Breeding biologies, seed production and species-rich bee guilds of (Cleomaceae) Cleome lutea
advertisement

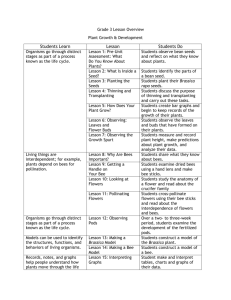
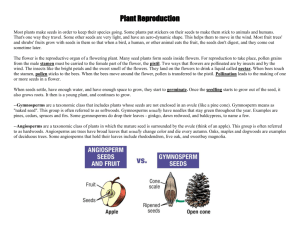
Plant Species Biology (2008) 23, 152–158 doi: 10.1111/j.1442-1984.2008.00224.x Breeding biologies, seed production and species-rich bee guilds of Cleome lutea and Cleome serrulata (Cleomaceae) JAMES H. CANE US Department of Agriculture–Agricultural Research Service, Bee Biology and Systematics Laboratory, Utah State University, Logan 84322–5310, United States of America Abstract The summer-blooming annual forbs Cleome lutea and Cleome serrulata (Cleomaceae) are native across the US Intermountain West and Rocky Mountains, respectively. Their farmed seed is sought to help rehabilitate western rangelands in those regions. This study of the reproductive biologies and pollinator faunas of C. lutea and C. serrulata is the first for this cosmopolitan family, the sister family to the Brassicaceae. Unlike the S-allele self-incompatibility systems of some Brassicaceae, both species of Cleome were found to be self-fertile and capable of some autogamy. Compared with selfing, outcrossing did not enhance seed set, seed viability or seedling vigor for either species (in fact, selfed progeny were more robust). Large, openly visited plants yielded >20 000 seeds each. Like several species of the sister family Capparaceae, flowers of both species first shed their pollen, secreted nectar and became receptive nocturnally. Although no nocturnal visitors were found, both Cleome species attracted a diverse array of diurnal native bees, wasps and butterflies. Among the many floral generalist bees that work Cleome flowers for pollen and nectar are two managed agricultural pollinators, Apis mellifera and Megachile rotundata. These observations bode well for pollinating C. lutea and C. serrulata in small commercial seed fields. It appears that diverse wild bees would benefit from the addition of native Cleome to restoration seed mixes, with the objective of sustaining native pollinator faunas during the first few years of postfire plant community rehabilitation. Keywords: Apiformes, Brassicaceae, Capparaceae, pollination, seedling fitness, self-compatibility. Received 3 January 2008; accepted 24 July 2008 Introduction The small cosmopolitan eudicot family Cleomaceae (300 species) is the sister family to the more diverse Brassicaceae; it also shares characters with the Capparaceae (= Capparidaceae), with which it has been formerly classified (Hall et al. 2002). Dominating the family Cleomaceae are the 180–200 species of the type genus Cleome (called ‘spider-flowers’ or ‘bee-plants’) that occur in many warmer regions of the world (Iltis 1957; Sanchez-Acebo 2005). Some are annual forbs common in disturbed habitats; others are woody, but short-lived. Knowledge of the breeding biologies of Cleome species might be relevant to Correspondence: James H. Cane Email: jim.cane@ars.usda.gov the evolution of self-incompatibility systems in the Brassicaceae, and is certainly needed to successfully farm Cleome seed for large-scale rangeland restoration projects. Native species of Cleome might have a unique role to play in plant community restoration in xeric valleys and plains of the US Intermountain West. Rehabilitating western USA rangeland plant communities has long included reseeding, but primarily with grasses and shrubs. Seed of several native forbs are now being grown commercially for this purpose, with more to follow (Cane 2008), but these are all herbaceous perennials that typically do not flower in the year after seeding. In contrast, Cleome are annuals that can provide bloom quickly and, if used by a diverse array of wild bees, might help sustain pollinator communities the year after an autumn restoration seeding. Larger quantities of affordable seed from Journal compilation © 2008 The Society for the Study of Species Biology No claim to original US government works P O L L I N AT I O N N E E D S O F CLEOME native Cleome species, if available from seed farmers, could be added to seed mixes that are used to rehabilitate vast burned rangelands of the Great Basin and neighboring biomes of western USA (Cane 2008). Farming seed crops generally requires pollinator supplementation to realize potential seed yields (Free 1993), but how much can depend on a plant species’ breeding biology. The Brassicaceae, sister group to the Cleomaceae, has a distinctive sporophytic incompatibility (SI) system (Charlesworth et al. 2005) and only pollen flow between plants sets seed. However, species of Cleome are early successional species at disturbed sites; such attributes in other species are often associated with selffertility and autogamy (Baker & Stebbins 1965). Thus, ecological versus phylogenetic inferences give contrasting predictions for the pollination needs of Cleome. To evaluate the pollination needs of a plant, the breeding biology of the plant must be understood. The objectives of the present study were to characterize the floral and breeding biologies of two of the six species in Cleome section Peritoma (Iltis 1957), Cleome lutea (yellow or Nevada bee-plant) and Cleome serrulata (Rocky Mountain bee-plant). The necessity or benefit of pollinators and cross-pollination was evaluated for producing fruits, fertile seeds and vigorous progeny. Flowering phenology and the timing of stigma receptivity and anther dehiscence were characterized. Data from museum specimens and limited field collections of flower visitors to these plants were also compiled to document pollinator species richness and the attributes of the pollinator guilds. Materials and methods Traits of Cleome lutea and Cleome serrulata Both species are robust, tap-rooted annuals native to the xeric valleys and plains of western North America. They are typically found at disturbed sites, such as waste places, margins of washes or barren sandy desert plains (Iltis 1957). Owing to its beauty, C. serrulata has been cultivated in gardens in and beyond its native range in western USA. Plants of both species are erect (3–25 dm tall), glabrous and malodorous. They invariably produce one or more compact, bracteolate terminal racemes. Individual racemes are indeterminate, continually producing new flowers for weeks during the summer. The round seeds (2–4 mm in diameter) are borne in siliques. The seeds lack an endosperm (Sanchez-Acebo 2005) and require moist cold stratification for embryo development and germination. Flowering Individual plants of both Cleome species produce two types of like-sized showy flowers (yellow for C. lutea and Plant Species Biology 23, 152–158 153 Fig. 1 Flower, fruits and seeds of Cleome lutea. Shown are the pistil atop its gynophore and the dehiscing anthers of this hermaphroditic flower, an immature and mature fruit (silique), and its dehiscent valves removed to reveal the loop-like replum and the content of mature dark seeds. Inset: X-ray positive of C. lutea seeds showing viable (X-ray dense) and non-viable (gray) embryos. magenta for C. serrulata). Hermaphroditic flowers have six stamens and a large pistil atop a stalked gynophore (Fig. 1). Staminate flowers have rudimentary pistils that never set pods (Stout 1923). However, Cleome are not andromonoecious. A staminate flower begins as a hermaphroditic bud; its pistil thereafter fails to develop fully. When a raceme is shunting resources to many maturing fruits, most of its flowers become staminate. Over the long flowering season, racemes alternate between the production of staminate and hermaphroditic flowers (Murneek 1937); thus, maturing siliques and shedding seed while continuing to bloom. Daily blooming phenologies were observed on plants grown in two common gardens in Logan, Utah, USA (41°45′ N, 111°48′ W). To judge stigma receptivity, excised pistils were individually inserted into a Pasteur pipette tip filled with hydrogen peroxide (J. Thomson, pers. comm., 1999). Receptivity was indicated by the visible generation of oxygen bubbles on the bare stigma, the result of peroxidase activity (Zeisler 1938). Breeding biology Seeds were commercially collected and pooled from wild populations in Utah. The seeds were shallowly planted outdoors in autumn 2003 at the common garden of the Bee Biology and Systematics Laboratory in Logan, Utah, USA. The following summer, the clay loam soils were infrequently irrigated as needed to maintain plant vigor. For each of the four pollination treatments, six well-separated individuals per species of similar size, vigor and bud Journal compilation © 2008 The Society for the Study of Species Biology No claim to original US government works 154 J . H . C A N E development were chosen, randomly assigned their treatment, and tagged. These plants were enclosed in 7 m ¥ 7 m ¥ 2 m walk-in field cages made of Lumite screening (Synthetic Industries, Chicopee, GA, USA) to exclude flower visitors. Individual pollinator exclusion bags were not used because, in earlier trials, we found that flowers on the crowded tall racemes of Cleome transfered pollen by passively rubbing or jostling against the bag netting. Once the flowers began to shed pollen, each new flower on every tagged raceme was marked and pollinated. During June and July, two manual pollination treatments were applied to newly opened flowers of the caged plants: geitonogamy (transfer of self pollen) and xenogamy (outcrossing). As younger crowded plants often produced single racemes, whose flowers proved difficult to tag individually, different plants received single treatments. Geitonogamous pollination involved rubbing the day’s recipient virgin stigmas with fresh anthers from an untagged flower from the same plant. Donor anthers for xenogamy were taken from untagged plants. Optical magnifying visors were used as necessary to visually confirm pollen transfer. Floral racemes were manually pollinated daily until a plant shifted from producing hermaphroditic to staminate flowers. Six other plants per species in the cages served as controls for autonomous self-pollination (autogamy). Apomixis was impractical to evaluate because the flowers would have needed to be nightly emasculated when they first opened. Adjacent to the pollination treatment cages, six additional tagged plants per species were used as positive controls that were freely visited by pollinators. The pollination treatments were compared by species using general linear model anova tests for percentage fruit (silique) set (arcsine or log10 transformed) and for their yields of dark seeds per silique. In general, only the dark seeds possessed developed embryos. The data transformations yielded acceptable normality (Shapiro–Wilk statistic, P > 0.01 or better) and homogeneous variances (Levene’s test, P > 0.04 or better). When differences were found among treatments (P ⱕ 0.05), the treatments were compared by Ryan–Enot–Gabriel–Welch (REGW) a posteriori tests (Ray & Sall 1985). Seed production Once siliques were mature, but before they dehisced, they were individually removed and returned to the laboratory. Siliques per raceme and the individual contents of the dark seeds were counted (Fig. 1). To estimate and compare seed viability, embryo development was compared for plump seeds differing in color (dark vs pale) using X-ray imagery (HP 4380 N Faxitron; Hewlitt Packard, Salt Lake City, UT, USA; 25 KV, 30 s exposure, medium grain industrial film) (Fig. 1) from lots of 20 seeds per species and color, representing all pollination treatments. Persistently pale seeds and pale seeds that had darkened 1 week after harvest were X-rayed again and their germination evaluated. Two lots of 10 dark seeds each per species were weighed. Three large, mature, intact plants of C. lutea were taken at the end of flower production. The life-time silique production per uncaged plant was determined by counting their complements of siliques and replums (a loop of tissue that persists after the valves of the siliques have dehisced) (Fig. 1). Lifetime seed production was then estimated by multiplying by the average numbers of seeds per silique for that species. Seedling vigor Harvested seeds of C. lutea and C. serrulata from manually selfed and outcrossed flowers were placed in cold (4°C) storage for 4 months and then planted individually in ‘conetainers’ in a heated glasshouse. On 19 April 2005, seedlings were measured for size (height, length and width of largest leaflet). Once the data were transformed (log10), the variances were homogeneous (Levene’s test) and the data were normally distributed (Kolmogorov– Smirnov test). The performance of plants from outcrossed versus selfed seed was compared by general linear model anova followed by REGW a posteriori tests where warranted (Ray & Sall 1985). Statistical significance in all cases was P ⱕ 0.05. Results Flowering The flowers of Cleome presented an unexpected phenology given the diverse diurnal pollinator fauna revealed by this and other studies (e.g. Messinger 2006). All new flowers of both species opened nocturnally, starting 1–3 h after sunset. No new flowers were added during daylight hours. For C. lutea, newly opened flowers from 10 plants were checked 150 mins after sunset on 13 July (23.30 hours mountain daylight savings time [MDST]). New pistils (n = 15) of C. lutea flowers were large, and each stigmatic tip was suffused with red pigment. Their pollen-free stigmas all produced frequent bubbles when immersed in hydrogen peroxide, indicative of their receptivity. The anthers of half of these flowers had begun to dehisce, each anther first rupturing at its distal tip. For C. serrulata, the petals had unfurled by approximately 90 mins after sunset on 7 August. One or more nectar droplets was visible in 90% of these opening flowers, although at this hour only 2 of 18 flowers had dehiscing anthers and none showed stigmatic peroxidase activity. Journal compilation © 2008 The Society for the Study of Species Biology No claim to original US government works Plant Species Biology 23, 152–158 P O L L I N AT I O N N E E D S O F CLEOME During the hours after sunset, no flower visitors were observed in the large uncaged plots of either species in the northern Utah common gardens, although the nights were warm and calm. By dawn, all new flowers of both species had opened and possessed fully dehisced anthers, receptive stigmas and large droplets of viscous nectar (large enough in C. serrulata to rain out of shaken plants). From an isolated C. lutea plant inspected just before sunrise, 11 of 17 stigmas from new flowers lacked pollen, and the remaining stigmas had <10 grains. Later that same sunny morning (10.30 hours MDST) after several hours of general bee activity, 14 of 15 stigmas bore pollen (most had >15 grains) on fresh flowers of the same plant. Thus, some stigmas of C. lutea receive pollen at night, apparently passively by autogamy; however, more thorough pollination occurred during morning bee activity. Breeding biology and seed production Both C. lutea and C. serrulata are fully self-fertile. Autogamy yielded considerable numbers of siliques and seeds for both species (Table 1). Autogamy is facilitated by the stamens coiling inward later in the day. For C. lutea, equivalent proportions of flowers set fruits in the autogamy, geitonogamy and xenogamy treatments; flowers accessible to pollinators were two–threefold more fruitful (Table 1). The average counts of fertile seeds per silique did not differ between treatments. In C. serrulata, autogamy was inferior to the other treatments, with autogamous flowers setting fewer siliques (F3,18 = 3.39, P < 0.04) each with fewer seeds (F3,228 = 20.49, P < 0.0001) (Table 1). Neither xenogamy nor pollinator access improved on geitonogamy for either fruit set or seeds per silique. Comparing the two species, siliques of C. serrulata produced threefold more mature seeds than those of C. lutea in all but the autogamy treatment (Table 1). 155 The X-ray images of seeds from both Cleome species revealed the folded embryo within. Images of seeds could be visually classed into two groups, those possessing an X-ray dense embryo (dark in positive image) (Fig. 1 inset) and those whose embryo was less dense (gray). Dark seeds invariably had an X-ray dense embryo and were all readily germinable, whether dark at harvest (20 of 20 images) or darkening in the week after harvest (20 of 20). Pale seeds at harvest sometimes lacked the visually distinct embryo (5 of 20); those that remained pale 1 week after harvest mostly lacked developed embryos (14 of 20). Mature dark seeds of both species were comparable in weight, averaging 6.7 mg each (for an estimated 150 000 seeds per kg). In the common garden, three large openly visited C. lutea plants produced an average of 1470 siliques each containing on average eight seeds, those of C. serrulata produced 1229 siliques with 21 seeds each. In a dense vegetated part of the plot, 22 C. lutea plants grew in 0.1 m2, yet even these averaged 6.6 ⫾ 3.6 racemes per plant. One big C. lutea raceme set large seeds from 80% of its 336 flowers. The C. lutea plants averaged 110 ⫾ 61 racemes per m2 of plot. Seedling vigor Progeny of C. lutea grown in the glasshouse from seed that was dark at harvest (‘early’) were similar in size to those from seed that darkened the week after harvest (‘late’). Seedling progeny from these two seed maturation classes (early or late) were equivalent in leaf and plant dimensions (6–10 seedlings measured per species; P > 0.1 to P > 0.8). If the seed was viable (dark), then whether it matured on or off the plant did not affect subsequent seedling vigor. Progeny from the two manual pollination treatments were not equivalent in vigor in either species. Seedlings Table 1 Comparison of fruit set, mature dark seeds per silique and progeny vigor for four pollination treatments of Cleome lutea and Cleome serrulata Reproductive response Cleome species Flowers setting fruit (%) lutea serrulata lutea serrulata lutea serrulata lutea serrulata Seeds per fruit Seedling height (cm) Seedling longest leaf (cm) Pollination treatment (⫾ SD)† AutoGeitonXenogamy ogamy gamy 23 ⫾ 15 34 ⫾ 15* 6⫾4 8 ⫾ 6* – – – – 31 ⫾ 15 56 ⫾ 14 7⫾4 19 ⫾ 10 17 ⫾ 3.6* 13 ⫾ 3.7* 35 ⫾ 5.6* 25 ⫾ 3.0 26 ⫾ 16 63 ⫾ 22 7⫾4 22 ⫾ 11 11 ⫾ 3.6 10 ⫾ 2.4 25 ⫾ 3.4 26 ⫾ 3.0 Sum of measured Freely visited Plants Flowers or fruits 76 ⫾ 13* 64 ⫾ 32 8⫾4 21 ⫾ 11 – – – – 47 22 47 22 70 48 70 48 1235 720 263 232 – – – – †All statistical comparisons are between treatments within species. *Values are statistically different from other treatments within their row (P ⱕ 0.05). Only seeds from manual pollinations were sown and compared for progeny vigor. SD, standard deviation. Plant Species Biology 23, 152–158 Journal compilation © 2008 The Society for the Study of Species Biology No claim to original US government works 156 J . H . C A N E resulting from geitonogamy were equal to, or larger than, those from xenogamy (Table 1). For C. lutea seedlings of like age, those from geitonogamy exceeded those from xenogamy in both height (F1,69 = 43.3, P < 0.001) and length of the longest leaf (F1,69 = 72.7, P < 0.001) (Table 1). For C. serrulata seedlings of like age, those from geitonogamy were 25% taller than outcrossed progeny (F1,47 = 6.7, P < 0.015), but seedlings from both treatments were equivalent in leaf length and leaflet width (Table 1). No xenogamy advantage was evident in these measures of seedling performance. Discussion Both Cleome species possess pollination traits that have long been considered favorable for colonists (Baker & Stebbins 1965). Self-fertility enables the first individual that colonizes a site to produce viable seed. The coiling stamens of older flowers facilitate autogamy. Furthermore, progeny from geitonogamous seed grew at least as vigorously as progeny from xenogamy. If pollinators are absent, perhaps following some ecological perturbation such as fire or flood, both Cleome species are capable of autogamy and facilitated self-pollination. Selection is expected to favor self-fertility of colonizing annuals like Cleome when pollinator services might be unreliable (e.g. soon after habitat perturbation) (Lloyd 1992). The rate of expansion and persistence of Cleome populations in nature is not known, but in our common gardens, the parent plants set massive numbers of readily germinable seeds that produced dense stands of seedlings the following year. If representative of wild populations, even when bees are present, these Cleome species are likely to be under serviced by their pollinators in some years of massive bloom, circumstances again favoring self-fertility and a degree of autogamy. Phylogenetic inference from sister families was not predictive of Cleome breeding biologies. These Cleome species did not share the distinct SI system found in their sister family, the Brassicaceae (Charlesworth et al. 2005). Meager evidence from the Capparaceae (sister group to Brassicaceae and Cleomaceae) was not illuminating either, for while Capparis flexuosa is self-fertile, C. verrucosa is reportedly self-incompatible (Zapata & Arroyo 1978). The nocturnal anthesis of these Cleome flowers is puzzling. Most species of Brassicaceae flower diurnally. A desert exception, Lyrocarpa coulteri, has drab, somewhat tubular flowers that release a heavy Gardenia-like scent after nightfall (J. Cane, pers. obs., 1994), attributes consistent with moth pollination. In contrast, flowers of both C. lutea and C. serrulata are vividly colored and scentless (to humans), attributes atypical for nocturnally pollinated flowers (Faegri & van der Pijl 1979). The flowers attracted no nocturnal visitors in the common gardens, but plenti- ful bees, wasps and butterflies during daylight hours, attributes shared with some other desert Cleomaceae (e.g. Wislizenia, Cleomella and Oxystylis). With reference to the Capparaceae, the flowers of Capparis ovata and Capparis spinosa are nocturnal, the former pollinated by sphingid moths, while those of C. spinosa (in Israel) are first visited nocturnally by pollen-foraging Proxylocopa bees, but later the next day by honey bees and other bees as well (Dafni et al. 1987). Perhaps nocturnal anthesis is a persistent ancestral attribute in these Cleome species. For now, no satisfying ecological or phylogenetic explanation is apparent for the peculiar flowering schedules of these two species of Cleome. Beginning in the morning, diverse polylectic (i.e. generalist) bees, butterflies and wasps opportunistically sought nectar and sometimes pollen from flowers of both Cleome species in the common gardens (Fig. 2). Bees have been extensively collected from wild C. lutea in southern Utah, both for this study and from 4 years of exhaustive bee surveys in Grand Staircase-Escalante National Monument (O. Messinger and T. Griswold, pers. comm., 2007). Polylectic bees were found to be prevalent and widespread on C. lutea, accounting for 3/5 of the collected specimens. Nearly half of the polylectic individuals sampled belonged to just three genera of small-bodied bees: Halictus, Hylaeus and Lasioglossum sensu lato, plus diverse Perdita that are likely to be specialists on other floral hosts. Bees less frequently collected from C. lutea represent 28 other native bee genera: Agapostemon, Andrena, Anthidiellum, Anthidium, Anthophora (Fig. 2), Ashmeadiella, Bombus, Ceratina, Colletes, Diadasia, Dianthidium, Dieunomia, Dufourea, Eucera, Exomalopsis, Habropoda, Heriades, Macrotera, Megachile, Melecta, Melissodes, Nomada, Osmia, Protandrena, Sphecodes, Stelis, Triepeolus and Xylocopa. Overall, 140 species of native bees have been taken from C. lutea in southern Utah, Fig. 2 Digger bee (Anthophora californica) hovering to collect pollen from the exserted anthers of a Cleome lutea flower. Journal compilation © 2008 The Society for the Study of Species Biology No claim to original US government works Plant Species Biology 23, 152–158 P O L L I N AT I O N N E E D S O F CLEOME collectively representing many of the native bee genera found in the western USA. The fauna at C. serrulata has not been so exhaustively sampled. Nonetheless, 62 species of native bees were collected in the Grand Staircase-Escalante National Monument survey, with 95% of those individuals representing 51 bee species shared with C. lutea (Messinger 2006). Together, the 162 native bee species found on these two Cleome species are a substantial fraction of the speciesrich bee community that inhabits the 730 000 hectares of the National Monument (Messinger 2006). In particular, one-third of the Monument’s entire fauna of polylectic bee species (58% of all 656 species) were collected visiting C. lutea and/or C. serrulata. These two floral hosts can be expected to attract and feed many additional polylectic bee species throughout their broad geographic ranges in the western USA (23 additional bee species in the general collections at the Bee Biology and Systematics Laboratory were collected from C. lutea and C. serrulata). Flowering patches of Cleome attract and feed diverse floral generalists from local bee communities, underscoring the potential value of these plants for native bee communities where summer floras are otherwise lacking bloom. Other species-rich floral guilds of bees often include numerous oligolectic species (taxonomic pollen specialists) because their hosts (e.g. Helianthus, Larrea, Salix, Phacelia) present a ‘predictable plethora’ that oligolectic populations can depend on for ample pollen (Wcislo & Cane 1996). In contrast, the two Cleome species examined in the present study appear to be opportunistic colonists whose annual bloom varies greatly with patchy annual rainfall. Only a single putative oligolege is associated with either Cleome species, the tiny bee Perdita zebrata. Perdita zebrata is common on C. lutea, accounting for half of the 2463 bees sampled from C. lutea in southern Utah. In turn, the Cleomaceae account for 90% of all 1100 host labels for this bee in the extensive US Department of Agriculture– Agricultural Research Service bee collections at Logan, suggestive of it being a Cleome specialist. Many of the P. zebrata specimens caught from C. lutea were males, suggesting that they use Cleome flowers as likely sites to find mates, a habit common among oligoleges (Eickwort & Ginsberg 1980). A local bee community study found that P. zebrata dominated C. lutea, rarely if ever visiting the other abundant flowering species used by diverse native floral generalists (Tepedino et al. 2008). Other than P. zebrata, the bee faunas found associated with C. lutea and C. serrulata all appear to be floral generalists. This accords well with the relative dearth of floral specialists associated with members of the Brassicaceae (Hurd 1979; Gomez & Zamora 1999), likely reflecting the fact that their populations are transient and their floral morphologies and rewards are unspecialized (Wcislo & Cane 1996). Plant Species Biology 23, 152–158 157 Farmed C. lutea and C. serrulata should produce prodigious quantities of seed. Multiplying together seeds per silique (Table 1), siliques per raceme and racemes per plant, each mature, well-pollinated C. lutea plant in the common garden produced an estimated 11 000 viable seeds (73 g fresh weight). Likewise, large plants of C. serrulata each produced an estimated 26 000 seeds (173 g). Conservatively, a farmed hectare of these two Cleome species could produce nearly 2000 kg/ha of seed, although the species’ indeterminate growth and flowering would prevent harvest of all of that seed at once. Adequately abundant pollinators will be necessary to realize such prodigious seed production by these two Cleome species; plants accessible to pollinators yielded three–fivefold more viable seed than those limited to autogamy (Table 1). Fortunately, these Cleome species are broadly attractive to a diverse array of generalist bees, including commercially managed species, such as hived honeybees or alfalfa leaf-cutting bees. General stewardship of otherwise unmanageable local ground-nesting bees on farms could also be beneficial. Over several years, such stewardship practices could foster adequate numbers of generalist bees to satisfy the pollination needs of fields planted with this crop. In turn, these Cleome species can feed diverse members of native bee communities on a farm, gradually multiplying their numbers to pollinate additional native summer seed crops. Ground-nesting bees comprise 90% of the individuals and 80% of the species taken from C. lutea in the wild. This bodes well for wild bee communities of the Intermountain West amid postfire restoration seedings because progeny of most of these ground-nesting bees should be deep enough underground to survive the surface heat of wildfires. Seeded in the autumn during postfire rangeland rehabilitation treatments, these Cleome species will germinate and bloom in the following summer, and their nectar and pollen will feed surviving members of mid-summer bee communities at a time when most seeded perennials will often still be in vegetative growth stages. As both Cleome species attract a diverse array of bees and set prodigious seed, a land manager’s choice will be guided by an individual Cleome species’ geographic range and its habitat requirements, particularly soil texture and moisture, rather than any difference in value for wild bee communities. Acknowledgments Faye Rutishauser, Melissa Weber, Morgan Yost and James McDonald contributed ably to all facets of the field and laboratory work. Terry Griswold kindly shared label data from bee surveys of the Grand Staircase region of southern Utah and Harold Ikerd assisted with database searches. Dr Hugh Iltis enthusiastically shared his Journal compilation © 2008 The Society for the Study of Species Biology No claim to original US government works 158 J . H . C A N E research insights for the Cleomaceae. I am especially grateful to Drs Vincent Tepedino and Nancy Shaw for their thorough and constructive critiques. This research was funded by the Great Basin Native Plant Selection and Increase Project through the USDI-BLM Great Basin Restoration Initiative and the USDA-FS Rocky Mountain Research Station. References Baker H. G. & Stebbins G. L. (1965) The Genetics of Colonizing Species. Academic Press, New York. Cane J. H. (2008) Pollinating bees crucial to farming wildflower seed for U.S. habitat restoration. In: James R. R. & Pitts-Singer T. L. (eds). Bees in Agricultural Ecosystems. Oxford University Press, New York, pp. 48–64. Charlesworth D., Vekemans X., Castric V. & Glemin S. (2005) Plant self-incompatibility systems: a molecular evolutionary perspective. New Phytologist 168: 61–69. Dafni A., Eisikowitch D. & Ivri Y. (1987) Nectar flow and pollinators’ efficiency in two co-occurring species of Capparis (Capparaceae) in Israel. Plant Systematics and Evolution 157 (3–4): 181–186. Eickwort G. C. & Ginsberg H. S. (1980) Foraging and mating behavior in Apoidea. Annual Review of Entomology 25: 421–446. Faegri K. & van der Pijl L. (1979) The Principles of Pollination Ecology. Pergamon Press, New York. Free J. B. (1993) Insect Pollination of Crops. Academic Press, New York. Gomez J. M. & Zamora R. (1999) Generalization vs specialization in the pollination system of Hormathophylla spinosa (Cruciferae). Ecology (Washington DC) 80: 796–805. Hall J. C., Sytsma K. J. & Iltis H. H. (2002) Phylogeny of Capparaceae and Brassicaceae based on chloroplast sequence data. American Journal of Botany 89: 1826–1842. Hurd P. D. Jr (1979) Superfamily apoidea. In: Krombein K. V., Hurd P. D. J., Smith D. R. & Burks B. D. (eds). Catalog of Hymenoptera in North America North of Mexico, 2nd edn. Smithsonian Institute Press, Washington, pp. 1741– 2209 Iltis H. H. (1957) Studies in the Capparidaceae. III. Evolution and phylogeny of the western North American Cleomoideae. Annals of the Missouri Botanical Garden 44: 77–119. Lloyd D. G. (1992) Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences 153: 370–380. Messinger O. (2006) A survey of the bees of Grand StaircaseEscalante National Monument, southern Utah: incidence, abundance, and community dynamics. Utah State University. Murneek A. E. (1937) The physiological basis of intermittent sterility with special reference to the spider flower. University of Missouri Agricultural Experiment Station Research Bulletin 106: 1–37. Ray A. A. & Sall J. P. (1985) SAS User’s Guide: Statistics. SAS Institute, Cary. Sanchez-Acebo L. (2005) A phylogenetic study of the new world Cleome (Brassicaceae, Cleomoideae). Annals of the Missouri Botanical Garden 92: 179–201. Stout A. B. (1923) Alternation of sexes and intermittent production of fruit in the spider flower (Cleome spinosa). American Journal of Botany 10: 57–66. Tepedino V. J., Bradley B. A. & Griswold T. L. (2008) Might flowers of invasive plants increase native bee carrying capacity? Intimations from Capitol Reef National Park, Utah. Natural Areas Journal 28: 44–50. Wcislo W. T. & Cane J. H. (1996) Floral resource utilization by solitary bees (Hymenoptera: Apoidea) and exploitation of their stored foods by natural enemies. Annual Review of Entomology 41: 195–224. Zapata T. R. & Arroyo M. T. K. (1978) Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica 10: 221–230. Zeisler M. (1938) Uber die Abgrenzung der eigentlichen Narbenfläche mit Hilfe von Reaktionen. Beihefte Zum Botanisches Zentralblatt A 58: 308–318. Journal compilation © 2008 The Society for the Study of Species Biology No claim to original US government works Plant Species Biology 23, 152–158