum, iron, calcium, and magnesium (Table 2). It
should be noted that iron is not the dominant element in the DWTS
although both treatment plants use ferric salts as primary coagulants. The higher amount of aluminum in the DWTS is attributed
to aluminosilicate minerals (in silt, sand and clay) retained during
the coagulation/flocculation/sedimentation and filtration processes.
High manganese content in the SDWTS is attributed to manganese
precipitation from the surface water in which manganese concentration is 0.2–0.5 mg L1. The detected trace inorganic constituents
concentrations for the SDWTS included copper (360 ± 71 lg g1),
chromium (45 ± 5.2 lg g1), lead (27 ± 1.6 lg g1), arsenic
(22 ± 0.6 lg g1), and selenium (4.4 ± 0 lg g1); and the detected
trace inorganic constituents concentrations for the GDWTS included
copper (288 ± 0 lg g1), chromium (119 ± 2.1 lg g1), lead
(2.8 ± 0.2 lg g1), arsenic (43 ± 1.3 lg g1), and selenium
(1.6 ± 0 lg g1).
The total alkalinity contributed from the dry and wet SDWTS
was similar (1.2–1.7 mg as CaCO3 per g dry solids), while the alkalinity contribution of the GDWTS (4.9–6.9 mg as CaCO3 per g dry
Table 2
Organic carbon and major metals in the DWTS (in mg per gram of dry solids mass).
Organic carbon
Al
Ca
Fe
Mg
Mn
Dry SDWTS
Dry GDWTS
220
17 ± 0.4
2.5 ± 0.8
3.3 ± 0.2
2.4 ± 0.2
30 ± 0.2
140
53 ± 1.4
17 ± 0.9
1.1 ± 0.0
13 ± 0.5
1.0 ± 0.0
solids) was approximately 4–5 times higher than that of the
SDWTS. The SDWTS was more acidic than the GDWTS with the
pHPZC in the order of dry SDWTS (4.8), wet SDWTS (5.4), and
GDWTS (6.9). The pHPZC values were similar to those reported by
Zhou et al. [46] (5.6–5.7) for aluminum-derived DWTS. As the
major constituents of DWTS were organic matter and aluminosilicate particles, the pHPZC values of the DWTS differed from that of
iron oxide precipitates, which is in the range of 7.5–9 [47]. Indeed,
the absence of characteristic peaks corresponding to crystalline
ferric hydroxide phases from X-ray diffraction spectra is consistent
with the disordered nature of the SDWTS and GDWTS phases.
3.2. Leaching of chemical constituents from the SDWTS
Sodium, phosphate, potassium, aluminum, manganese, ammonium, chloride, sulfate, and nitrate were detected in all or some
leachates of the SDWTS, while no arsenic, bromide, calcium, fluoride, iron, magnesium, nitrite, or selenium were detected in any
of the SDWTS leachates. Higher amounts of organics and inorganic
constituents leached from the dry SDWTS than from the wet
SDWTS, as shown in Fig. 2 and Table 3, and their concentrations
decreased significantly with processed bed volumes of deionized
water. Substantial amount of organics leached from the dry
SDWTS; the DOC concentration reached 140 mg L1 at the beginning of the testing and declined to below 10 mg L1 after 120
bed volumes. In contrast, less than 6 mg L1 DOC leached from
the wet SDWTS throughout the testing, that is 0.79 mg carbon leached per gram of dry solid mass, whereas the organics leached
from the dry DWTS was 8 times higher (6.74 mg carbon leached
per gram of dry SDWTS). Similarly, 62 mg L1 ammonium leached
from the dry SDWTS at the beginning of the testing as compared to
22 mg L1 from the wet SDWTS, resulting in average of 1.5 and
1.08 mg ammonium per gram of dry solids mass leached from
the dry and wet SDWTS, respectively. Nitrate concentration was
below 5 mg L1 in the leachates of the dry SDWTS whereas it
was below 0.1 mg L1 for the wet SDWTS throughout the testing.
Because ammonium was not detected during surface water treatment, the high amount of ammonium in the SDWTS is the result
of the decomposition of organic matter in the solids. The pH of
the effluent was slightly acidic, increasing from 5.76 to 7 during
the leaching test.
The leaching tests indicate that the potential risks from the use
of the SDWTS are primarily associated with the leaching of organic
constituents and ammonia. The risks can be alleviated when wet
SDWTS is used as adsorbent, or the initial treated effluent with
high organic content is discarded. The leaching results are consistent with previous studies [29,30] showing that arsenic is firmly
bonded to solids and does not leach from the wet and dry SDWTS
to deionized water. In this study DOC was used as an indicator to
characterize the leaching of organic constituents that are composed of mainly NOM removed from surface water sources. However, it is worth identifying the composition of organic carbon
further, using advanced analytical methods to understand the
potential risks of organic leachate from the SDWTS.
256
X. Xu et al. / Journal of Colloid and Interface Science 445 (2015) 252–261
160
70
dry SDWTS
wet SDWTS
140
120
50
NH4+ (mg·L-1)
DOC (mg·L-1)
dry SDWTS
wet SDWTS
60
100
80
60
40
30
40
20
20
10
0
0
0
100
200
300
400
500
600
700
0
100
200
18
dry SDWTS
wet SDWTS
80
500
600
700
dry SDWTS
wet SDWTS
16
70
14
60
K (mg·L-1)
Mn (mg·L-1)
400
Bed volumes
Bed volumes
90
300
50
40
12
10
8
30
6
20
4
10
2
0
0
0
100
200
300
400
500
600
0
700
100
200
Bed volumes
300
400
500
600
700
Bed volumes
35
dry SDWTS
wet SDWTS
200
160
120
25
20
15
80
10
40
5
0
dry SDWTS
wet SDWTS
30
Cl- (mg·L-1)
SO42- (mg·L-1)
240
0
0
100
200
300
400
500
600
700
0
100
200
300
400
500
600
700
Bed volumes
Bed volumes
Fig. 2. Leaching of organics (DOC) and ions (manganese, sulfate, ammonium, potassium, and chloride) from dry and wet SDWTS as a function of bed volumes at 23 °C.
3.3. Effect of pH on arsenate sorption
H3 AsO4 H2 AsO4 þ Hþ ðpKa1 ¼ 2:19Þ
The impact of pH on arsenate sorption to the DWTS was
investigated at pH values of 5.5 (adjusted with hydrochloric acid
solution) and 7.8 (ambient pH of the RO concentrate). Many RO
systems operate at acidic pH to prevent membrane scaling. The
testing pH values of 5.5 and 7.8 are within the operating pH
range of typical RO concentrates in desalination facilities. The
pH of the solution also affects the charge of DWTS, resulting
in different electrostatic interactions between arsenate and the
solids.
Arsenate sorption efficiency increased substantially when the
pH of the RO concentrate decreased from 7.8 to 5.5 (Fig. 3). The
solution pH affects the speciation of arsenate in aqueous solutions
(controlled by the acid dissociation constant, pKa) and the surface
charge of DWTS (determined by the pHpzc). As described by the
three equilibrium reactions, arsenate species exist primarily as H22
AsO
4 (3 < pH < 7) or HAsO4 (7 < pH < 11) [48]:
þ
H2 AsO4 HAsO2
4 þ H ðpKa2 ¼ 6:94Þ
3
þ
HAsO2
4 AsO4 þ H ðpKa3 ¼ 11:5Þ
Table 3
Total amount of detected chemical constituents leached from the SDWTS (in mg per
gram of dry solids mass).
DOC
Al
Na
K
Mn
NH+4
Cl
NO
3
SO2
4
Dry SDWTS
Wet SDWTS
6.74
0.013
2.38
0.50
5.12
1.50
2.84
0.825
7.83
0.79
0.011
0.93
0.28
0.89
1.08
0.80
0.011
3.33
257
X. Xu et al. / Journal of Colloid and Interface Science 445 (2015) 252–261
100%
100%
(a)
90%
80%
80%
Percent removal
Percent removal
(b)
90%
70%
60%
50%
40%
30%
20%
70%
60%
50%
40%
30%
20%
pH 7.8
pH 5.5
10%
pH 7.8
pH 5.5
10%
0%
0%
0
100 200 300 400 500 600 700 800
0
10
20
Bed volumes
30
40
50
60
70
80
90
Bed volumes
100%
(c)
90%
Percent removal
80%
70%
60%
50%
40%
30%
20%
pH 7.8
pH 5.5
10%
0%
0
100 200 300 400 500 600 700 800
Bed volumes
Fig. 3. Percent arsenate removal as a function of bed volumes and pH at 23 °C by (a) dry SDWTS at an MLR of 23.5 L kg1 h1; (b) wet SDWTS at an MLR of 13.9 L kg1 h1; and
(c) dry GDWTS at an MLR of 23.5 L kg1 h1. Error bars represent the standard deviation of duplicate samples.
3.4. Effect of initial concentration on arsenate sorption
To evaluate the effect of different arsenate concentrations on
DWTS sorption capacity, arsenate (Na2HAsO47H2O, reagent grade,
Fisher Scientific, Fairlawn, NJ) was spiked into the RO concentrate
to simulate high arsenate concentrations in natural groundwater. It
should be noted that the arsenate concentration varied significantly from 39 to 75 lg L1 among the batches of RO concentrate
100%
90%
80%
Percent removal
At pH 5.5, approximately 93% of arsenate exists as H2AsO
4,
while at pH 7.8, approximately 92% as HAsO2
4 . When the pH
increases from 5.5 to 7.8, arsenate is converted from monovalent
to divalent anions, resulting in stronger electrostatic repulsion
between HAsO2
4 ions and the negatively charged DWTS. The surface charge of the DWTS is determined by the transfer reactions
of proton between solution and solids. At pH 5.5, the dry SDWTS
is slightly negatively charged (pHPZC 4.8), the wet SDWTS is near
neutral (pHPZC 5.4), and the GDWTS is positively charged (pHPZC
6.9). Because of the strong inner-sphere complexes formed
between monovalent H2AsO
4 ions and the ferric hydroxide components in the solids, all DWTS maintained high removal efficiency
(greater than 70%) at pH 5.5 after 24 h of continuous operation.
As pH increases to 7.8, the surface charge of the DWTS becomes
increasingly negative. For the dry SDWTS, arsenate percent
removal decreased from 77% to 29%, whereas for the dry GDWTS
it reduced to 12%, after 700 bed volumes of RO concentrate being
processed. Greater electrostatic repulsion between the solids and
divalent H2AsO2
4 ions resulted in lower sorption capacity of the
DWTS, compared to that at pH 5.5.
70%
60%
50%
40%
30%
dry GDWTS, As 408 µg·L-1
dry SDWTS, As 335 µg·L-1
dry GDWTS, As 42 µg·L-1
dry SDWTS, As 75 µg·L-1
20%
10%
0%
0
200
400
600
800
Bed volumes
Fig. 4. Percent arsenate removal by dry SDWTS and GDWTS as a function of bed
volumes and initial arsenate concentration at a pH of 5.5, MLR of 23.5 L kg1 h1,
and 23 °C. Error bars represent the standard deviation of duplicate samples.
collected at different times from the RO desalination plant due to
variation of groundwater quality.
The experiments were conducted with the dry SDWTS and
GDWTS at pH 5.5, MLR 23.5 L kg1 h1, and 23 °C (Fig. 4). At high
arsenate concentrations of 335 lg L1 (dry SDWTS) and 408 lg L1
(GDWTS), the percent removal of arsenate decreased from 95.5% to
78.6% after 700 bed volumes for both DWTS. For low arsenate concentrations of 75 lg L1 (dry SDWTS) and 42 lg L1 (dry GDWTS),
258
X. Xu et al. / Journal of Colloid and Interface Science 445 (2015) 252–261
8.65 lg h1 for dry GDWTS at high arsenate concentrations
(Fig. 5). Despite the differences between the two types of DWTS
with regard to water source, plant operation, and chemical composition, no significant differences were observed using these solids
for sorption of arsenate with various concentrations as shown in
Figs. 4 and 5.
Arsenic uptake rate (µg·g-1·h-1)
10
9
8
7
6
5
3.5. Arsenate sorption during long-term operation
y = 0.0217x - 0.3359
R² = 0.9989
4
3
2
1
0
0
200
400
Initial arsenic concentration
600
(µg·L-1)
Fig. 5. Correlation between initial arsenate concentration and uptake rate by dry
SDWTS and GDWTS at pH 5.5, MLR 23.5 L kg1 h1, and 23 °C.
both DWTS exhibited similar percent removals, declining from
over 80% to 65–69% after 700 bed volumes. With the increase of
arsenate concentration in the RO concentrate, the number of arsenate ions at the solid-solution interface also increases, resulting in
the increase in removal efficiency. Total arsenate sorption capacities (as arsenic) achieved in 24 h continuous operation were normalized to the mass of dry solids (per gram), which were
calculated as 0.029 mg for dry SDWTS and 0.017 mg for dry
GDWTS at low arsenate concentrations; and 0.16 mg for dry
SDWTS and 0.21 mg for dry GDWTS at high arsenate
concentrations.
Higher solute concentration can increase the arsenate concentration gradient between sorbent and solution, thus enhancing
the driving force for arsenate diffusion and sorption from the RO
concentrate to the DWTS pores. Previous study by Papelis demonstrated that the rate of ion sorption on large, porous sorbents, such
as DWTS, could be limited by mass transfer, and specifically by
intraparticle diffusion [49]. The increase in arsenate concentration
in aqueous solution enhanced the driving force for intraparticle
diffusion, thus improving the arsenate uptake rate by the DWTS,
as shown in Fig. 5. Under steady state conditions the average arsenate uptake rate per gram of dry solids mass increased linearly as a
function of initial arsenate concentration, that is, 1.20 lg g1 h1
for dry SDWTS and 0.69 lg g1 h1 for dry GDWTS at low arsenate
concentrations; and 6.82 lg g1 h1 for dry SDWTS and
100%
90%
Percent removal
80%
70%
60%
50%
40%
30%
20%
10%
0%
0
250
500
750
1000 1250 1500
Bed volumes
Fig. 6. Percent arsenate removal by wet SDWTS as a function of bed volumes at a
pH of 5.5, MLR of 13.9 L kg1 h1, and 23 °C. Error bars represent the standard
deviation of duplicate samples.
In order to investigate arsenate removal efficiency for a longer
period of time, a 16-day continuous-flow, once-through experiment was conducted using the wet SDWTS (Fig. 6). During the
experiment, arsenate concentrations in the RO concentrates collected at different times from the desalination plant fluctuated
between 39 and 75 lg L1.
After treating more than 1300 bed volumes of RO concentrate at
pH 5.5, arsenate removal leveled off at 48% and the sorption capacity achieved 0.27 mg (as arsenic) per gram of dry solids. The arsenate sorption capacity for RO concentrate appears lower in this
study than the data reported in the literature. Gibbons and Gagnon
examined arsenic sorption in batch and column experiments using
a low salinity groundwater that had an average arsenic concentration of 43 lg L1 and a pH of 8.1 [35]. In batch sorption experiments, Langmuir isotherm modeling was used to determine that
a ferric-based SDWTS and a commercial granular ferric hydroxide
(GFH) sorbent had maximum sorptive capacities of 2230 and
640 mg As/g dry solids, respectively. In a column sorption experiment, the ferric-DWTS achieved arsenic removal of >26,000 bed
volumes before breakthrough above 10 lg L1, whereas the effluent arsenic concentration from the GFH column was below the
method detection limit at 28,000 bed volumes [35]. Although there
are some differences in hydraulic retention times (12.5 min vs.
3.4 min in this study), initial arsenic concentration (38 lg L1 vs.
66 lg L1 in this study), and column geometry, the major factors
contributing to the lower arsenic sorption capacities in our study
are the significant differences in ferric mass in the DWTS and feed
water quality.
In our study, the amount of iron in the DWTS for arsenate sorption is very low (1.1 mg g1 and 3.3 mg g1 of dry solids for GDWTS
and SDWTS, respectively), approximately 100–500 times lower
than the ferric mass in the study by Gibbons and Gagnon
(277.3 mg g1 and 528.8 mg g1 of dry solids for the DWTS and
GFH) [35]. Such low ferric mass in the DWTS could result in low
arsenic sorption capacity in our study. If the arsenic sorption
capacity is normalized with respect to ferric mass in the solids,
the arsenate sorption capacity of the wet SDWTS in our study
would be at least 82 mg As per g Fe, which falls within the maximum sorption capacity range of 8–158 mg As per g Fe reported
by Gibbons and Gagnon for different types of ferric-based DWTS
and GFH [35].
Another important reason for the low arsenate sorption capacity is the presence of competing anions in the RO concentrate and
the solids, including sulfate and NOM. In the study by Gibbons and
Gagnon, the salt concentration in the feed water was not reported,
but can be assumed to be low because the groundwater was from a
drinking water well. In the RO concentrate, the TDS concentration
was approximately 10 g L1, with sulfate concentration of
1300 mg L1. Given that the molar ratio of S:As is 23,660 in the
RO concentrate, sulfate ions can compete with arsenate ions for
the sorption sites in the DWTS, although sulfate ions are assumed
to form only weak outer-sphere complexes whereas arsenate ions
can form strong inner-sphere complexes.
The NOM present in both RO concentrate and DWTS may play
an important role in arsenate speciation in water and sorption to
the DWTS [50]. NOM, mainly negatively charged at neutral pH,
contains functional groups such as carboxylic, esteric, phenolic,
259
X. Xu et al. / Journal of Colloid and Interface Science 445 (2015) 252–261
100%
90%
Percent removal
80%
70%
60%
50%
40%
30%
T 35℃, pH 5.5
T 35℃, pH 7.8
T 23℃, pH 5.5
T 23℃, pH 7.8
20%
10%
0%
0
50
100
Bed volumes
Fig. 7. Percent arsenate removal by wet SDWTS as a function of bed volumes,
temperatures, and pH at MLR of 13.9 L kg1 h1. Error bars represent the standard
deviation of duplicate samples.
quinone, amino, nitroso, sulfhydryl, hydroxyl, and other moieties
[51]. Besides the competition for sorption sites with arsenate,
NOM could form aqueous and surface inner-sphere complexes
with soluble cationic metals and the NOM-metal complexes could
associate strongly with arsenate in RO concentrate by a metalbridging mechanism, inhibiting arsenate from forming surface
complexes with the DWTS [52]. The average DOC concentration
in the RO concentrate was 4.1 mg L1, and DOC was also leaching
from the DWTS, thus reducing arsenate sorption to the DWTS.
The impact of water chemistry on arsenate adsorption by the
DWTS needs further investigation.
3.6. Effect of temperature on arsenate sorption
The effect of temperature on DWTS sorption was investigated
using the wet SDWTS at MLR of 13.9 L kg1 h1 and pH of 7.8
and 5.5 (Fig. 7). At the initial pH of the RO concentrate, arsenate
removal efficiency increased slightly with increasing temperature,
which is not expected if the chemical interactions between different arsenic species and the DWTS are the rate limiting process for
arsenate sorption. Previous thermodynamic studies by Banerjee
et al. demonstrated that under steady-state the sorption of arsenate and arsenite onto granular ferric hydroxide (GFH) is a spontaneous and endothermic process [32]. The activation energy values
100%
3.7. Effect of hydraulic retention time (HRT)
The effect of HRT on arsenate removal was investigated using
the dry SDWTS at two conditions: (1) at a pH of 7.8 with an HRT
of 1.1 (MLR 46.9 L kg1 h1), 2.1 (MLR 23.5 L kg1 h1), and
3.2 min (MLR 13.9 L kg1 h1) using 42.2 g solids; and (2) pH of
5.5 with an HRT of 2.1 min (MLR 23.5 L kg1 h1) using 42.2 g solids, and 8.1 min (MLR 6.2 L kg1 h1) using 126.6 g solids (Fig. 8).
Longer HRT and lower MLR improved arsenate sorption because
of longer contact time and increased opportunity for interactions
between arsenate and the SDWTS. At pH 7.8 and 350 bed volumes
the average removal efficiencies achieved 32.5%, 46.1%, and 59.8%
for HRTs of 1.1, 2.1, and 3.2 min, respectively. After that, the effect
of HRT on arsenate sorption decreased and leveled off at 20–28%
after additional 1000 bed volumes (Fig. 8a). Similarly, at pH 5.5,
arsenate removal at an HRT of 8.1 min increased the average
removal efficiency by 10% compared to an HRT of 2.1 min
(Fig. 8b). Although longer retention time allowed more opportunities for arsenate to sorb onto the solids, the impact of HRT declined
as the solids saturated with arsenate and other species sorbed from
100%
HRT 1.1 minutes
HRT 2.1 minutes
HRT 3.2 minutes
90%
70%
60%
50%
40%
30%
HRT 2.1 minutes
HRT 8.1 minutes
90%
80%
Percent removal
80%
Percent removal
obtained for arsenate at pH 6.5 was about 35% less than that
obtained at pH 7.5, resulting in a faster adsorption rate and better
removal at pH 6.5 than at pH 7.5. The equilibrium adsorption coefficients of arsenate increased by 335% and 262% at pH 6.5 and 7.5,
respectively, when temperature increased from 20 °C to 40 °C.
Maximum arsenate adsorption capacity of GFH also increased from
3.13 mg/g to 4.57 mg/g with increasing temperature [32]. However, our column experiments did not observe this significant temperature impact on arsenate sorption. At pH 5.5, the removal
efficiency increased by less than 5% for the same bed volumes
when water temperature increased from 23 °C to 35 °C. At pH
7.8, the removal efficiency increased by less than 10%, except at
the beginning of the experiment, where it increased by 13%. The
results are in agreement with the study by Zhou et al. where metal
sorption by DWTS increased by less than 5% between 20 °C and
60 °C [46].
Banerjee’s et al. study was conducted at batch operation under a
steady-state and an equilibrium condition (24 h), whereas our column experiments were conducted under a non-steady state with
an HRT of 3.2 min. It suggests that the continuous-flow arsenate
sorption process is diffusion-controlled, in which the activation
energies would be substantially lower than the chemosorption
process, therefore major temperature effects would not be
expected.
70%
60%
50%
40%
30%
20%
20%
10%
10%
(a)
(b)
0%
0%
0
300
600
900
Bed volumes
1200
1500
0
200
400
600
800
Bed volumes
Fig. 8. Percent arsenate removal by wet SDWTS as a function of bed volumes and HRT at 23 °C and (a) pH of 7.8 and (b) pH of 5.5. Error bars represent the standard deviation
of duplicate samples.
260
X. Xu et al. / Journal of Colloid and Interface Science 445 (2015) 252–261
While the disposal of used DWTS with high arsenic content was
not evaluated in this study, this is an important issue that needs
further investigation.
100%
90%
Percent removal
80%
70%
Acknowledgments
60%
50%
40%
30%
20%
10%
0%
dry SDWTS, pH 7.8
dry SDWTS, pH 5.5
wet SDWTS, pH 7.8
wet SDWTS, pH 5.5
0 2 4 6 8 10 12 14 16 18 20 22 24 26
Volume processed (L)
Fig. 9. Percent arsenate removal by dry and wet SDWTS as a function of bed
volumes and pH of 5.5 and 7.8, at 23 °C. Error bars represent the standard deviation
of duplicate samples.
the RO concentrate. Thus, the differences in percent removal
decreased as the number of bed volumes increased.
3.8. Effect of moisture content of the SDWTS
The moisture content of DWTS changes over time and depends
on the dewatering/drying processes in a water treatment plant.
Dry DWTS may be preferred because of the reduced solid volume
that needs to be transported. The impact of the DWTS moisture
content on arsenate removal was evaluated with the wet SDWTS
(MLR 13.9 L kg1 h1) and the dry SDWTS (MLR 23.5 L kg1 h1)
at pH 5.5 and 7.8, and 23 °C (Fig. 9). The wet SDWTS exhibited
slightly higher arsenate removal efficiency than the dry SDWTS
by an average of 6% and 12% at pH 5.5 and 7.8, respectively, during
a 24-h continuous operation.
Water contained in the wet SDWTS facilitated the hydrolysis of
iron, aluminum, and cations on the surface, contributing to higher
sorption efficiency. The lower removal efficiency of the dry SDWTS
could be attributed to the loss of surface-active hydroxyl sites after
drying at 105 °C, or the air trapped in the dry solids, which prevents the contact between the concentrate and the solids. As sorption time passes, the dry SDWTS is wetted by the RO concentrate
and should behave similarly to the wet SDWTS after saturation.
This assumption is consistent with the experimental results; at
pH 5.5 and 7.8, the removal efficiency of the dry SDWTS and the
wet SDWTS overlapped after treating approximately 14 L and
10 L of the RO concentrate, respectively.
4. Conclusions
This study demonstrates that DWTS is effective for removing
arsenate from RO concentrate. Arsenate sorption by the DWTS is
mainly affected by pH and initial arsenate concentration, while
hydraulic retention time, temperature, and moisture of the DWTS
have marginal impacts on arsenate removal. The two types of
DWTS show negligible differences in arsenate sorption, although
higher ferric mass in DWTS is anticipated to enhance sorption
capacity. The competition by other ions in the RO concentrate
may hinder the sorption of arsenate on DWTS. The leaching tests
indicate that the potential risks associated with the use of DWTS
include the leaching of organic contaminants and ammonia. The
risks can be alleviated when wet DWTS is used as sorbent, or the
initially treated effluent with high organic content is discarded.
Support for this study was provided by the National Science
Foundation Engineering Research Center Program under
Cooperative Agreement EEC-1028968 (ReNUWIt). The authors
thank John Balliew, Hector Gonzalez and Mike Fahy with El Paso
Water Utilities, and Ashley Dalton with Golden Drinking Water
Treatment Plant for technical support. The authors also acknowledge Mark Chidester with New Mexico State University for
assistance in chemical analysis.
References
[1] C. Charcosset, Desalination 245 (2009) 214–231.
[2] N. Ghaffour, T.M. Missimer, G.L. Amy, Desalination 309 (2013) 197–207.
[3] P. Xu, J.E. Drewes, C. Bellona, G. Amy, T.-U. Kim, M. Adam, T. Heberer, Water
Environ. Res. 17 (2005) 40–48.
[4] P. Xu, J.E. Drewes, D. Heil, Desalination 225 (2008) 139–155.
[5] P. Xu, M. Capito, T.Y. Cath, J. Hazard. Mater. 260 (2013) 885–891.
[6] P. Xu, T. Cath, A.P. Robertson, M. Reinhard, J.O. Leckie, J.E. Drewes, Environ. Eng.
Sci. 30 (2013) 502–514.
[7] M. Matsui, C. Nishigori, S. Toyokuni, J. Takada, M. Akaboshi, H. Ochi, M.
Ishikawa, S. Imamura, J. Dermatol. Sci. 16 (Supplement 1) (1998) S54.
[8] R.C. Dart, Medical Toxicology, Williams & Wilkins, Lippincott, 2004.
[9] T. Viraraghavan, K.S. Subramanian, J.A. Aruldoss, Water Sci. Technol. 40 (1999)
69–76.
[10] E.O. Kartinen Jr, C.J. Martin, Desalination 103 (1995) 79–88.
[11] R.Y. Ning, Desalination 143 (2002) 237–241.
[12] R. Güell, C. Fontàs, V. Salvadó, E. Anticó, Sep. Purif. Technol. 72 (2010) 319–
325.
[13] S.K. Maji, A. Pal, T. Pal, J. Hazard. Mater. 151 (2008) 811–820.
[14] K.C. Makris, D. Sarkar, J.G. Parsons, R. Datta, J.L. Gardea-Torresdey, J. Hazard.
Mater. 171 (2009) 980–986.
[15] C. Wang, Y. Qi, Y. Pei, Chem. Eng. J. 209 (2012) 379–385.
[16] C. Wang, Z. Wang, L. Lin, B. Tian, Y. Pei, J. Hazard. Mater. 203–204 (2012) 145–
150.
[17] C. Wang, Y. Pei, Chemosphere 88 (2012) 1178–1183.
[18] E. Lombi, D.P. Stevens, M.J. McLaughlin, Environ. Pollut. 158 (2010) 2110–
2116.
[19] L. Lin, X. Xu, C. Papelis, T.Y. Cath, P. Xu, Sep. Purif. Technol. 134 (2014) 37–45.
[20] A.O. Babatunde, Y.Q. Zhao, Crit. Rev. Environ. Sci. Technol. 37 (2007) 129–164.
[21] M.L. Pierce, C.B. Moore, Water Res. 16 (1982) 1247–1253.
[22] K.C. Makris, D. Sarkar, R. Datta, Chemosphere 64 (2006) 730–741.
[23] M.K. Gibbons, G.A. Gagnon, Water Res. 44 (2010) 5740–5749.
[24] S.K. Gupta, K.Y. Chen, J. Water Poll. Contr. Fed. 50 (1978) 493–506.
[25] R.D. Wauchope, J. Environ. Qual. 4 (1975) 355–358.
[26] N.T. Livesey, P.M. Huang, Soil Sci. 131 (1981) 88–94.
[27] D.D. Sarkar, K.C. Makris, M.T. Parra-Noonan, R. Datta, Environ. Int. 33 (2007)
164–169.
[28] K.C. Makris, W.G. Harris, G.A. O’Connor, T.A. Obreza, H.A. Elliott, Environ. Sci.
Technol. 39 (2005) 4280–4289.
[29] K.C. Makris, D. Sarkar, J.G. Parsons, R. Datta, J.L. Gardea-Torresdey, J. Hazard.
Mater. 171 (2009) 980–986.
[30] K.C. Makris, D. Sarkar, J.G. Parsons, R. Datta, J.L. Gardea-Torresdey, J. Colloid
Interface Sci. 311 (2007) 544–550.
[31] M. Shafiquzzaman, M.S. Azam, J. Nakajima, Q.H. Bari, Desalination 261 (2010)
41–45.
[32] K. Banerjee, G.L. Amy, M. Prevost, S. Nour, M. Jekel, P.M. Gallagher, C.D.
Blumenschein, Water Res. 42 (2008) 3371–3378.
[33] J. Pattanayak, K. Mondal, S. Mathew, S.B. Lalvani, Carbon 38 (2000) 589–596.
[34] O.S. Thirunavukkarasu, T. Viraraghavan, K.S. Subramanian, Water Air Soil Poll.
142 (2003) 95–111.
[35] M.K. Gibbons, G.A. Gagnon, Water Res. 44 (2010) 5740–5749.
[36] T.S. Singh, K.K. Pant, Sep. Purif. Technol. 36 (2004) 139–147.
[37] S. Ouvrard, M.-O. Simonnot, M. Sardin, Ind. Eng. Chem. Res. 41 (2002) 2785–
2791.
[38] V. Lenoble, O. Bouras, V. Deluchat, B. Serpaud, J.-C. Bollinger, J. Colloid Interf.
Sci. 255 (2002) 52–58.
[39] B. Daus, R. Wennrich, H. Weiss, Water Res. 38 (2004) 2948–2954.
[40] T.-F. Lin, J.-K. Wu, Water Res. 35 (2001) 2049–2057.
[41] G.S. Murugesan, M. Sathishkumar, K. Swaminathan, Bioresour. Technol. 97
(2006) 483–487.
[42] Standard Methods for the Examination of Water & Wastewater, American
Public Health Association, American Water Works Association, and Water
Environment Federation, 2005.
[43] J.M. Cook, M.J. Gardner, A.H. Griffiths, M.A. Jessep, J.E. Ravenscroft, R. Yates,
Mar. Pollut. Bull. 34 (1997) 637–644.
X. Xu et al. / Journal of Colloid and Interface Science 445 (2015) 252–261
[44]
[45]
[46]
[47]
[48]
K. Sakurai, Y. Ohdate, K. Kyuma, Soil Sci. Plant Nutr. 34 (1988) 171–182.
A. Jain, K.P. Raven, R.H. Loeppert, Environ. Sci. Technol. 33 (1999) 1179–1184.
Y.-F. Zhou, R.J. Haynes, Water Air Soil Pollut. 215 (2010) 631–643.
G. Sposito, The Surface Chemistry of Soils, Oxford University Press, 1984.
M. Habuda-Stanić, B. Kalajdžić, M. Kuleš, N. Velić, Desalination 229 (2008) 1–9.
261
[49] C. Papelis, Environ. Sci. Technol. 29 (1995) 1526–1533.
[50] B.T. Hart, Environ. Technol. Lett. 2 (1981) 95–110.
[51] A.D. Redman, D.L. Macalady, D. Ahmann, Environ. Sci. Technol. 36 (2002)
2889–2896.
[52] P. Thanabalasingam, W.F. Pickering, Environ. Pollut. 12 (1986) 223–246.





